Assessing the effect of hydrogen on the electronic properties of 4H-SiC
Yuanchao Huang(黄渊超) Rong Wang(王蓉) Yiqiang Zhang(张懿强)Deren Yang(杨德仁) and Xiaodong Pi(皮孝东)
1State Key Laboratory of Silicon Materials and School of Materials Science and Engineering,Zhejiang University,Hangzhou 310027,China
2Institute of Advanced Semiconductors&Zhejiang Provincial Key Laboratory of Power Semiconductor Materials and Devices,Hangzhou Innovation Center,Zhejiang University,Hangzhou 311200,China
3School of Materials Science and Engineering&College of Chemistry,Zhengzhou University,Zhengzhou 450001,China
Keywords: 4H-silicon carbide,hydrogen,electronic properties,passivation
1. Introduction
As a leading third-generation semiconductor,silicon carbide (SiC) is leaping in an explosive development to meet the increasing demand of electrical vehicles, 5G communications and renewable-energy systems. There exist over 250 polymorphs for SiC,among which 4H-SiC has attracted great attention owning to its wide bandgap, high carrier mobility,high thermal conductivity, and high stability.[1—3]4H-SiC ingots are usually grown by the physical vapor transport(PVT)method, during which hydrogen gas (H2) is used to optimize the surface of the seed crystal and tune the growth condition of 4H-SiC.[4—7]4H-SiC epilayers are often homoepitaxially grown by chemical vapor deposition (CVD), during which H2is widely used as the carrier gas.[8—11]It should be also noted that H impurities are frequently incorporated during the processing of 4H-SiC-based devices.[12—15]Therefore, understanding the effect of H on the electronic properties of 4H-SiC is critical to the development of 4H-SiC technologies.
Considerable efforts have already been devoted to investigate H in 4H-SiC.[16—30]For n-type 4H-SiC, it was found that H—ion implantation, H2-plasma treatment, and hightemperature H2annealing could reduce the concentration of electrons and increase the resistivity of 4H-SiC.[20—22]The reduction of the electron concentration was controversially attributed to the formation of NC—H complexes, as well as the creation of electron traps such as H interstitials and intrinsic defects.[20]The hole concentration of p-type 4H-SiC also decreased after high-temperature H2annealing,[22—25]as a result of H2annealing-induced decomposition of AlSi—H complexes and the creation of positively charge H,[22—24]which differed from the behavior of H in n-type 4H-SiC. H2-plasma treatment was found to reduce the photoluminescence of both Alrelated defects and N—Al complexes.[25]But the underlying mechanism remains ambiguous. Moreover, intrinsic carbon vacancies (VC) were found to seriously reduce carrier lifetime in 4H-SiC.[26—29]It was experimentally found that H2-atomsphere annealing was actually not capable of passivating VCin 4H-SiC.[32,33]However, it was theoretically proposed that H might passivate VC.[34—36]It is apparent that a clear picture on H in 4H-SiC remains elusive.
In this work, we systemically explore the effect of H on the electronic properties of both n-type and p-type 4H-SiC.The passivation of H on intrinsic defects such as VCand silicon vacancies(VSi)in 4H-SiC is also evaluated. We find that

2. Computational methodology
First-principles calculations are carried out by the projector-augmented wave(PAW)method,as implemented in the Viennaab initiosimulation package (VASP).[37,38]The wave functions are expanded by using the plane waves up to a kinetic energy cutoff of 500 eV.The Perdew—Burke—Ernzerhof revised for solids(PBEsol)functional with the GGA exchange correlation is adopted for the structural relaxation.[39]The supercell size and atomic positions are fully relaxed until the total energy per cell and the force on each atom converge to less than 1×10-6eV and 0.01 eV/°A,respectively. For accurate bandgap energy and defect level description, the hybrid density functional of Heyd,Scuseria,and Ernzerhof(HSE06),which mixes 25% of screened Hartree—Fock exchange to the PBE exchange functional, is employed during the calculation of electronic properties.[38]H impurities are modeled in 128-atom 4H-SiC supercells. For Brillouin zone integration,the Monkhorst—Pack scheme with aΓ-centered 2×2×2 specialk-points mesh is used.[39]Defect formation energies of H are calculated by the well-established mixedk-point scheme.[42,43]
3. Results and discussion
3.1. Configurations


Fig.1. Relaxed structures of H in 4H-SiC.HC,HSi,,i ,,andare denoted by gray,orange,purple,yellow,cyan,and pink balls,respectively. Si and C atoms are denoted by red and white balls,respectively.

Fig.2. (a)Accessible range of chemical potentials(green area)for equilibrium incorporation of H in 4H-SiC,(b)calculated formation energies of H in 4H-SiC.
During the incorporation of H in 4H-SiC,thermodynamic equilibrium growth conditions require a series of limitations on the achievable values for chemical potentials of the constituents (μi). Firstly, the values ofμSiandμCare limited to maintain the stable 4H-SiC:

where ΔHf(CH4) are formation energy of CH4. By solving Eqs.(1)—(3),we can get the accessible range for values of the chemical potentials,as shown by the green regions in Fig.2(a).We then take the Si-rich limit[point A in Fig.2(a)]and the Crich limit growth condition [point B in Fig. 2(a)] to calculate the formation energies of H in 4H-SiC.



