Thermo-physical Characteristics of Nickel-coated Aluminum Powder as a Function of Particle Size and Oxidant
LEE Sanghyup, NOH KwanyoungLIM Jihwan, and YOON Woongsup
Thermo-physical Characteristics of Nickel-coated Aluminum Powder as a Function of Particle Size and Oxidant
LEE Sanghyup1,*, NOH Kwanyoung1, LIM Jihwan2, and YOON Woongsup1
12
Aluminum particles 15–25 µm in size are widely used in fuel propellants and underwater propulsion systems in national defense research. However, these particles are covered with an aluminum oxide film, which has a high melting point, so ignition is difficult. To improve the ignitability of high-energy aluminum powder and to understand the reaction phenomenon as a function of particle size(15–25 µm, 74–105 µm, and 2.38 mm) and oxidizer(air, CO2, and argon), the natural oxide films are chemically removed. The particles are then coated with nickel using an electro-less method. The degree of nickel deposition is confirmed qualitatively and quantitatively through surface analysis using scanning electron microscopy/energy dispersive spectroscopy. To characterize the nickel coatings, elemental analysis is also conducted by using X-ray diffraction. Thermogravimetric analysis/differential scanning calorimetry (TGA/DSC) enable comparisons between the uncoated and coated aluminum, and the reaction process are investigated through fine structural analysis of the particle surfaces and cross sections. There are little difference in reactivity as a function of oxidant type. However, a strong exothermic reaction in the smaller nickel-coated aluminum particles near the melting point of aluminum accelerates the reaction of the smaller particles. Explanation of the reactivity of the nickel-coated aluminum depending on the particle sizes is attempted.
nickel-coated aluminum, TGA/DSC, aluminum ignition, combustion, intermetallic reaction
1 Introduction
Aluminum has been widely used to increase the energy of solid propellants owing to its high enthalpy[1–6]. It also used as an industrial material owing to its good corrosion resistance, workability, and thermal conductivity, along with a specific gravity of only 2.7 g/cm3[7]. In addition, aluminum has been researched for underwater propulsion systems that use seawater as an oxidizer[8]. It is also important as a fuel for lunar exploration and Mars missions[9]and as a fuel for power generation and hydrogen production from a reaction with water[10]. The high-temperature combustion of aluminum is considered to be important in the self-propagating high-temperature synthesis of such materials and in the production of metal oxide[11–13]. However, there are limitations to the application of aluminum. The formation of an aluminum oxide film as a result of oxidation on the particle surface at lower temperatures increases the ignition temperature of the particles. Therefore, the disadvantage of using aluminum as a fuel is that it naturally oxidizes to form Al2O3, which has a high melting point (=2400 K). Furthermore, this oxide film is known to cause agglomeration of aluminum within solid rocket motors during combustion, which leads to incomplete combustion of the aluminum and the formation of slag that deters steady burning[14–15]. Thus, due to either an increase in the burning time or a decrease in the burning rate of aluminized energetic materials, the combustion efficiency of these materials is reduced. Therefore, it is difficult to directly use aluminum as a fuel. To overcome such problems, there are three possibilities: volume modification of the aluminum by chemically active elements, complete removal of Al2O3from the particle surface, or application of a coating with controlled properties to the particle surface[16]. Various research groups have been interested in the third method, and nickel has mainly been used as the coating material because of its various advantages[17]. Some groups have used copper[18], carbon[19], iron[20], graphite[21], or Teflon[22]as a coating material. However, in many other cases, nickel was used as a surface coating material. Through a self-propagating, high-temperature synthesis (SHS) reaction between nickel and aluminum, a Ni–Al alloy is produced. The bond between the metal atoms is produced by the enthalpy difference between the melting point of aluminum and the heat generated by the SHS reaction. This exothermic energy causes enhanced aluminum particle ignition. The reason for using nickel as a coating material is its corrosion resistance, where a thin coating of nickel on an aluminum surface prevents the oxidization of aluminum during general storage conditions. The nickel also forms an initial warming phase[16, 23]. In addition, the melting point of nickel(1728.15 K) is higher than that of aluminum(933.45 K), so nickel provides thermophysical protection at high temperatures, along with good ignitability because its melting point is much lower than that of aluminum oxide[24]. Moreover, there have been reports that when the aluminum surface is coated with nickel, there is reduced agglomeration of the unburnt aluminum that hinders burning[25–26]. However, few studies closely examine the reaction process of nickel-coated aluminum particles. Most of the previous studies have focused on CO2, argon, the burnt gas of the propellant, and the combustion of dust clouds of aluminum particles in an air environment. In terms of particle size, most of these studies have been limited to millimeter-sized aluminum particles. Many researchers have also provided insight on the ignition and burning of nickel-coated single aluminum particles from research conducted on micron-sized particles with laser ignition in air[27–28]. However, there was only a very short time to analyze the ignition characteristics due to the experimental environment and a lack of sufficient details, so it was difficult to observe what was happening on the particle surface in terms of ignition details. In such circumstances, it is reported that the ignition temperature increased due to oxygen despite the nickel coating on the aluminum surface, but a deeper understanding of this phenomenon was lacking[29]. In terms of ignition mechanisms, many researchers have mentioned that aluminum is oxidized and ignition occurs as the shell structure of the nickel surrounding the aluminum is broken by thermal stress[16, 30]. Reactions inside the particles resulting in the formation of NiAl3were also detected, which indicates that the melt spreading of aluminum plays a major role in the ignition of nickel-coated aluminum particles[31]. In a combustion synthesis experiment to produce nickel aluminide using a process in which nickel and aluminum particles are physically mixed together, it was shown that the ignition process was connected to the phenomenon of molten aluminum spreading over the surfaces of the nickel particles[31]. Several research groups conducting ignition experiments on nickel-coated aluminum powder have reported that as the nickel coating is broken by thermal expansion, the molten aluminum leaks out and flows over the nickel surface. Heat is supplied by the SHS reaction, leading to ignition[32]. The exothermic intermetallic reactions between liquid aluminum and solid nickel lowered the ignition temperature, thus greatly decreasing the ignition delay time. This reaction has been identified as the major factor in promoting ignition[27]. The experimental conditions, however, a lack of sufficient details required to reveal ignition details.
The goal of the present study was to investigate aluminum reactivity as a function of particle size and oxidizing environment. Especially, aluminum particles 15–25 µm in size are widely used in fuel propellants and underwater propulsion systems in national defense research. So we focus on 15–25 µm sized aluminum. To accomplish this, nickel coating of aluminum particles of various sizes using an electroless in-house coating process was carried out and the samples were characterized by XRD analysis. SEM/EDS provided quantitative and qualitative analysis of the nickel coating. TGA/DSC enabled comparisons between uncoated and coated aluminum. We also explain the reaction process using fine structural analysis of the surfaces and cross sections of the particles.
2 Experimental
The aluminum powder particles used in this study had a spherical shape, and 99.99% purity. Fig. 1 is a diagram of the entire coating process. The particle-size distributions were measured using an AccuSizer 780AD, and the results are presented in Fig. 2.

