Interaction between systemic iron parameters and left ventricular structure and function in the preserved ejection fraction population: a two-sample bidirectional Mendelian randomization study
Xiong-Bin MA ,Yong-Ming LIU,Yan-Lin LV ,Lin QIAN
1. The First Clinical Medical College of Lanzhou University,Lanzhou,Gansu,China;2. Geriatric Cardiovascular Department and Gansu Clinical Research Center for Geriatric Diseases,First Hospital of Lanzhou University,Gansu,China
ABSTRACT BACKGROUND Left ventricular (LV) remodeling and diastolic function in people with heart failure (HF) are correlated with iron status;however,the causality is uncertain.This Mendelian randomization (MR) study investigated the bidirectional causal relationship between systemic iron parameters and LV structure and function in a preserved ejection fraction population.METHODS Transferrin saturation (TSAT),total iron binding capacity (TIBC),and serum iron and ferritin levels were extracted as instrumental variables for iron parameters from meta-analyses of public genome-wide association studies.Individuals without myocardial infarction history,HF,or LV ejection fraction (LVEF) < 50% (n=16,923) in the UK Biobank Cardiovascular Magnetic Resonance Imaging Study constituted the outcome dataset.The dataset included LV end-diastolic volume,LV endsystolic volume,LV mass (LVM),and LVM-to-end-diastolic volume ratio (LVMVR).We used a two-sample bidirectional MR study with inverse variance weighting (IVW) as the primary analysis method and estimation methods using different algorithms to improve the robustness of the results.RESULTS In the IVW analysis,one standard deviation (SD) increased in TSAT significantly correlated with decreased LVMVR(β=-0.1365;95% confidence interval [CI]: -0.2092 to -0.0638;P=0.0002) after Bonferroni adjustment.Conversely,no significant relationships were observed between other iron and LV parameters.After Bonferroni correction,reverse MR analysis showed that one SD increase in LVEF significantly correlated with decreased TSAT (β =-0.0699;95% CI: -0.1087 to -0.0311;P=0.0004).No heterogeneity or pleiotropic effects evidence was observed in the analysis.CONCLUSIONS We demonstrated a causal relationship between TSAT and LV remodeling and function in a preserved ejection fraction population.
Heart failure (HF) is a clinically heterogeneous disease with high incidence,mortality,and socioeconomic burden.[1]Owing to developments in medical and health care,the aging trend is becoming increasingly evident in the population.Similarly,with the increasing incidence of diseases such as hypertension,diabetes,and heart disease,the population with heart failure and preserved ejection fraction (HFpEF) is increasing.[2-4]However,despite the confirmed diagnosis of HFpEF,its treatment remains challenging,partly owing to its diverse pathophysiological mechanisms.[5]Investigating risk variables associated with the occurrence and development of HFpEF,particularly modifiable risk factors,should be expedited regarding medical obstacles.
Iron deficiency (ID) is associated with various organic disorders,including HFpEF.Approximately 59% of patients with HFpEF have ID,which may be attributable to the influence of chronic inflammation and oxidative stress on iron storage.[6-8]A systematic review showed that iron insufficiency is associated with lower exercise tolerance and quality of life in patients with HFpEF.[9]Iron substantially influences mitochondrial function and is essential for cardiomyocyte metabolism.Under normal conditions,iron is involved in complex energy production processes in cardiomyocyte mitochondria and an essential substrate for enzymes that remove reactive oxygen species.Therefore,ID affects heart function and may exacerbate pathological cardiac remodeling.[10-13]Gao,et al.[14]concluded that ID was significantly associated with left ventricular (LV) diastolic function in patients with HFpEF,and the results were not influenced by other factors.In populations with preserved EF,whether systemic iron parameters and LV structure are associated is unknown.Although randomized controlled trials are the gold standard for causal inference,this approach is rarely used in the early stages of research owing to factors such as long lead times and high costs.
In the context of rapidly developing large-scale genome-wide association studies (GWAS),Mendelian randomization (MR) analysis is a promising method for causal inference.[15]Genetic variation is randomly distributed at conception;thus,confounding variables or reverse causation are less likely to affect the exposure in MR analysis as it uses genetic instruments.Therefore,it is a method similar to randomized controlled studies.In addition,MR analysis enables the use of many GWAS samples.
Given that iron affects LV structure and function,we conducted a two-sample bidirectional MR study to investigate the interaction between systemic iron parameters and LV structure and function in populations with preserved EF.This will facilitate the understanding of ID as a pathogenic factor in HFpEF.
METHODS
Study Design
Figure 1 shows a detailed illustration of the MR study strategy.This study rigorously followed the key principles for reporting epidemiological observational research and STROBE-MR guidelines.[16,17]
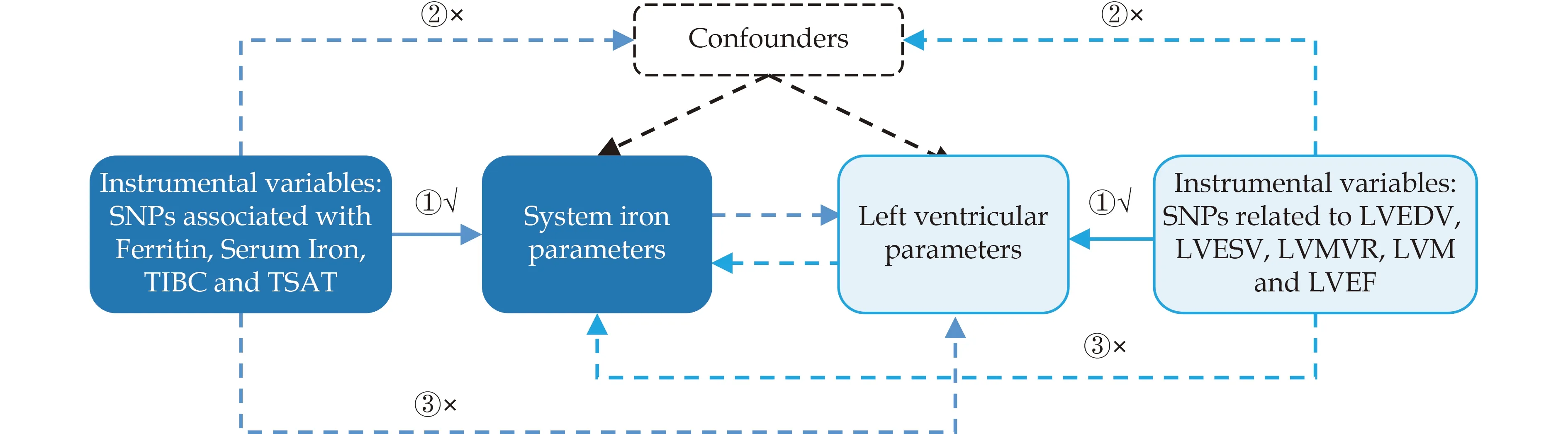
Figure 1 Main assumptions underlying the Mendelian randomization study design. Three assumptions should be met: ①The SNPs should be closely related to exposures;②: The SNPs selected should be independent of confounders;③: The SNPs should affect results only through exposure and not through direct correlation.SNP: single nucleotide polymorphism.