Fig. 3. Single-electron levels of dominant H interstitials, dopants, and intrinsic defects in 4H-SiC.Pink and blue regions indicate the CB and the VB of 4H-SiC,respectively. The up and down arrows denote the occupied spinstates of electrons on the defect levels.
3.2. H in n-type 4H-SiC
The high dopability of N in 4H-SiC endows 4H-SiC a great success in high power electronics. For n-type 4H-SiC substrates, the resistivity in the order of 10-3Ω·cm is highly desired to guarantee its conductivity. During the homoepitaxy of n-type 4H-SiC layers, the electron concentration of the ntype buffer layer should be as high as possible to guarantee the conversion efficiency of basal plane dislocations.[48,49]However, the doping efficiency of N still lags behind the demand of ideal power-device applications. In this section, we evaluate the effects of compensation (or passivation) of H on the electronic properties of n-type 4H-SiC.



Fig. 4. (a) Calculated formation energies of NC, NC—H, HSi i—te , and Hbi cin 4H-SiC,(b)calculated binding energy of the NC—H complex in 4H-SiC.
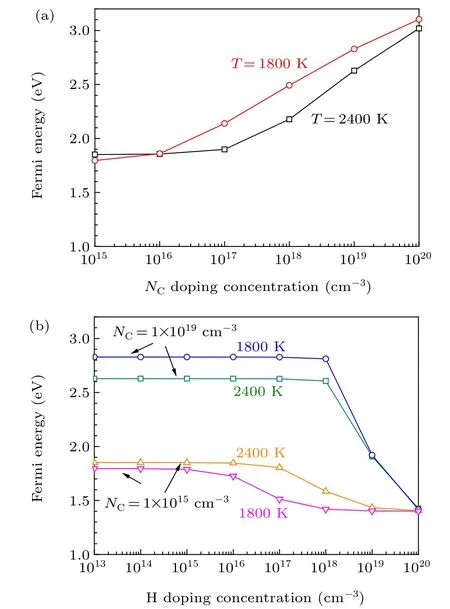
Fig. 5. (a) Fermi energies of 4H-SiC grown at 1800 K and 2400 K as functions of the concentration of N, (b) Fermi energies of 4H-SiC grown at 1800 K and 2400 K as functions of the concentration of H under different background concentrations of N.
The typical temperatures of PVT growth and CVD homoepitaxy of 4H-SiC are 2400 K and 1800 K,respectively.[1]Therefore,we investigate the effects of H on the Fermi energy and carrier concentration of n-type 4H-SiC at the temperatures of 2400 K and 1800 K. As shown in Fig. 5(a), the Fermi energy of 4H-SiC grown under 1800 K is slightly higher than that of 4H-SiC grown under 2400 K,due to lower concentration of thermally excited electrons at lower temperatures. This indicates that the hole concentration of 4H-SiC grown under 1800 K is higher than that of 4H-SiC grown under 2400 K.When the concentration of N increases from 1015cm-3to 1016cm-3,the increase of Fermi energy of 4H-SiC grown under 1800 K is more significant than that for 4H-SiC grown under 2400 K. For 4H-SiC grown under lower temperatures,the hole concentration is lower, which means that it is easier to tune the Fermi energy of 4H-SiC by increasing the concentration of N. When the concentration of N increases to 1019cm-3, the Fermi energy of 4H-SiC grown under 1800 K and 2400 K increase to 2.6 eV and 2.8 eV,respectively.Taking 4HSiC with the N-doping concentration of 1019cm-3as an example,we find that the effect of unintentional incorporated H exerts negligible effect on the n-type doping of 4H-SiC.Only when the concentration of H exceeds 1018cm-3, the Fermi energy of 4H-SiC begins to decrease [Fig. 5(b)]. When the concentration of H increases to 1020cm-3, the Fermi energy of 4H-SiC is pinned at 1.4 eV due to the self-compensation of H[Fig.5(b)].
3.3. H in p-type 4H-SiC
The p-type 4H-SiC substrates are of great importance to the development of n-channel bipolar devices based on 4H-SiC, which hold great promise for ultra-high voltage (>10 kV) applications.[50—53]Al owns the lowest ionization energy among all group-III elements in 4H-SiC,which makes it as the most popular p-type dopant in 4H-SiC.It is well known that the lowest defect configuration of Al is AlSi. Therefore,we investigate the interaction of H with AlSiin this section,the effect of dominant intrinsic defect of VCis also taken into consideration.

Fig.6.(a)Calculated formation energiesofAlSi,AlSi—H,VC,HSi i—te,andHbi c in 4H-SiC,(b)calculatedbinding energyofthe AlSi—Hcomplexin4H-SiC.