Fig. 1. Schematic of the pre-treatment and nickel-plating processes

Fig. 2. Characterization of particles used in the work
Fig. 2 also displays the SEM image of the aluminum powder after the oxide film was removed. These spherical aluminum particles were preprocessed as follows. The particles were dipped in a mixed solution of chromium(VI) oxide(CrO3, Sigma-Aldrich) and phosphoric acid(H3PO4, Sigma-Aldrich) at 50℃ for 10 min to remove the oxide film on their surfaces[33]. To prevent re-oxidation of the aluminum surface during the nickel-coating process, the interval between the processes was minimized. After vacuum drying at 80℃ for 5 h, the isothermal–isohumidity was maintained in a vacuum desiccator before the experiments. The electroless coating method in this study utilized a coating solution comprising a mixture of nickel sulfate, hydroxyl-ammonium, glycin, citric acid, borated dimethylamine complex, and distilled water. The coating temperature was 338 K. To enable the fabrication of a nickel coating of uniform quality and thickness, which minimizes the influence of coating variations on the ignition process, a shaking water bath was used and agitation was performed at 100 r/min. The coating temperature was maintained by a proportional-integral- derivative(P.I.D.) control. Water cleansing and degreasing was repeated before and after all coating processes to prevent sample contamination. The coated particles were separated from the bath, washed in distilled water several times, and finally dried in a vacuum oven at 80℃ for 5 h. A previous study reported that complete combustion occurs when the nickel fraction is less than the stoichiometric ratio of the synthetic reaction; thus, preliminary experimental results on the coating thickness as a function of time were used to form a 0–35 wt% nickel coating film[30]. SEM/EDS(JEOL, JCM6000) were used for surface analysis of the nickel-coated aluminum powder. The uniformity of the coating film as a function of time was qualitatively assessed by SEM/EDS image analysis. The chemical element composition was verified by XRD (RIGAKU, Ultima IV) with Cu Kα radiation (=1.540 2 Å). The coating thickness analysis was done by inductively coupled plasma optical emission spectrometry (ICP-OES; PerkinElmer, OPTIMA 8300). The element analysis was carried out by EDS using the atomic number correction(ZAF)[34]. TGA/DSC(TA Instruments, SDT-Q600) was used for thermophysical analysis. At the time of the experiment, the mass of the specimens was approximately 40 mg and they were heated at a rate of 20 K/min. The temperature range during analysis was approximately 30–1800 K. To compare the reactivity as a function of the particle size, we used nickel-coated aluminum particles 15–25 µm, 74–105 µm, and 2.38 mm in size. We compared the reaction of each sample group in air, CO2, and argon environments for the analysis.
3 Result and Discussion
The shape and morphology of a nickel-coated aluminum particle is displayed in Fig. 3. It confirms that a nickel coating was successfully deposited onto the surface of the aluminum particle following removal of the natural oxide film. The nickel coating was fairly homogeneous in all the samples. XRD analysis was used to measure the intensity spectrum generated from the diffraction of irradiated X-rays of the coated sample surface. The reference databases used to compare the elemental patterns were JCPDS[35].

Fig. 3. SEM micrographs of nickel-coated aluminum particle at (a) low (x2700) and (b) high (x5000) magnification.

Fig. 4. XRD pattern of (a) nickel-coated aluminum and (b) pure aluminum
It is well known that sintering nickel with aluminum involves a 1:1 intermetallic reaction and that ignition characteristics decrease when the mass fraction of nickel exceeds approximately 50%[30]. As a fuel for propulsion systems, the nickel coating should be thin because its only role is as a protective film over aluminum. It also serves to accelerate the reaction without energy loss. Therefore, the mass fraction of nickel should be less than 50% in order to improve the ignition characteristics of aluminum produced with the SHS reaction, and all experiments in this study met this criterion.
Fig. 5 shows the nickel-coated aluminum particles before and after TGA/DSC measurements for the different oxidizers. The nickel oxide layer of the smaller aluminum particles(15–25 µm) collapsed in the air and CO2oxidizers. For the larger aluminum particles(74–105 µm), although partial formation of NiAl3by the SHS reaction between the aluminum and nickel was observed because of cracks in the NiO layer, the shape of the NiO oxide layer was retained. The largest aluminum particles(2.38 mm) formed NiO and NiAl3. Cracks are visible with this size particle, and the Al2O3layer is seen between the cracks. The aluminum that leaked out through the cracks was oxidized, forming Al2O3on the surface as a result of low-temperature oxidation. The formation of small amounts of NiAl3can be seen inside the layer. However, we confirmed that the larger nickel-coated aluminum particles (74–105 µm and 2.38 mm) retained the shape of the NiO oxide layer in all oxidizer environments, whereas the smaller nickel-coated aluminum particles did not. We therefore precisely analyzed the effect of particle size for each of the oxidizers.