MR analysis was used to investigate the causal relationship between systemic iron parameters and LV structure and function (LV end-diastolic volume(LVEDV),LV end-systolic volume (LVESV),LV mass(LVM),LVM-to-end-diastolic volume ratio (LVMVR),and LV ejection fraction (LVEF)) in the population with preserved EF.Systemic iron parameters included transferrin saturation (TSAT),total iron binding capacity (TIBC),and serum iron and ferritin levels.This study was based on pooled data from published GWAS on iron and LV parameters.Notably,the study strategy adhered to three assumptions: (1) instrumental variables (IVs) are strongly connected to exposure,(2) confounding variables associated with exposure and results should not be associated with IVs,and (3) IVs can only act on outcomes through exposure.[18]Our study population primarily comprised individuals of European ethnicity to avoid racial mismatch and increase the robustness of the analysis.Detailed information on the data can be found in the Supplementary Table S1.Obtaining additional ethical approval or participant agreement was not required for this study because it was based on published work and accessible databases.
Data Source
We used data from a meta-analysis of the largest GWAS of iron steady-state biomarkers to date and extracted four iron parameter GWAS summary datasets,including TSAT (n=131,471),TIBC (n=135,430),serum iron (n=163,511),and ferritin (n=246,139).[19]
Our study outcomes were derived from the most comprehensive GWAS data available on LV structure and function in the preserved EF population,which included 16,923 Europeans without myocardial infarction,HF,or LVEF < 50%.We extracted summarylevel data from cardiac magnetic resonance scans of the genetic loci for five LV imaging phenotypes as our outcome data,including LVEDV,LVESV,LVM,LVMVR,and LVEF.To adjust the GWAS dataset for influencing factors,we followed the adjustment strategy described in the original study.[20]
Genetic IV Selection Criteria
We performed a series of rigorous screening processes to select suitable single nucleotide polymorphisms (SNPs) based on systematic iron and LV parameters in the GWAS database.
From the summary results of two GWAS,we selected SNPs with a strong correlation (P< 5 × 10-8),independent inheritance (r2< 0.001,kb=10,000),and no linkage disequilibrium (LD).[21]In addition,we removed SNPs with palindromic alleles to prevent inadvertent bias.Furthermore,we excluded some confounding SNPs with a threshold ofP<5 × 10-8based on the Phenoscanner database to avoid specific confounding factors.Finally,the MR-PRESSO method was used to detect and remove aberrant SNPs.
To assess the strength of the association between exposure and SNPs,we computed F-statistics for each SNP.An F-statistic >10 indicated sufficient statistical strength for the genetic variables.[22]The F-statistic is calculated using the following formula: F=R2(N-2)/(1-R2).[23]Here,R2represents the exposure variance explained using the selected SNPs,and N represents the sample size of the gene for each iron parameter.
Genetic Correlation and Direction Validation
MR results may produce false positives when a genetic correlation between traits exists.[24,25]Although SNPs related to the outcome were eliminated during the instrument selection process,combining SNPs insignificantly associated with the LV parameters of the preserved LVEF population could pose some genetic risks.Therefore,to assess whether the discovered causal relationship was influenced by a shared genetic architecture,LD score regression (LDSC)was used to assess the genetic correlation between iron and LV parameters identified in the population with preserved EF.[26]
Furthermore,we used the Steiger test to validate whether the observed causal relationship was biased owing to reverse causation.[27]The Steiger test assumes that the IV accounts for more variation in exposure than in outcome variation.
If the IV satisfies this requirement,the direction of the instrument is regarded as “TRUE”;otherwise,it is regarded as “FALSE”.We performed MR analysis after eliminating SNPs with a “FALSE” orientation using the Steiger test.
MR Analysis
All MR analyses were conducted using Two-Sample MR (version 0.5.6) and MRPRESSO (version 1.0)in the R software (version 4.2.3).Genetic correlationanalysis was performed using LDSC software (version 1.0.1).After Bonferroni correction,aP-value <0.05 or ≥ 0.0025 was deemed nominally significant,and the association with aP-value < 0.0025 (0.05/20)was considered a significant relationship.The analyses in the other directions were the same.A flowchart of the MR study is shown in Figure 2.[28]
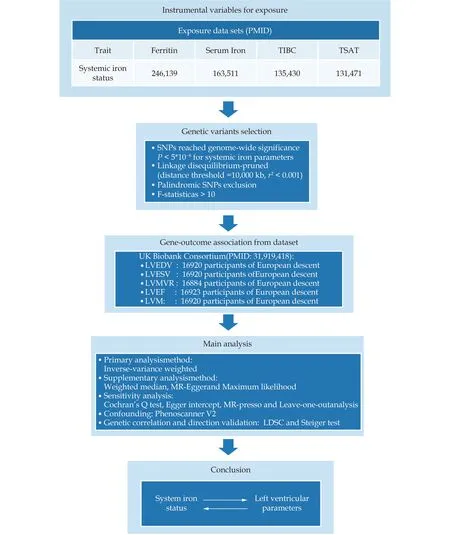
Figure 2 Flow chart and data source of the current Mendelian randomization study. LVEDV: left ventricular end-diastolic volume;LVEF: left ventricular ejection fraction;LVESV: left ventricular end-systolic volume;LVMVR: left ventricular mass-to-end-diastolic volume ratio;LVM: left ventricular mass;SNP: single nucleotide polymorphism.