3.4. H passivation of VC
When 4H-SiC is applied in power electronics, VChas been identified as the carrier-lifetime killer in bipolar devices based on 4H-SiC.[26—30]By trapping carriers in its deep defect level, VCseverely reduces the carrier lifetime and thus the blocking voltage of the 4H-SiC drift layer.[53]Various approaches such as carbon ion implantation followed by thermal annealing,thermal oxidation,and annealing with a carbon cap,have been proposed to eliminate VCin 4H-SiC.[54—57]For the sake of reducing processing complexity and cost,we evaluate whether H can passivate the defect states of VC.
As shown in Fig. 2, passivating VCby 4 H atoms can effectively eliminate the defect level of VCand potentially improve the carrier lifetime in 4H-SiC. Therefore, we calculate the defect formation energies of VC—nH (n=1—4) to verify whether H is capable of passivating VCand enhance the carrier lifetime of 4H-SiC.As shown in Fig.7(a),the defect formation energies of VC—nH(n=1—4)are all larger than that of pure VC.Although the deep defect states of VC—4H disappears in 4H-SiC, VC—4H has the highest formation energy among all VC—nH (n= 1—4) complexes. This indicates that equilibrium incorporation of H cannot eliminate the defect states of VC. Nonequilibrium approaches, such as H ion implantation or irradiation may capable of passivating VCby H. The binding energy of VC—nH(n=1—4)complexes are calculated byEb(VC—nH)=ΔHf(VC)+nΔHf(Hi)-ΔHf(VC-nH).As shown in Fig.7(b),when the Fermi energy of 4H-SiC is in the range from 0.44 eV to 3.03 eV, the binding energies of VC—4H is positive,indicating the VC—4H complex is stable against decomposition.This means that nonequilibrium passivation of VCby H is effective to eliminate the defect states of VCand thus enhance the carrier lifetime of moderately doped 4H-SiC with Fermi energy ranging from 0.44 eV to 3.03 eV.

Fig.7. (a)Formation energies and(b)binding energies of VC—nH(n=1—4)complexes in 4H-SiC.
3.5. H passivation of VSi
Since 2015, VSihas been manipulated as isolated spin qubits for quantum computing.[58—63]Isolated VSicolor centers were created by laser writing,ion implantation or electron irradiation.[58]It was found that both the photoluminescence(PL) wavelength and intensity were not uniform throughout the VSiarray of 4H-SiC.[64,65]In this section,we evaluate the possible reason of H passivation on the nonuniform PL emission of VSiarrays in 4H-SiC.
As shown in Fig. 8(a), (VSi—4H)0and (VSi—3H)-complexes have the lowest formation energies in p-type and n-type 4H-SiC, respectively. This indicates that H would passivate VSiand change the optical properties of VSidefects in 4HSiC.The occupied defect state of VSilies in 0.72 eV above the VBM of 4H-SiC, which agrees well with experimental and theoretical results.[40,41]When VSiis passivated by 4 H atoms,the defect state disappears from the bandgap of 4H-SiC.This gives rise to the disappear of PL emission of VSi. When VSiis passivated by 3 H atoms, the defect states shift to 0.70 eV above the VBM of 4H-SiC. This results in the change of PL emission wavelength and intensity for the VSiarrays in 4HSiC.[66]
We also calculate the binding energies of VSi—nH (n=1—4) complexes byEb(VSi—nH) = ΔHf(VSi)+nΔHf(Hi)-ΔHf(VSi—nH). As shown in Fig.8(b),the binding energies of VSi—nH (n=1—4) complexes are all positive. This indicates that once the complexes are formed, VSi—nH (n=1—4) complexes are stable against decomposition. Because of the low binding energies of VSi—nH(n=1—4)complexes,thermal annealing is needed to decompose VSi—nH(n=1—4)complexes,and promote the uniformity for the PL properties of VSiarray in 4H-SiC.

Fig.8. (a)Formation energies and(b)binding energies of VSi—nH(n=1—4)complexes in 4H-SiC.
4. Conclusion
In conclusion, we have systematically investigated the role of H in the electronic properties of 4H-SiC. We have
Acknowledgments
Project supported by the National Key Research and Development Program of China(Grant No.2018YFB2200101),the National Natural Science Foundation of China (Grant Nos. 91964107 and U20A20209), the “Pioneer” and “Leading Goose” Research and Development Program of Zhejiang Province,China(Grant No.2022C01021),and partial support from the National Natural Science Foundation of China for Innovative Research Groups(Grant No.61721005). The National Supercomputer Center in Tianjin is acknowledged for computational support.
- Chinese Physics B的其它文章
- A nonlocal Boussinesq equation: Multiple-soliton solutions and symmetry analysis
- Correlation and trust mechanism-based rumor propagation model in complex social networks
- Gauss quadrature based finite temperature Lanczos method
- Experimental realization of quantum controlled teleportation of arbitrary two-qubit state via a five-qubit entangled state
- Self-error-rejecting multipartite entanglement purification for electron systems assisted by quantum-dot spins in optical microcavities
- Pseudospin symmetric solutions of the Dirac equation with the modified Rosen–Morse potential using Nikiforov–Uvarov method and supersymmetric quantum mechanics approach