Fig. 5. Cross-sectional SEM images of the nickel-coated aluminum powders as a function of particle size after TGA and DSC experiments in air, CO2, and argon environments
3.1 Influence of particle size in air oxidizer
In this study, in order to investigate the effect of the particle size of aluminum in the air, we performed thermo-physical analysis. To facilitate understanding of the effect of aluminum particle size on the ignition mechanism in air, TGA and DSC analysis were conducted using air as the oxidizer within a temperature range of 300–1800 K. Fig. 6 displays the TGA and DSC curves of 99.9% pure nickel (2.2–3.0 µm particle size, Alfa-Aesar). Pure nickel undergoes a gradual exothermic reaction at 600–1100 K as a result of the heat generated from the combination of oxygen and nickel to form bright-green nickel oxide (NiO)[36].
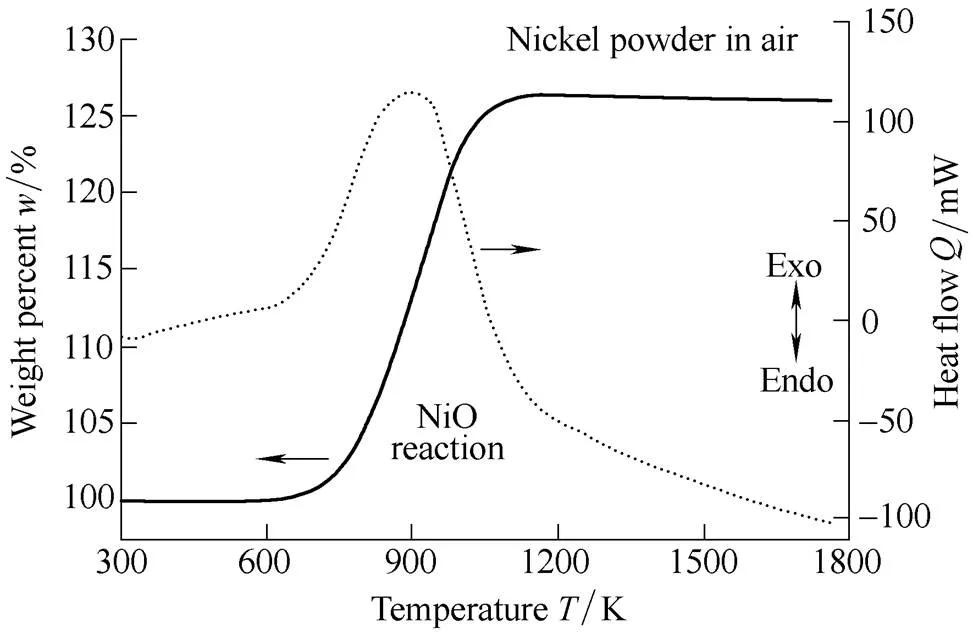
Fig. 6. TGA and DSC results of nickel powder in air
The heat of formation is Δf,298= –239.7 kJ/mol, resulting in an increase in mass of approximately 26.3% as compared to the initial mass. NiO develops porosity as oxygen combines on the surface of the pure nickel powder and simultaneously combines with the surrounding NiO powder.
As seen in Fig. 7, the presence of NiO was confirmed from the XRD analysis Fig. 8 displays the TGA and DSC results of the pure aluminum following oxide film removal presented at a heating rate of 20 K/min. It can be seen in the TGA curve that there was minimal weight increase from 600 K to 773 K. However, when the temperature was increased to 800 K, a stepwise weight increase is observed. The figure shows that there are three weight increase stages: an initial slow oxidation stage, a sharply increasing oxidation stage, and the last oxidation stage. The initial slow oxidation stage occurs between 800 and 940 K and results in a weight increase of ~3%. It also shows that aluminum melts at 920 K. This illustrates that the mainly solid aluminum powder oxidizes during the initial slow oxidation stage and that the formed alumina phase may be an amorphous oxide layer. Unreacted aluminum powder is coated with the amorphous oxide layer, which transitions to a γ-Al2O3layer. In this stage, molten aluminum is oxidized quickly and may form γ-Al2O3[37]. The newly formed γ-Al2O3does not cover the entire aluminum surface owing to its density(3.6–3.67 g/cm3), which is greater than that of amorphous alumina(3–3.1 g/cm3). This causes openings in the oxide layer, exposing bare aluminum[38]. However, thermal cracking of the protective initial oxide film and its healing by a heterogeneous reaction, a homogeneous reaction followed by the formation of an oxide film(=healing) during particle heating.
As shown Fig. 8, the sharp oxidation stage demonstrates a weight increase of ~10% in the temperature region of 930–1320 K. The exposed aluminum spots are rapidly oxidized, and the oxidation rate reaches a maximum at approximately 1200 K, the temperature at which the reactions are most active. It is very interesting that there are obvious shoulder peaks in the DSC curves. This suggests that the sharp oxidation stage also produces an alumina phase that is different from γ-Al2O3, perhaps δ and θ phases. Finally, it means that the oxide layer transitions to α-Al2O3. The last oxidation stage occurs at 1350 K, after which the oxidation rate decreases. In this stage, the thickness of the α-Al2O3layer is increasing. The TGA curves continue to increase up to 1600 K. This means that the aluminum powder is not fully oxidized at this temperature. In summary, we confirmed that a 13% increase in mass occurred in the air-oxidized environment at approximately 1800 K. In addition, a strong endothermic reaction can be seen near 900 K as a result of aluminum melting. It is also confirmed that a strong exothermic reaction occurs at approximately 1100–1300 K because of a phase change to α-Al2O3on the particle surface.

Fig. 7. XRD pattern of nickel powder (NiO) after TGA and DSC experiments

Fig. 8. TGA and DSC results of pure aluminum powder in air
Fig. 9 shows the TGA and DSC analysis results from samples of 15–25 µm-sized nickel-coated aluminum particles as a function of nickel content(wt%). As previously mentioned, a rapid increase in the entire particle mass was observed as the temperature increased. This result indicates that a rapid reaction occurred in the air-oxidized environment resulting from the production of NiO and an intermetallic sintering reaction. In addition, the phase change observed in the nickel-coated aluminum at approximately 1250 K occurred more rapidly as the mass fraction of the coated nickel increased owing to the energy produced by the subsequent acceleration of the intermetallic reaction. This phenomenon is the result of the reaction between NiO and the alumina produced on the surface. A comparison of the results of DSC analysis of pure and nickel-coated aluminum indicates the occurrence of a weak exothermic reaction from the production of NiO near 600 K and a strong exothermic reaction from the intermetallic reaction near 900 K. It is estimated that a strong exothermic reaction occurred at approximately 920 K, the melting point of aluminum, which improved the reaction of the aluminum with nickel. To verify this, we analyzed a variety of particle sizes of the nickel-coated aluminum powder.

Fig. 9. TGA and DSC curves of pure aluminum and nickel-coated aluminum powder in air with respect to the nickel content(wt%)
Fig. 10 shows the TGA/DSC analysis results according to the various particle sizes of the nickel-coated aluminum in air. From the TGA results and similar to the trend seen with the 15–25 µm nickel-coated aluminum, we confirmed that the mass of the final reaction products for the 74–105 µm and 2.38 mm nickel-coated aluminum increased as a function of the nickel content. Unlike pure aluminum, as the nickel content increased, it became more difficult to distinguish the difference between increases in mass and the equilibrium stage. This phenomenon was caused by the continuous SHS reaction between aluminum and nickel as a function of the temperature. Similar to the smaller nickel-coated aluminum particles, the larger nickel-coated aluminum particles exhibited a weak exothermic reaction at 600 K owing to the heat of formation of NiO, but at the aluminum melting point at 920 K, the curve of heat flow shows the opposite result. The reason is as follows. Because the heat capacity of the smaller nickel-coated aluminum particles is lower than that of the larger particles, the liquefaction of the aluminum is accelerated. In the cracks of the nickel and nickel oxide layers, molten aluminum reacts with solid-state nickel. Also, the NiAl3intermetallic reaction occurs over a large area. Heat is generated by the intermetallic reaction, and there appear to be additional intermetallic reactions of Ni2Al3, Ni3Al, and NiAl, with strong exothermic reactions created by the high-temperature thermal environment. To verify whether these intermetallic reactions are the result of the reaction between aluminum and nickel rather than the effect of the air oxidizer, the same experiment was carried out in an argon environment. In the argon environment, there is no bonding with an oxidizer, so the exothermic reaction is seen to be solely the result of the SHS reaction.