Statistical Analysis for MR
In this MR study,the inverse variance weighting(IVW) analysis method was the most essential for identifying the overall causal effect.First,the IVW method is used to compute the Wald ratio to evaluate the influence of each SNP on the outcome,and it enables the use of the inverse variance of the SNPs as weights to determine the overall causal effect.[29]The fixed-effects model may run the danger of providing unrealistically exact estimates when heterogeneity is present.Hence,we chose to employ the random-effects model as the primary method.[30]To guarantee the reliability of the findings,we used the weighted median,MR-Egger,maximum likelihood method,and MR-PRESSO as supplementary estimation methods.The weighted median method offers a reliable estimate;however,it requires that efficient genetic instruments account for more than half of the weight.[31]By determining if the intercept of the correlation between exposure and result differs from zero,MR-Egger can be used to identify the overall horizontal pleiotropy of genetic instruments.[32]To further measure the reliability of the results,a maximum likelihood approach was employed.[33]After identifying causal effects using the methods mentioned earlier,we performed sensitivity analyses using the MR Egger intercept test to evaluate horizontal pleiotropy and Cochran's Q test to evaluate heterogeneity.Subsequently,outlier SNPs were detected and removed using the MR-PRESSO method,and the estimates after removing outlier SNPs were provided.[34]Finally,we refined the leave-one-out analysis to evaluate whether the variables significantly influenced the relationship between IVs and outcome.
RESULTS
Effect of Systemic Iron Parameters on LV Parameters in the Preserved EF Population
Selection of IVs
We selected 27,27,35,and 62 SNPs from the GWAS database to perform genetic predictions of TSAT,TIBC,serum iron,and ferritin levels,respectively.All SNPs were not associated with the outcome.We excluded SNPs missing from the outcome dataset and those with palindromic alleles.Subsequently,based on the Phenoscanner database,we removed certain SNPs associated with confounding variables and had a threshold ofP< 5×10-8.Finally,after using the MR-PRESSO method to detect and remove aberrant SNPs,the final number of SNPs included in the analysis was determined.The numbers of SNPs at each screening stage are presented in Supplementary Table S2.
In addition,the F-statistic was used to assess the IV strength,and the results were as follows: TSAT(38-1677),TIBC (44-1664),serum iron (38-1932),and ferritin (43-792).Therefore,the F-statistics for all four iron parameters were > 10,indicating sufficient statistical strength to act as IVs (Supplementary Tables S3-S6).[22]
Effects of Systemic Iron Parameters on LV Parameters in the Preserved EF Population
Effects of TSAT on LV parameters
In the IVW analysis,each one standard deviation increase in TSAT was significantly correlated with decreased LVMVR (β=-0.1365;95% CI: -0.2092 to-0.0638;P=0.0002).Furthermore,each one standard deviation increase in TSAT was nominally significantly associated with increased LVESV (β=0.0779,95% CI: 0.0054 to 0.1505;P=0.035) (Table 1).
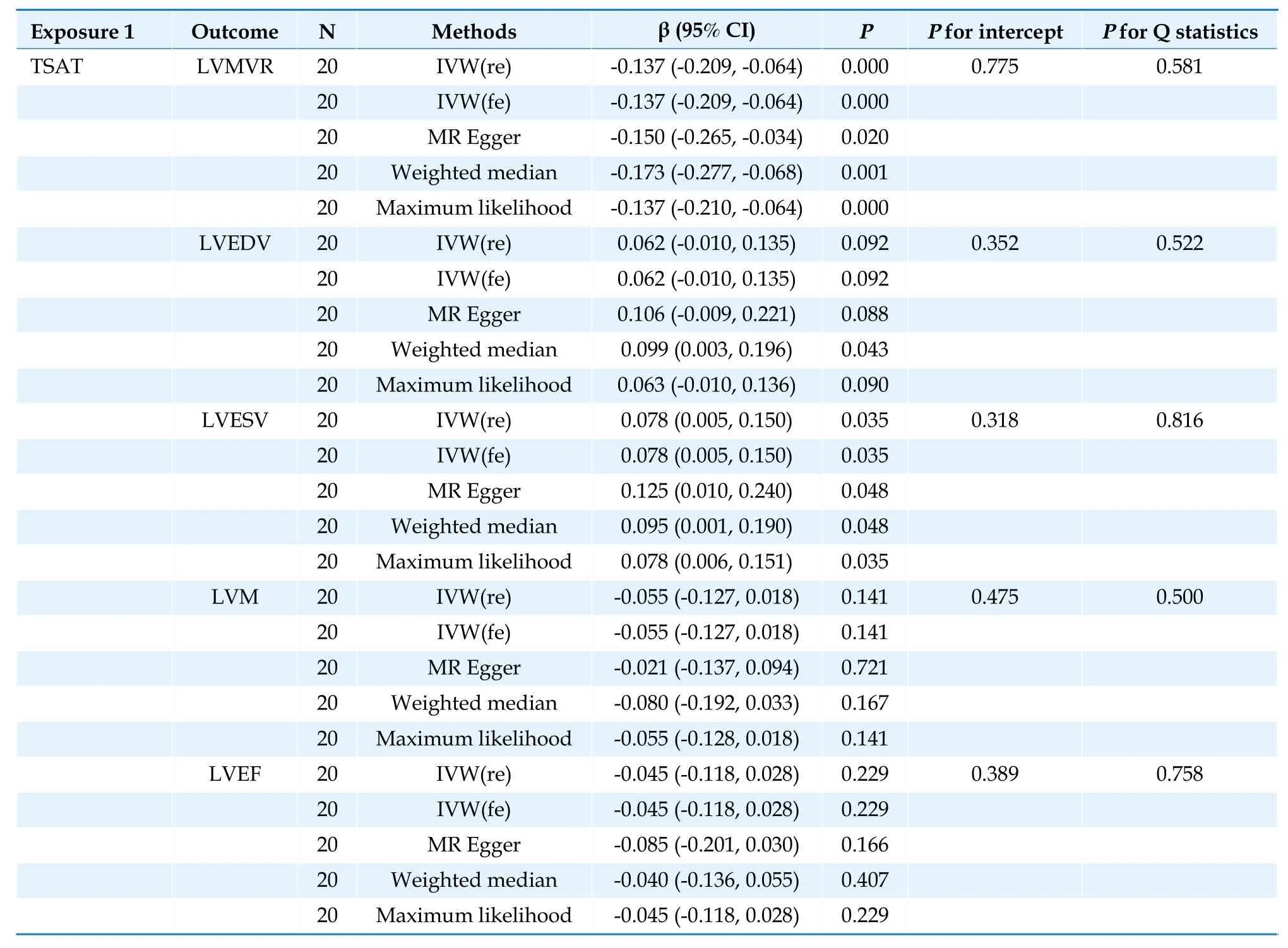
Table 1 Causal association between transferrin saturation and left ventricular parameters.