Fig. 10. TGA and DSC curves of 15–25 µm, 74–105 µm, and 2.38 mm nickel-coated aluminum powder in air
As presented at Fig. 11, the 15–25 µm-sized nickel- coated aluminum showed a strong exothermic reaction as a result of the intermetallic reaction between aluminum and nickel at approximately 920 K, the melting point of aluminum. In addition, an endothermic reaction was observed by NiAl3melted at approximately 1130 K and Ni2Al3melted at approximately 1420 K. We were able to identify a variety of intermetallic reactions. On the other hand, the larger nickel-coated aluminum particles showed that endothermic reactions occurred at the melting point of aluminum; intermetallic exothermic reactions were not observed. The smaller nickel-coated aluminum particles were able to fully overcome the endotherm originating from melted aluminum and the heat flow turned exothermic, whereas for the 74–105 µm and 2.38 mm particles, this effect is absent and an endotherm is seen. Therefore, it was confirmed that the SHS reaction of Ni–Al does not always accelerate ignition in nickel-coated aluminum and that the effect is dependent on the particle size and reactive gas.
The nickel-coated 15–25 µm-sized aluminum powder was divided according to the mass change and the characteristics of the heat flow (in and out) from the TGA/DSC observations. The heating temperature was increased from 750 to 1762 K, and quenching was done to observe the morphology of the formed products and changes in the components. Figs. 12 and 13 show the SEM/EDS and XRD results, respectively. In the initial state, we clearly distinguished the nickel layer and aluminum.

Fig. 11. TGA and DSC curves of pure aluminum and nickel-coated aluminum powder in argon

Fig. 12. Cross-sectional view of the 15–25 µm-sized nickel- coated aluminum powder in air as a function of temperature observed under SEM and EDS at (a) 750, (b) 930, (c) 1050, (d) 1170, (e) 1350, (f) 1500, and (g) 1762 K

Fig. 13. XRD pattern in air as a function of temperature for 15–25 um-sized nickel-coated aluminum powder at (a) 750, (b) 930, (c) 1050, (d) 1170, (e) 1350, (f) 1500, and (g) 1762 K
We determined that NiO was formed by the reaction of nickel and oxygen at 750 K and that a strong exothermic reaction occurred at 930 K and 1050 K, near the melting point of aluminum, by a variety of intermetallic reactions of nickel and aluminum. This was attributed to the solid-state inter-diffusion that occurs between aluminum and nickel particles at temperatures around the eutectic temperature. These solid-state reactions form predominantly aluminum- rich compounds namely NiAl3(Δf,298= –150.6 kJ/mol) and Ni2Al3(Δf,298= –282.4 kJ/mol). These reactions are exothermic, which subsequently heat the sample from 750 K to the eutectic temperature of 930 K, thus triggering a reaction. The formation of these compounds can also lead to swelling of the sample and the Kirkendall effect owing to the unbalanced diffusivities of nickel and aluminum[39]. It was also reported that a pre-combustion (=ignition) stage exists for high heating rates at approximately 980 K owing to the formation of NiAl (Δf,298=–118.4 kJ/mol) layers, which effectively increases the temperature of the sample to the eutectic temperature[40]. The formation of NiAl phases can essentially act as a buffer that dilutes exothermic reactions. When the temperature reaches the melting point of aluminum, the reaction between aluminum and solid nickel starts at the interface with the formation of a solid NiAl3compound owing to the higher mobility of molten aluminum atoms. At some temperature, the difference in the thermal expansion coefficient, between the solid nickel shell (= 10–5K–1) and the molten aluminum (= 10–4K–1) core leads to cracking of the shell. It show that at this stage, aluminum melt spreads all over the solid nickel surface, increasing the contact area, which intensifies the reaction rate, leading to the effective reaction of the particle. This in turn results in larger mechanical stress in the particle, causing shell cracking at that in the resulting reaction of Ni2Al3, the formation of NiAl was promoted by NiAl3melted at approximately 1170 K and the exothermic reaction resulted from the α-Al2O3(Δf,298= –1675.7 kJ/mol) generated by the reaction with partial aluminum at 1350 K. When the temperature reached 1500 K, the intermetallic reaction became more active and NiAl2O4(Δf,298= –1921.5 kJ/mol) was generated by the high-temperature environment. When it reached 1762 K, the NiO oxide film was destroyed, leaving voids and a hollow structure. From this experiment, the small nickel-coated aluminum particles, different from large size ones, was observed that the reaction occurs a lot of SHS reaction. Through this, it was confirmed that the difference of reactivity in the air oxidizer depends on the particle size of the nickel-coated aluminum. In order to determine whether the particle size effect was the same in the CO2oxidizer, the same experiment was conducting using CO2
3.2 Influence of particle size in CO2oxidizer
In order to investigate the effect of the particle size of aluminum in the CO2oxidizer, we performed thermo-physical analysis. To facilitate understanding of the effect of aluminum particle size on the ignition mechanism in CO2, TGA and DSC analysis were conducted in a CO2-oxidized environment within a temperature range of 300–1800 K. Fig. 14 displays the heat and weight change of 99.9% pure nickel. Similar to the results of the air experiment, pure nickel undergoes an exothermic reaction in CO2. This phenomenon is caused by heat generated from the combination of oxygen and nickel during the formation of nickel oxide. Because of this, the mass increased approximately 25% as compared to the initial mass. The porosity of NiO increased as oxygen combined on the surface of the pure nickel powder and simultaneously combined with the surrounding NiO powder. Unlike the results of the air experiment, the exothermic reaction occurred across a broad temperature range. In the air experiment, the flat TGA curve of NiO production appeared at 1100 K, but in the CO2experiment, it started at approximately 1650 K. However, XRD analysis confirmed that only NiO was produced(Fig. 15), as seen in Fig. 7 for air. This difference of temperature is known to be caused by CO[41]. However, in this study, it was difficult to find out clearly, and therefore additional experiment and analysis is required.

Fig. 14. TGA and DSC results of nickel powder in CO2.

Fig. 15. XRD pattern of NiO after TGA and DSC experiments
Fig. 16 displays TGA and DSC results at heating rates of 20 K/min of the pure aluminum following oxide film removal. In the CO2oxidizer, the TGA results of pure aluminum followed the same trend as that of air, with three stages of weight increase: the initial slow oxidation stage, the sharp oxidation stage, and the last oxidation stage. Similar to the air experiment, we confirmed that mass increase occurred in the CO2-oxidized environment at approximately 1800 K. The mass increase of the pure aluminum in a CO2-oxidized environment is due to the production of alumina induced by heat deterioration on the surface. In addition, a strong endothermic reaction can be seen near 900 K as a result of aluminum melting. Further, it is confirmed that a strong exothermic reaction occurred at approximately 1100–1300 K because of a phase change to the α-Al2O3on the particle surface.
Fig. 17 shows the TGA and DSC analysis results of the 15–25 µm-sized nickel-coated aluminum as a function of the nickel content. Similar to the air experiment, a rapid increase in the entire particle mass was observed as the temperature increased. This result indicates that a rapid reaction occurred in the CO2-oxidized environment resulting from the production of NiO and an intermetallic sintering reaction. In addition, the phase change observed in the nickel-coated aluminum at approximately 1250 K occurred more rapidly as the mass fraction of the coated nickel increased owing to the energy produced by the subsequent acceleration of the intermetallic reaction. This phenomenon is a result of the reaction between NiO and alumina produced on the particle surface. A comparison of the results of DSC analysis of the pure and nickel-coated aluminum indicates the occurrence of a weak exothermic reaction from the production of NiO near 600 K and a strong exothermic reaction from the intermetallic reaction near 900 K. Similar to the air experiment, there is a strong exothermic reaction from the intermetallic reaction near 900 K in CO2. It is estimated that a strong exothermic reaction occurring at approximately 920 K, the melting point of aluminum, improves the reaction of the aluminum with nickel. To verify this, we analyzed a variety of particle sizes of nickel-coated aluminum.
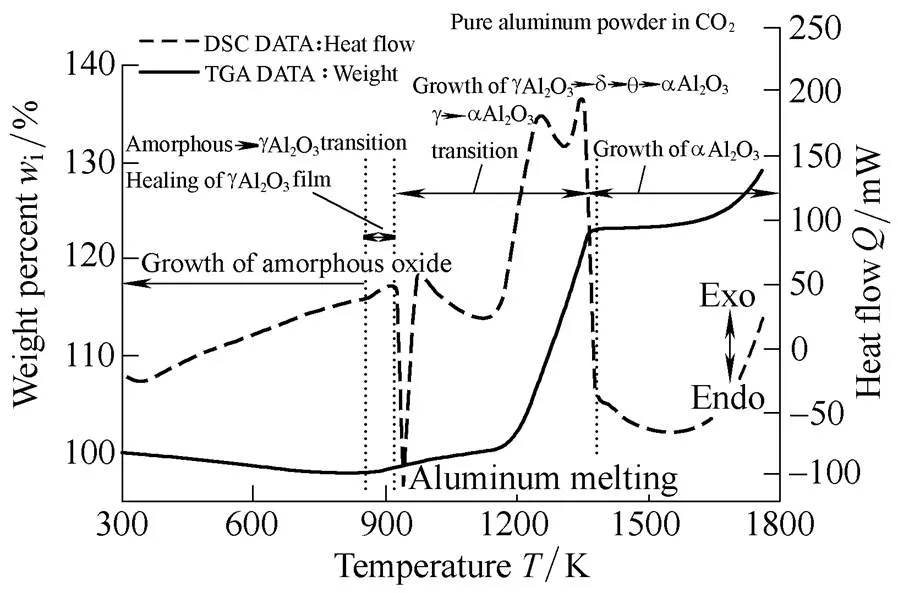
Fig. 16. TGA and DSC results of pure aluminum powder in CO2

Fig. 17. TGA and DSC curves of pure aluminum and nickel-coated aluminum powder in CO2with respect to the nickel content
Fig. 18 shows the TGA/DSC analysis results as a function of the various particle sizes of the nickel-coated aluminum in the CO2environment. The thermo-physical analysis results of the three particle sizes show tendencies similar to those of the air oxidizer. From the TGA results, we confirmed that the mass of the final reaction product of the larger nickel-coated aluminum particles increased as a function of the nickel content. Unlike the results seen with pure aluminum, as the nickel content increased, it was difficult to distinguish clearly the increasing mass and the stage at which the mass leveled off. This phenomenon was caused by a continuous SHS reaction between aluminum and nickel as a function of the temperature. Similar to the 15–25 µm-sized nickel-coated aluminum particles, the 74–105 µm and 2.38 mm-sized nickel-coated aluminum particles exhibited a weak exothermic reaction at 600 K as a result of the heat of formation of NiO, but in the aluminum melting point at 920 K, the curve of heat flow shows the opposite result. Similar to air oxidizer, the heat capacity of the nickel-coated 15–25 µm-sized aluminum particles is lower than that of the larger-size samples, and so the liquefaction of the aluminum was accelerated. In the cracks in the nickel and nickel oxide layer, molten aluminum reacts with solid-state nickel, and this intermetallic reaction occurs over a large area. Heat is generated by the intermetallic reaction, in addition to the intermetallic reactions of Ni2Al3, Ni3Al, and NiAl, and a strong exothermic reaction is created instantly by the high-temperature environment. The 15–25 µm-sized nickel- coated aluminum powder was able to fully overcome the endotherm originating from aluminum melting and the heat flow resulting from the exothermic reactions. With the 74–105 µm and 2.38 mm samples, this effect is absent and an endotherm is seen. Therefore, it was confirmed that the SHS reaction of Ni–Al does not always accelerate ignition in nickel-coated aluminum, and the effect may or may not occur depending on the particle size and reactive gas.