Effects of serum iron on LV parameters
In the IVW analysis,each 1 standard deviation increase in serum iron was nominally correlated with increased LVESV (β=0.0779,95% CI: 0.0054 to 0.1505;P=0.035),higher LVEDV (β=0.1117,95% CI: 0.0231 to 0.2004;P=0.014),and lower LVMVR(β=-0.1171,95% CI: -0.2075 to -0.0266;P=0.011)(Table 2).
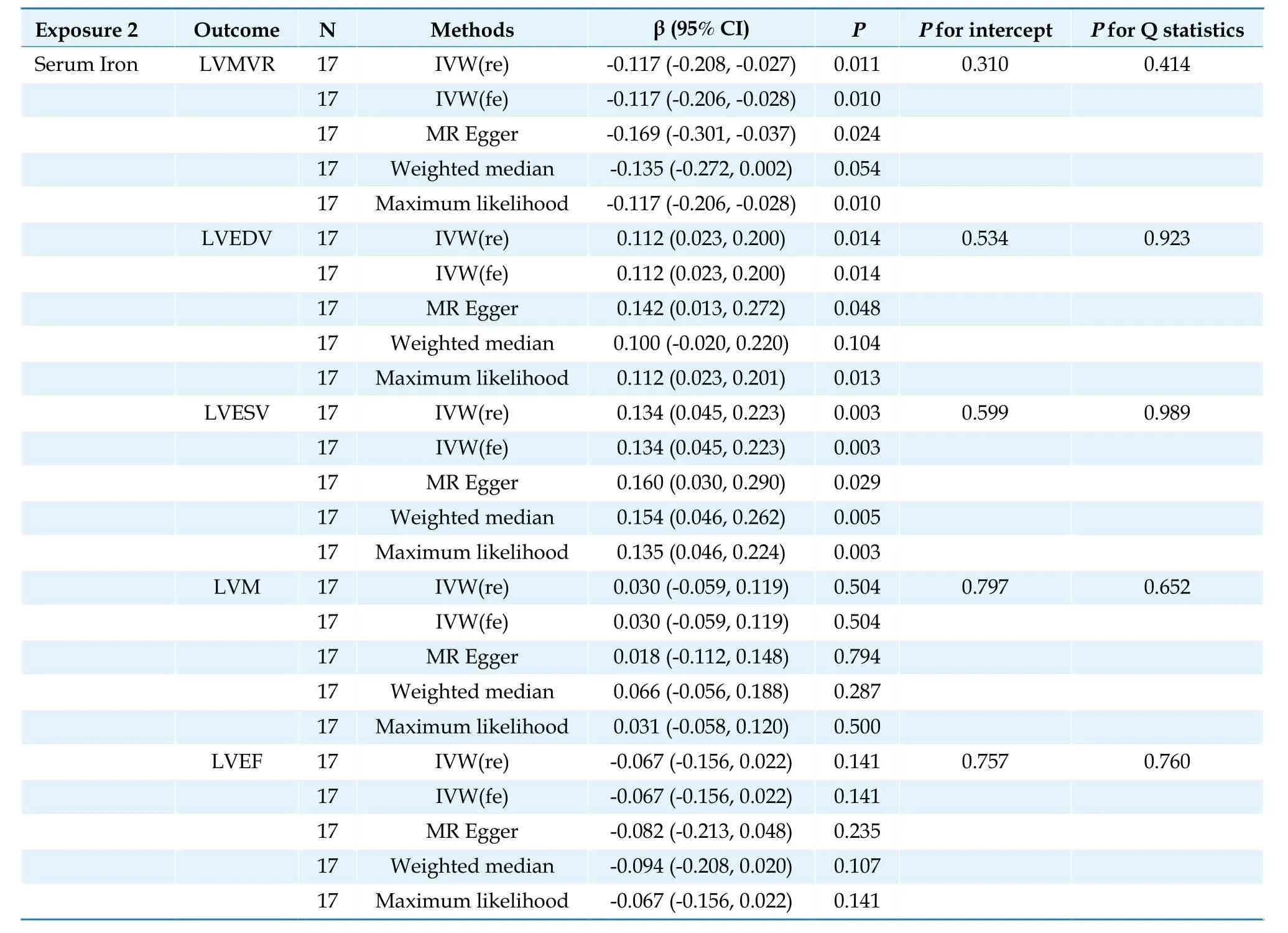
Table 2 Causal association between serum iron and left ventricular parameters.
Effect of TIBC on LV parameters
In the IVW analysis,each 1 standard deviation increase in TIBC was nominally significantly correlated with increased LVMVR (β=0.1023,95% CI:0.0028 to 0.2018;P=0.044) (Table 3).