Fig. 18. TGA and DSC curves of 15–25 µm, 74–105 µm, and 2.38 mm nickel-coated aluminum powder in CO2
The nickel-coated 15–25 µm-sized aluminum powder was divided according to the mass change and the characteristics of the heat flow (in and out) in the TGA/DSC observation. The heating temperature was increased sequentially to 750, 930, 1050, 1170, 1350, 1500 and 1762 K and quenching was done to observe the morphology of the formed products and changes in the components. Figs. 19 and 20 show the SEM/EDS and XRD results, respectively. Similar to the results of the air experiment, in the initial state, we clearly distinguish a nickel layer and aluminum. Unlike the air experiment, however, the production of NiO at 750 K could not be confirmed because the reaction with oxygen starts at temperatures higher than 800 K, as shown in the pure nickel experimental results presented in Fig. 14.
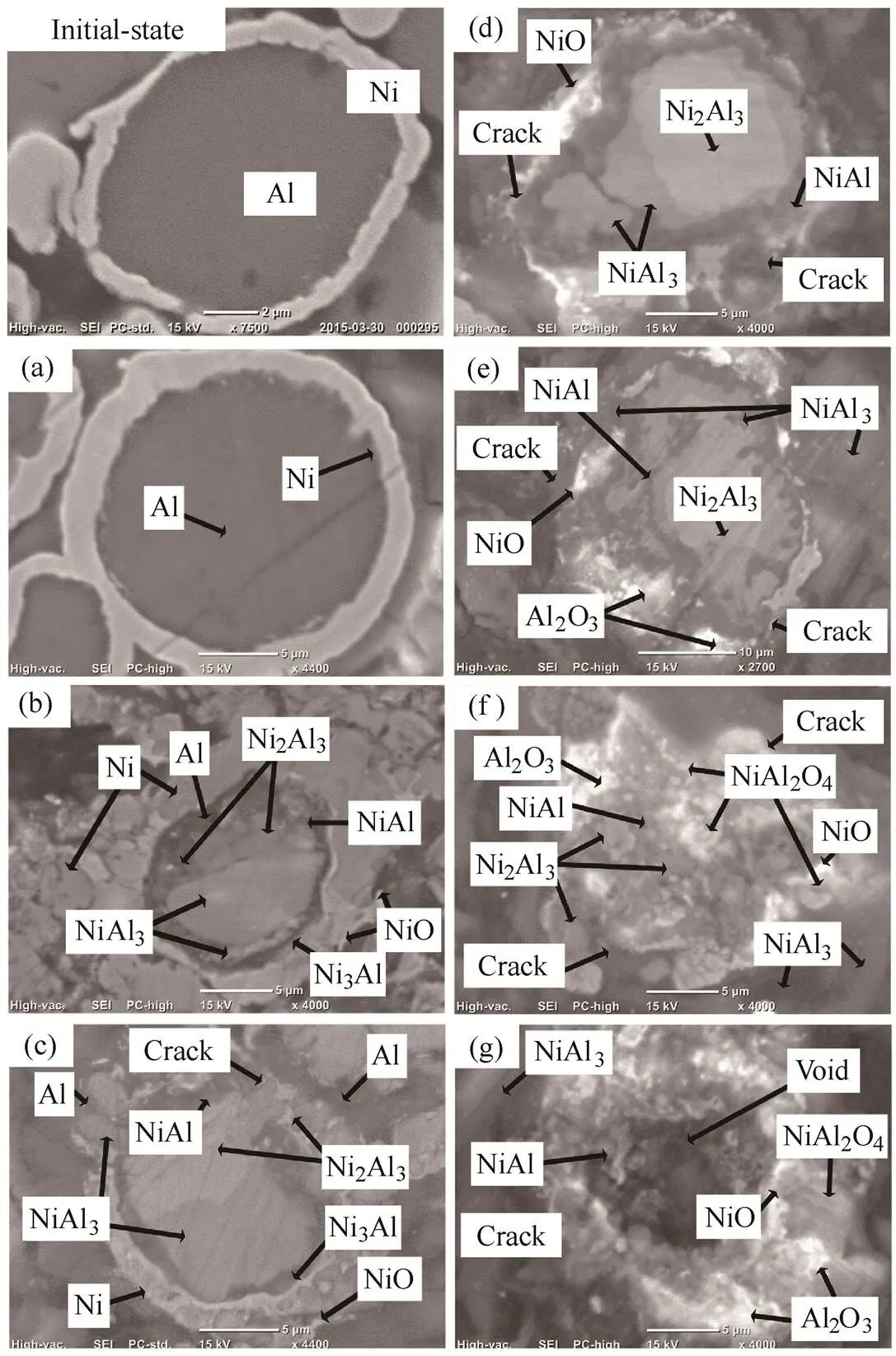
Fig. 19. Cross-sectional view of the 15–25 µm-sized nickel- coated aluminum powder in CO2 as a function of temperature observed under SEM and EDS at (a) 750, (b) 930, (c) 1050, (d) 1170, (e) 1350, (f) 1500, and (g) 1762 K
However, similar to the air oxidizer, it was confirmed that a strong exothermic reaction is generated by a various intermetallic reactions of nickel and aluminum at 930 K and 1050 K, near the melting point of aluminum. This was attributed to the solid-state inter-diffusion that occurs between aluminum and nickel particles at temperatures around the eutectic temperature. These solid-state reactions form predominantly aluminum-rich compounds, namely, NiAl3and Ni2Al3.

Fig. 20. XRD patterns in CO2 as a function of temperature for 15–25 µm-sized nickel-coated aluminum powder at (a) 750, (b) 930, (c) 1050, (d) 1170, (e) 1350, (f) 1500, (g) 1762 K
These reactions are exothermic, which subsequently heat the sample from 750 K to the eutectic temperature of 930 K, thus triggering a reaction. The formation of these compounds can also lead to swelling of the sample and the Kirkendall effect owing to the unbalanced diffusivities of nickel and aluminum[39]. It has also been reported that a pre-combustion (=ignition) stage exists for high heating rates at approximately 980 K owing to the formation of NiAl layers, which effectively increases the temperature of the compact to the eutectic temperature[40]. The formation of NiAl phases can essentially act as a buffer that dilutes exothermic reactions. When the temperature reaches the melting point of aluminum, the reaction between aluminum and solid nickel starts at the interface with formation of the solid NiAl3compound owing to the higher mobility of molten aluminum atoms. The difference in the thermal expansion coefficient leads to cracking of the shell. Microstructural investigations show that at this stage, aluminum melt spreads all over the solid nickel surface, increasing the contact area, which intensifies the reaction rate, leading to effective reaction of the particle.
This leads to larger mechanical stress on the particle, causing the shell to crack at lower temperatures. A crack is generated on the NiO layer by multiple intermetallic reactions and aluminum reacts rapidly with the oxidizer. It was observed that for the resulting reaction of Ni2Al3, NiAl was promoted by NiAl3melted at approximately 1170 K and the exothermic reaction of α-Al2O3was generated by the reaction with partial aluminum at 1350 K. When the intermetallic reaction reaches 1500 K, it becomes more active and NiAl2O4generated by the high-temperature environment is observed. When the reaction reaches 1762 K, the NiO oxide film is destroyed, creating voids and a hollow structure. From this experiment, the reaction of the 15–25 µm-sized nickel-coated aluminum was an SHS reaction. It was thus confirmed that the difference of reactivity in the CO2oxidizer depended on the particle size of the nickel-coated aluminum.
From the experimental results with air and CO2oxidizers, the differences owing to the oxidizer type were minimal, whereas the effect of the particle size was significant. Unlike the large aluminum particles, strong exothermic reactions occurred in the 15–25 µm-size nickel-coated aluminum near the melting point of aluminum. The smaller size aluminum particles had good reactivity as compared to pure aluminum.
The reason for this can be explained by the thermodynamic properties. Heat energy is released by the intermetallic reaction of aluminum and nickel. This heat energy is conveyed inside the aluminum to quickly increase the overall temperature of the particle. The specific heat capacity of the metals(aluminum and nickel) and NiO becomes constant as the temperature increases below 2400 K, whereas that of aluminum oxide gradually increases[42]. Pure aluminum is covered with aluminum oxide formed by the initial low temperature oxidation, and this aluminum oxide has a large specific heat capacity. In contrast, when aluminum is coated with nickel and nickel oxide, which has a relatively low specific heat capacity, surplus heat energy is supplied, which increases the overall temperature of the particle.
Therefore, nickel-coated aluminum has a higher reactivity than pure aluminum. In this study, we confirmed that the particle size had more of an effect than the oxidizer. The reaction of forming NiAl3for the larger size nickel-coated aluminum particles is weakly exothermic, and the small amount of heat locally induced by the increase in temperature is liberated. Because of the solid–solid reaction, the heat release rate of such a reaction is kinetically diffusion-controlled, especially governed by the preferential diffusion of aluminum atoms[43]. In the case of the larger nickel-coated aluminum particles, the diffusivity of the atoms is very low because of the smaller contact area, and so the heat-release rate is quite slow. As the temperature increases to near the eutectic point of Al–NiAl3, aluminum atoms become more active. As the reaction progresses, the amount of generated heat increases and more aluminum particles began to melt; thus, an endothermic peak appears at approximately 920 K.
Although there are some intermetallic exothermic reactions, the endothermic reaction is dominant. The SHS reactions mainly depend on the following facts related to the smaller aluminum particles. The smaller particles have a higher surface area, resulting in more contacts that facilitate the SHS reaction. The smaller particles also possess more surface atoms with higher diffusivities and surface energies, which results in more reactions and a higher heat-release rate. They also have a short diffusion distance, which also facilitates a rapid reaction. The enthalpy of the smaller particles facilitates the diffusion and melting of aluminum particles, thus accelerating the reaction. The smaller particles are also characterized by a large amount of interfaces and higher stored energy, thereby exhibiting higher chemical activity. Thus, the surface atoms of aluminum particles diffuse rapidly at even lower temperatures. With increasing temperature, these aluminum atoms quickly disengage from the surfaces of the particles, move to the surfaces of the nickel particles, and react with the nickel atoms at their interfaces via solid-state inter-diffusion. Because of short diffusion length, NiAl3forms rapidly. With further increases in temperature, the diffusion and melting of aluminum accelerates. The transient liquid rapidly spreads throughout the structure, consumes the elemental powders, and the formation of the intermediate phase Ni2Al3occurs. This results in the release of a larger amount of heat, which further accelerates the reactions. Therefore, the liquid zone gradually broadens as a result of these reactions and the increasing temperature.
In a thermally sufficient environment, the NiAl3and Ni2Al3phases react with the remaining nickel and aluminum, inducing the final strong exothermic reaction to form NiAl. A rapid reaction occurs and the NiO layer collapses by the strong exothermic reaction. Therefore, the small heat capacity of the small nickel-coated aluminum particles results in a higher reactivity than the large nickel-coated aluminum particles. In this study, we compared the reactivity of nickel-coated aluminum particles 15–25 µm, 74–105 µm, and 2.38 mm in size in air, CO2, and argon environments. However, there was little difference in the reactivity as a function of the type of oxidant. However, the smaller particles showed a strong exothermic reaction near the melting point of aluminum, which accelerated the reaction of the smaller particles. Through this, we confirmed the size effect of the nickel-coated aluminum particles.
4 Conclusions
(1) The reactivity of nickel-coated 15–25 µm-sized aluminum particles in various oxidizers is explained. To improve the ignition of high-energy aluminum powder, pure oxide films(alumina, Al2O3) were chemically removed and nickel coatings were deposited using an electroless method. The degree of coating was qualitatively and quantitatively confirmed by surface analysis using SEM/EDS and XRD chemical component analysis. Thermal analysis using TGA/DSC enabled comparisons between the uncoated and coated aluminum.
(2) The reaction process is explained through fine structural analysis of the surfaces and cross sections of the particles. There was little difference in reactivity as a function of oxidant type. However, a strong exothermic reaction occurred in the smaller nickel-coated aluminum particles near the melting point of aluminum, which accelerated the reaction of the smaller particles. Through this, we confirmed the size effect of the nickel-coated aluminum.