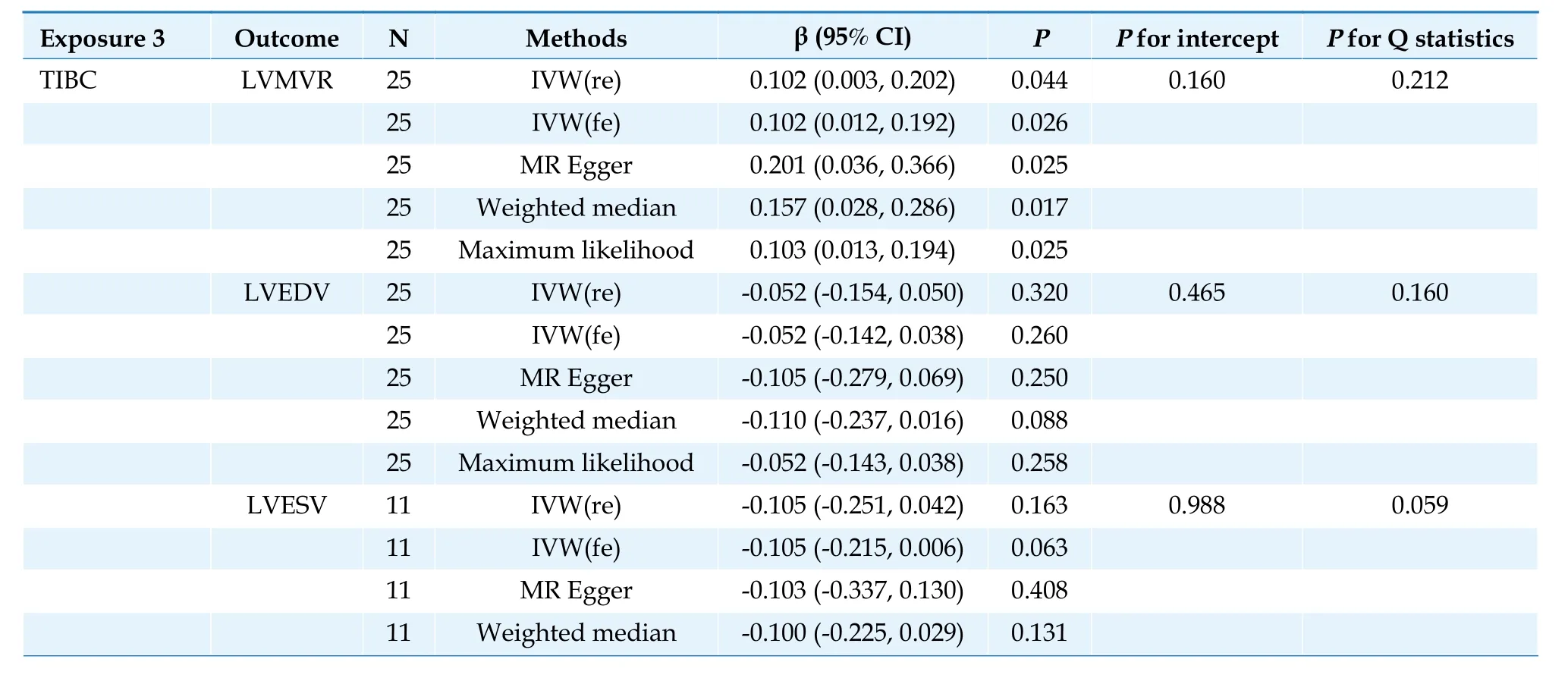
Table 3 Causal association between total iron binding capacity and left ventricular parameters.
Effect of ferritin on LV parameters
In the IVW analysis,no results supported a significant relationship between ferritin levels and changes in LV parameters (Table 4).
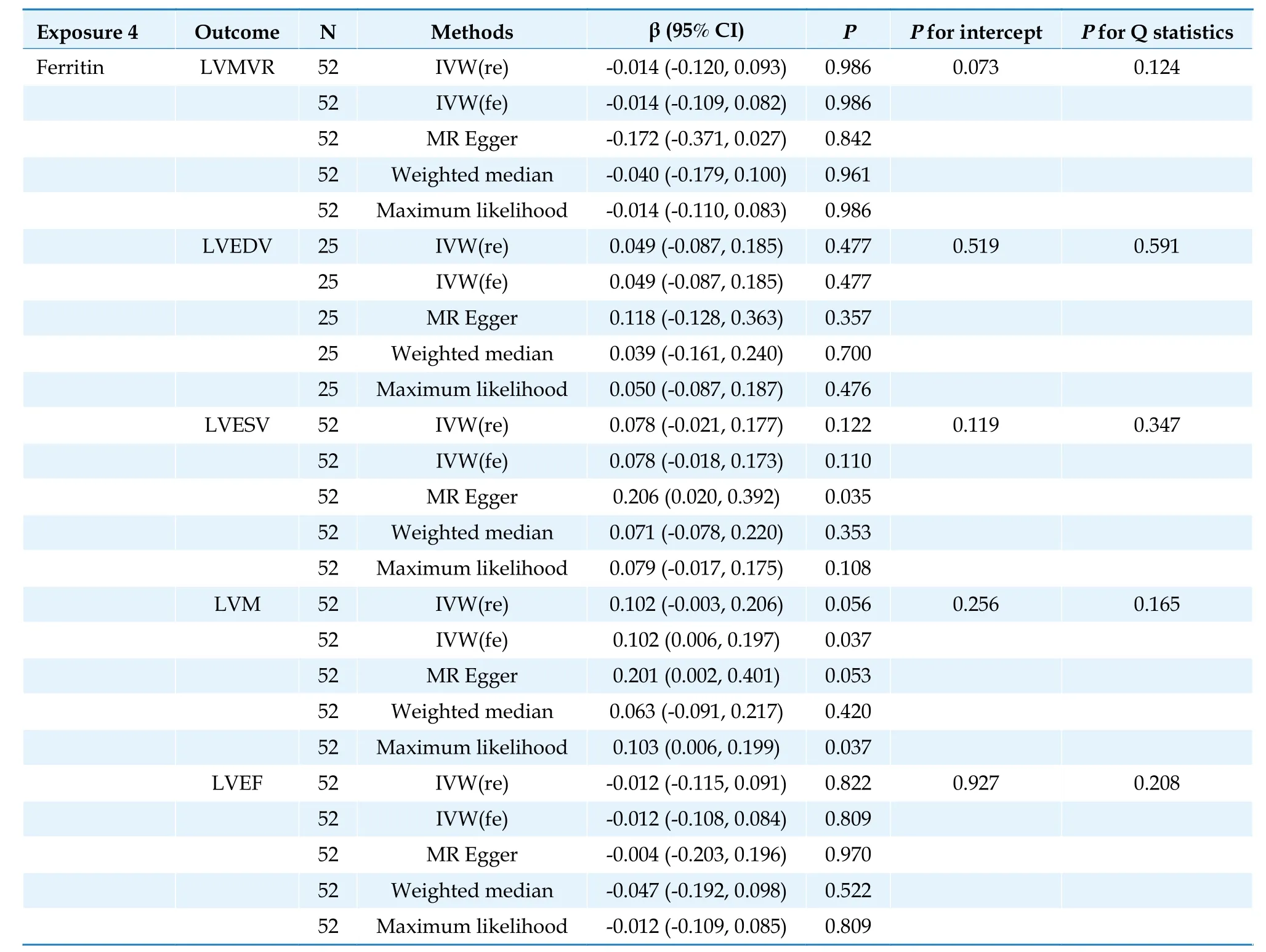
Table 4 Causal association between ferritin and left ventricular parameters.
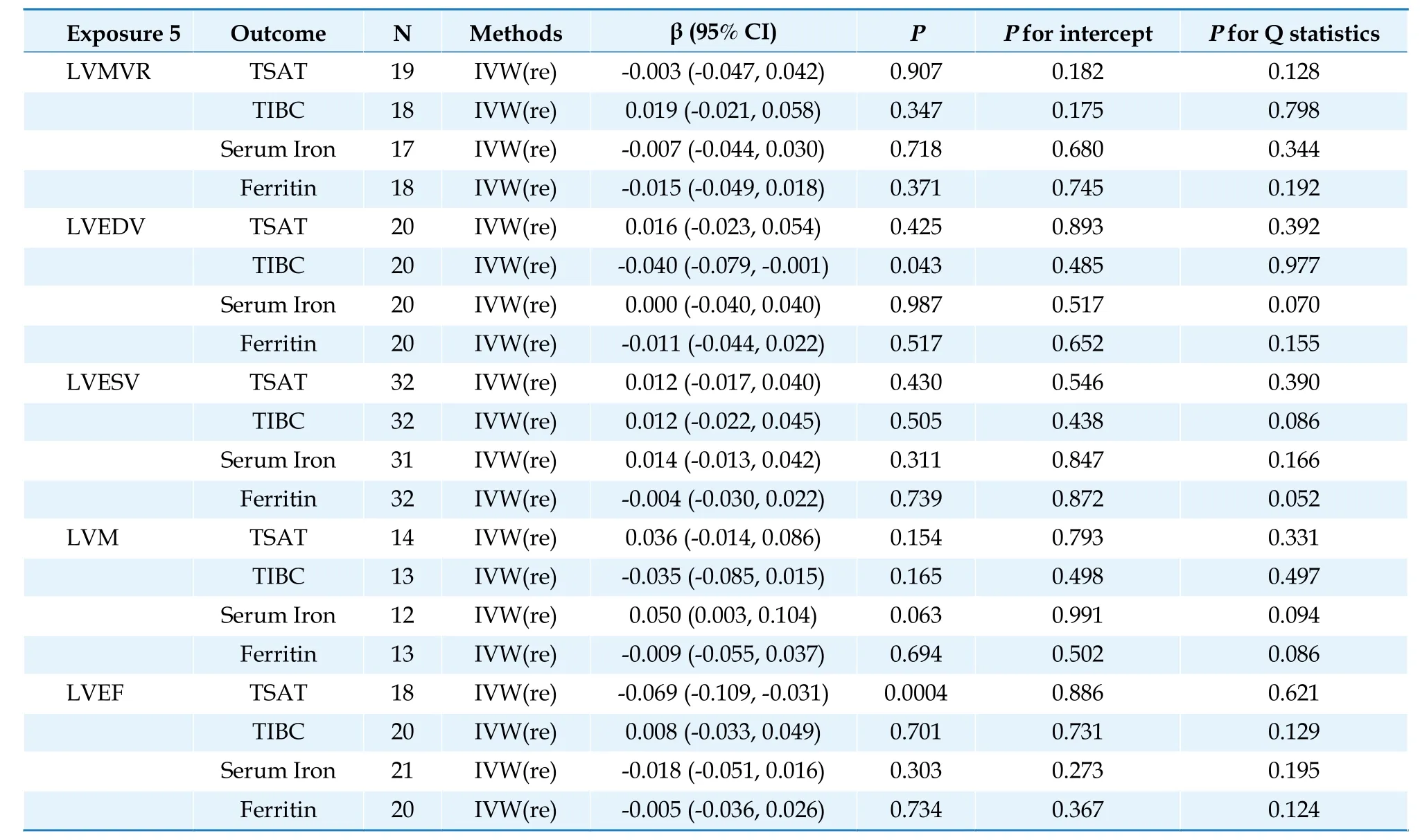
Table 5 Causal association between left ventricular parameters and iron parameters.
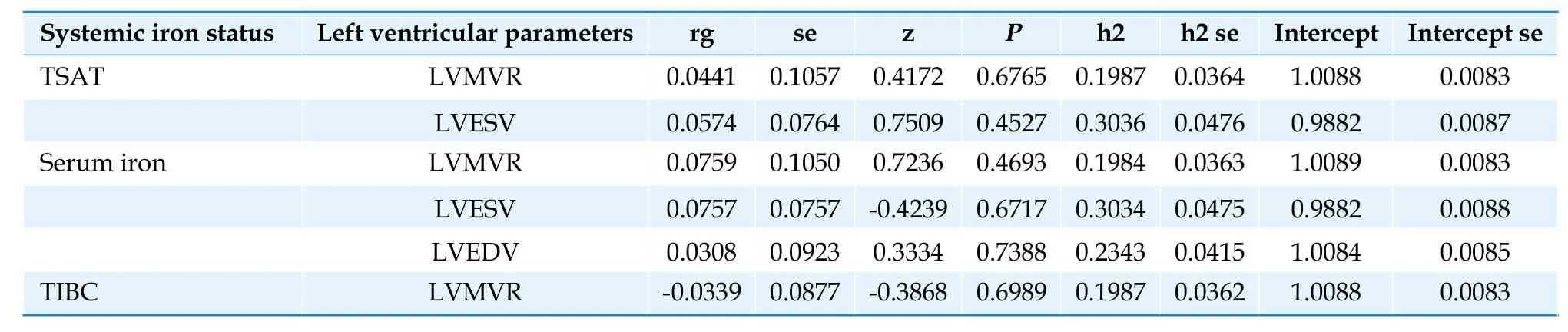
Table 6 Genetic correlation between iron parameters and left ventricular parameters.