(3) The results of this study could be used in the munitions industry for the fabrication of a solid propellant or a thermobaric bomb using aluminum.
[1] BECKSTEAD M W.[R]. Provo UT: Brigham Young University, 2004.
[2] SUNDARAM D S, YANG V, ZARKO V E. Combustion of nano aluminum particles (Review)[J].2014, 51(2): 173–196.
[3] FEDOROV A V, KHARLAMOVA Y V. Ignition of an aluminum particle[J]., 2003, 39(5): 544–547.
[4] GREMYACHKIN V M. Theory of ignition of metallic particles[J].1983, 19(3): 259–263.
[5] STARIK A M, KULESHOV P S, SHARIPOV A S, et al. Numerical analysis of nanoaluminum combustion in steam[J].2014, 161(6): 1659–1667.
[6] TEIPEL U.[M]. Weinheim: Wiley-VCH, 2005.
[7] LEE H Y, KIM G Y. Nickel aluminides coating on aluminum substrate using a reaction synthesis process[J]., 2015, 21(1): 147–152.
[8] GREINER L.[M]. HANSEN F A A. Arlington: Compass Publications, 1967.
[9] INGENITO A, BRUNO C. Using aluminum for space propulsion [J]., 2004, 20(6): 1056–1063.
[10] FRANZONI F, MILANI M, MONTORSI L, et al. Combined hydrogen production and power generation from aluminum combustion with water: analysis of the concept[J]., 2010, 35(4): 1548–1559.
[11] YETTER R A, RISHA G A, SON S F. Metal particle combustion and nanotechnology[J]., 2009, 32(2): 1819–1838.
[12] KORCHAGIN M A, GRIGOR`EVA T F, BOKHONOV B B, et al. Solid-state combustion in mechanically activated SHS systems. II. Effect of mechanical activation conditions on process parameters and combustion product composition[J]., 2003, 39(1): 51–58.
[13] ASSOVSKII I G, STRELETSKII A N, KOLESNIKOV- SVINAREV V I. Mechanism of formation of the condensed phase in aluminum combustion in carbon dioxide[J]., 2005, 405(1): 235–239.
[14] PRICE E. SIGMAN R. Combustion of aluminized solid propellants[J]., 2000, 185: 663–687.
[15] KARASEV V V, ONISHCHUK A A, GLOTOV O G, et al. Charges and fractal properties of nanoparticles—Combustion products of aluminum agglomerates[J]., 2001, 37(6): 734–736.
[16] YAGODNIKOV D, VORONETSKII A. Experimental and theoretical study of the ignition and combustion of an aerosol of encapsulated aluminum particles[J]., 1997, 33(1): 49–55.
[17] YAVOR Y, ROSENBAND V, GANY A. Reduced agglomeration resulting from nickel coating of aluminum particles in solid propellants[J]., 2010, 9(6): 477–492.
[18] BREITER A, MAL`TSEV V, POPOV E. Means of modifying metallic fuel in condensed systems[J]., 1990, 26(1): 86–92.
[19] ERMOLINE A, SCHOENITZ M, DREIZIN E, et al. Production of carbon-coated aluminium nanopowders in pulsed microarc discharge[J]., 2002, 13(5): 638.
[20] ANDRZEJAK T A, SHAFIROVICH E, VARMA A. Ignition of iron-coated and nickel-coated aluminum particles under normal-and reduced-gravity conditions[J]., 2008, 24(4): 805–813.
[21] PAI B C, ROHATGI P K. Production of cast aluminium-graphite particle composites using a pellet method[J]., 1978, 13(2): 329–335.
[22] SARKAR D K, FARZANEH M, PAYNTER R W. Superhydrophobic properties of ultrathin rf-sputtered Teflon films coated etched aluminum surfaces[J]., 2008, 62(8): 1226–1229.
[23] HUANG Y S, ZENG X T, HU X F, LIU F M. Corrosion resistance properties of electroless nickel composite coatings[J]., 2004, 49(25): 4313–4319.
[24] YAWS C L.[M]. Norwich: William Andrew, 2008.
[25] BABUK V, VASSILIEV V, SVIRIDOV V. Propellant formulation factors and metal agglomeration in combustion of aluminized solid rocket propellant[J]., 2001, 163(1): 261–289.
[26] YAVOR Y. Effect of nickel coating on aluminum combustion and agglomeration in solid propellants[C]//GANY A., Harford, Connecticut, July 21–23, 2008. Reston: AIAA, 2008: 5255.
[27] SHAFIROVICH E, BOCANEGRA P E, CHAUVEAU C, et al. Ignition of single nickel-coated aluminum particles[J]., 2005, 30(2): 2055–2062.
[28] SHAFIROVICH E, MUKASYAN A, THIERS L, et al. Ignition and combustion of Al particles clad by Ni[J]., 2002, 174(3): 125–140.
[29] ANDRZEJAK T A, SHAFIROVICH E, VARMA A. Ignition mechanism of nickel-coated aluminum particles[J]., 2007, 150(1–2): 60–70.
[30] MORSI K. Review: reaction synthesis processing of Ni–Al intermetallic materials[J]., 2001, 299(1): 1–15.
[31] THIERS L, MUKASYAN A S, VARMA A. Thermal explosion in Ni-Al system: Influence of reaction medium microstructure[J]., 2002, 131(1): 198–209.
[32] MUKASYAN A S, LAU C, VARMA A. Gasless combustion of aluminum particles clad by nickel[J]., 2001, 170(1): 67–85.
[33] YANAGISHITA T, KIMURA S, NISHIO K, et al. Fabrication of Hollow Spheres with Porous Structures by Anodization of Small Al Particles[J]., 2008, 1(8): 084001.
[34] BASTIN G F, HEIJLIGERS H J M, VAN LOO F J J. The performance of the modified Φ (ϱz) approach as compared to the love and scott, ruste and standard ZAF correction procedures in quantitative electron probe microanalysis[J]., 1984, 6(1): 58–68.
[35] JCPDS Powder Diffraction Files, PCPDFWIN Software[CP]. Swarthmore, Pennsylvania: Joint Committee on Powder Diffraction Standards–International Centre for Diffraction Data, 1996.
[36] BINNEWIES M.[M]. MILKE E. Weinheim: Wiley-VCH, 2002.
[37] JEURGENS L, SLOOF W, TICHELAAR F, et al. Thermodynamic stability of amorphous oxide films on metals: Application to aluminum oxide films on aluminum substrates[J]., 2000, 62(7): 4707.
[38] TRUNOV M A, SCHOENITZ M, ZHU X, et al. Effect of polymorphic phase transformations in Al2O3 film on oxidation kinetics of aluminum powders[J]., 2005, 140(4): 310–318.
[39] JANSSEN M M P, RIECK G D. Reaction diffusion and Kirkendall-effect in the nickel-aluminum system[J]., 1967, 239(9): 1372.
[40] PLAZANET L, NARDOU F. Reaction process during relative sintering of NiAl[J]., 1998, 33(8): 2129–2136.
[41] BRANDSTADT K, FROST D L, KOZINSKI J A. Nano-and micrometer-scale aluminum powder oxidation in carbon dioxide[J]., 2007, 6(1): 59–74.
[42] LINSTROM P J.[M]. MALLARD W. Gaithersburg: NIST, 2001
[43] DONG S, HOU P, YANG H, et al. Synthesis of intermetallic NiAl by SHS reaction using coarse-grained nickel and ultrafine-grained aluminum produced by wire electrical explosion[J]., 2002, 10(3): 217–223.
Biographical notes
LEE Sanghyup, born in 1982, is currently a postdoctoral program at. He received his PhD degree from, in 2016. His main research interests include energetic material combustion, measurement of flame characteristic with optical technique.
E-mail: ilikecola@yonsei.ac.kr
NOH Kwanyoung, born in 1986, is currently a PhD candidate at. He received his master degree on mechanical engineering in, in 2015.
E-mail: graymerc@yonsei.ac.kr
LIM Jihwan, born in 1978, is currently an engineer at Hanwha Corp., Korea. He received his PhD degree from, in 2014. His main research interests include energetic material combustion, measurement of flame characteristic.
E-mail: imjihwan.lim@gmail.com
YOON Woongsup, born in 1960, is currently a professor at. He received his PhD degree from, in 1989. His main research interests include energetic material combustion, measurement of flame characteristic.
Tel: +82-2-2123-7856; E-mail: wsyoon@yonsei.ac.kr
Received March 18, 2016; revised August 5, 2016; accepted August 11, 2016
Supported by Defense Acquisition Program Administration and Agency for Defense Development(Grant Nos. UD110095CD, UD130038GD)
© Chinese Mechanical Engineering Society and Springer-Verlag Berlin Heidelberg 2016
10.3901/CJME.2016.0811.092, available online at www.springerlink.com; www.cjmenet.com
E-mail: ilikecola@yonsei.ac.kr
 Chinese Journal of Mechanical Engineering2016年6期
Chinese Journal of Mechanical Engineering2016年6期
- Chinese Journal of Mechanical Engineering的其它文章
- Surface Topography and Roughness of High-speed Milled AlMn1Cu
- Integrated Simulation Method for Interaction between Manufacturing Process and Machine Tool
- Engineering the Smart Factory
- Exploring Barriers and Opportunities in Adopting Crowdsourcing Based New Product Development in Manufacturing SMEs
- Development of the Supply Chain Oriented Quality Assurance System for Aerospace Manufacturing SMEs and Its Implementation Perspectives
- Collaborative Simulation Method with Spatiotemporal Synchronization Process Control