The three complementary estimation methods consistently exhibited similar correlations (Supplementary Figures 1-4).However,no results supported a significant causal relationship between TSAT and LVM;LVEDV and LVEF;TIBC and LVEDV;LVESV,LVM,and LVEF;or serum iron and LVM and LVEF (Tables 1-3;Figure 3).
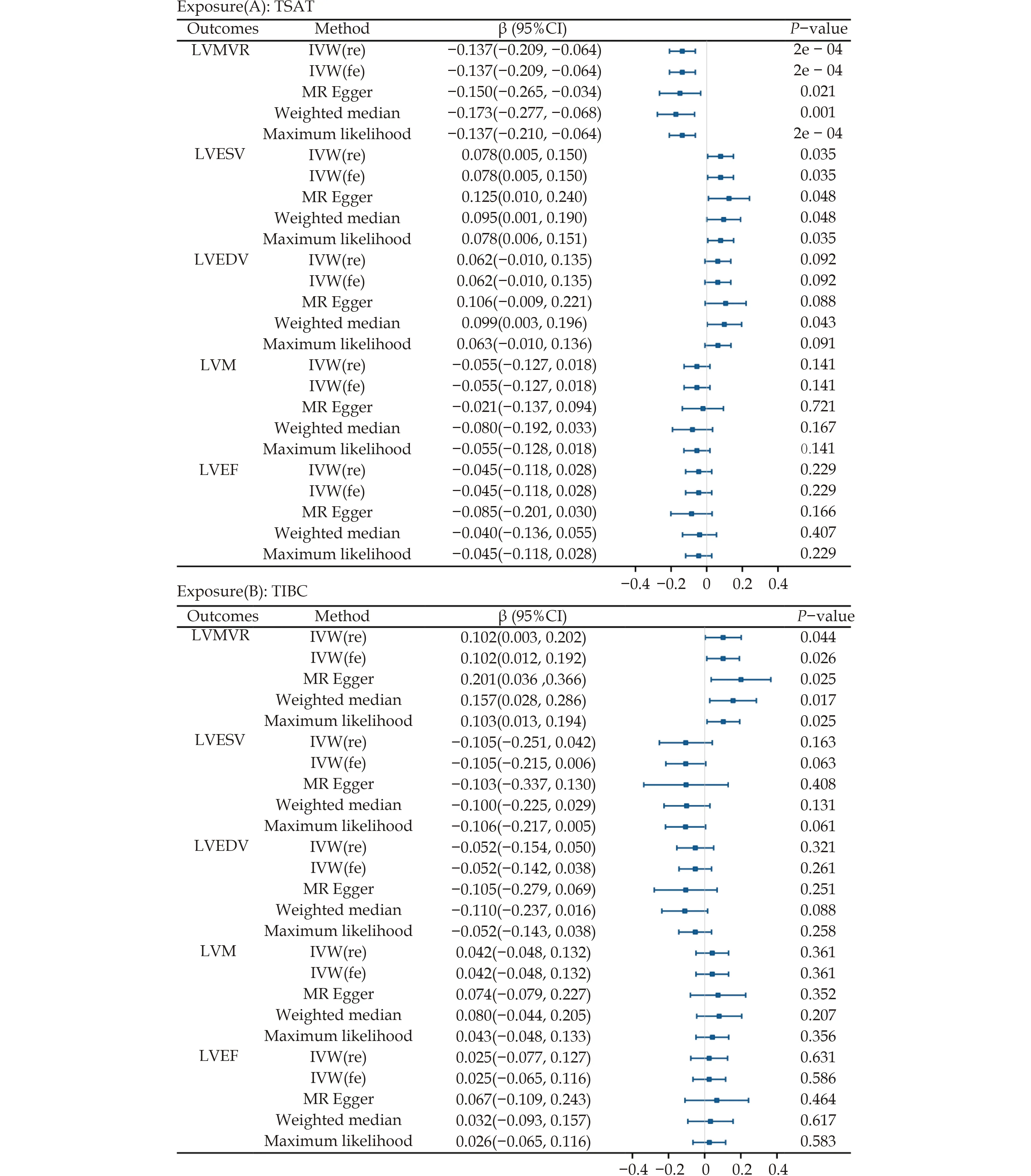
Figure 3 Forest plot of Mendelian randomization analysis between iron parameters and left ventricular parameters. LVEDV: left ventricular end-diastolic volume;LVEF: left ventricular ejection fraction;LVESV: left ventricular end-systolic volume;LVMVR: left ventricular mass-to-end-diastolic volume ratio;LVM: left ventricular mass.
Effects of LV Parameters in the Preserved EF Population on Systemic Iron Parameters
Selection of IVs
The following conditions were used: (1) there was no LD in the IVs (r2< 0.001);(2) the IVs were close to each other (10,000 kb);and (3) the IVs demonstrated genome-wide significance (P< 1×10-5).
We selected the IVs for LVEDV (25 SNPs),LVESV(38 SNPs),LVM (20 SNPs),LVMVR (35 SNPs),and LVEF (26 SNPs) from the LV parameter GWAS database.Repeating the selection procedure produced the final SNPs.The number of SNPs identified at each screening stage is shown in Supplementary Table 7.

Table 7 Steiger direction testing from iron parameters to left ventricular parameters.
In addition,the F-statistic ranges were: LVEDV(19-43),LVESV (19-81),LVM (19-54),LVMVR(19-59),and LVEF (19-62).As mentioned above,the F-statistics for all five LV parameters were > 10,indicating sufficient statistical strength to act as IVs.Information on the SNPs related to LV parameters is detailed in Supplementary Tables 8-12.

Table 8 Steiger direction testing from left ventricular parameters to iron parameters.
Effects of LV Parameters on Systemic Iron Parameters in the Preserved EF Population
Each one standard deviation increase in LVEF was significantly correlated with decreased TSAT (β=-0.0699;95% CI: -0.1087 to -0.0311;P=0.0004)after Bonferroni adjustment.In addition,each one standard deviation increase in LVEDV was nominally significantly associated with decreased TIBC (β=-0.0399,95% CI: -0.0786 to -0.0012;P=0.043) (Table 5;Figure 4).We observed no causal relationships between genetically determined LV (LVESV,LVM,LVMVR,and LVEF) and other iron parameters in our database.The scatterplots are displayed in Supplementary Figures 5-9.
Sensitivity Analysis
The MR-Egger and IVW methods showed no significant heterogeneity (P> 0.05) in the MR analyses after multiple screenings in the forward or reverse MR analysis.No horizontal multi-directionality was found in the MR-Egger regression results (P> 0.05),and the leave-one-out analysis plots did not show potential SNP-driven causalities between exposure and outcome.All data were analyzed using funnel plots to determine the presence of horizontal pleiotropy.No heterogeneity or outliers were detected using the MR-PRESSO global or outlier test (forward MR: Tables 1-4,Supplementary Tables S13-S16,Supplementary Figures 10-17;Reverse MR: Table 5,Supplementary Tables S17-S21,Supplementary Figures 18-27).
Genetic Correlation and Direction Validation
Using LDSC,we found little evidence of a genetic correlation between TSAT and LVMVR (rg=0.0441,se=0.1057,P=0.6765) and LVESV (rg=0.0574,se=0.0764,P=0.4527);serum iron and LVEDV (rg=0.0308,se=0.0923,P=0.7388),LVESV (rg=0.0757,se=0.0757,P=0.6717),and LVMVR (rg=0.0759,se=0.1050,P=0.4693);and TIBC and LVMVR (rg=-0.0339,se=0.0877,P=0.6989),proving that shared genetic elements did not affect MR results.[35]To confirm the direction of the influence of exposure on the outcome,the Steiger test was conducted.The calculated causality was not skewed using reverse causality according to the SteigerP-value (Tables 6-8;Supplementary Table S22).
DISCUSSION
This two-sample bidirectional MR study investigated the causal relationship between systemic iron status and LV structure and function in the preserved EF population,The main finding was that each one standard deviation increase in TSAT is causally associated with lower LVMVR in the preserved EF population.Moreover,our reverse MR analysis showed that each one standard deviation increase in LVEF was causally associated with a lower TSAT.
Basic and clinical trials have shown that ID causes systolic and diastolic dysfunctions and pathological myocardial remodeling in cardiomyocytes.Similarly,the trials showed that iron supplementation reverses the negative effects of ID on cardiomyocytes,which may be attributable to ID causing mitochondrial oxidative dysfunction,impaired energy balance,and impaired resistance to oxidative stress.[36-39]Zhang,et al.[40]used cardiac magnetic resonance imaging (MRI) to investigate myocardial iron status in patients with HF and concluded that myocardial ID is highly prevalent in patients with advanced HF and exacerbates cardiac rational remodeling.
ID also increases the risk of illness,all-cause death,and repeated all-cause hospitalization in patients with HF.[41-43]Several studies in patients with HF with reduced EF and ID demonstrated that intravenous ferric carboxymaltose improves symptoms,quality of life,and hospitalization for recurrent cardiovascular disease and positively affects the improvement of cardiac remodeling and LV systolic function.[44-46]Accordingly,guidelines for administering intravenous iron carboxymaltose to patients with ID and chronic HF with reduced EF have been recommended to reduce symptoms,improve health- related quality of life,and increase exercise tolerance.However,owing to inadequate evidence,this recommendation has not been extended to patients with HFpEF.[47]An effective explanation for the benefit of intravenous iron supplementation in patients with HF and ID can be found in a randomized controlled trial involving MRI to assess myocardial iron.This study found significant short-term changes in myocardial iron levels when patients with HF and ID were administered intravenous iron.[48]Nevertheless,an MR analysis on the genetic relationship between systemic iron status and HF in genetics showed results that were inconsistent with those of previous studies.This does not exclude associations with the study limitations,such as insufficient sample size or failure to stratify studies by HF subtype or severity.[49]
Our findings substantially aligned with those of previous studies.We further confirmed the causal relationship between higher TSAT and lower LVMVR in the preserved EF population,which may reveal a causal relationship between circulating iron parameters,LV diastolic dysfunction,and pathological remodeling by directly affecting cardiac morphology.Elevated LVMVR may reflect LV centripetal remodeling and LV diastolic dysfunction.[50,51]
HFpEF has a pathophysiological mechanism represented by diastolic dysfunction,and individuals with high LVEF (> 60%) are reportedly characterized by LV centripetal hypertrophy and myocardial systolic and diastolic stiffness.[52]As research progressed,several studies agreed that ID affected LV diastolic function and exercise capacity in patients with HFpEF,whereas intravenous iron supplementation significantly improved these functions and capacities,which may be attributable to the fact that iron supplementation improves oxidative stress-related endothelial dysfunction.[14,53]To date,many studies have investigated whether intravenous iron therapy effectively reverses myocardial remodeling and prevents myocardial remodeling owing to ischemic or non-ischemic injury,which is of great interest.[54,55]However,studies on the relationship between circulating iron parameters and myocardial iron are limited,and findings vary between studies;thus,the significance of circulating iron parameters as representatives of myocardial iron content remains unclear.[54,56]Although the relationship between the two is unclear,different studies have shown that ID,defined using circulating iron parameters,aids in diagnosing patients with HF and ID.[47]We look forward to large high-quality studies to further reveal the relationship.
The forward MR analysis revealed a causal relationship between TSAT,TIBC,serum iron,and LVMVR but not ferritin.This may be because ferritin is the only biomarker of iron storage in the body in the non-inflammatory state.[19,57]The population in this study included middle-aged and older adults,with an average age of 62.5 years.Although patients with myocardial infarction and HF were excluded from this study,chronic diseases such as hypertension,diabetes,and osteoarthritis were not excluded and might have influenced the results as they are all strongly associated with chronic inflammation.Chronic inflammation increases ferroregulin secretion,which reduces iron absorption and causes the release of ferritin into the blood,decoupling ferritin from ID.[58-61]To address this,it has been suggested that a highly specific definition of ID is required for diagnosis.because different ID definitions may provide inconsistent conclusions regarding prevalence and disease prognosis.[62-64]
The reverse MR analysis revealed a relationship between higher LVEF and lower TSAT in the preserved EF population.However,only few highquality studies have been published on the effects of LVEF on circulating iron levels.Iron is an essential micronutrient that can harm the body at excessively high or low levels;therefore,the body creates ingenious systems to maintain whole-body iron concentrations within the desired physiological range.Numerous studies have shown that hepcidin and transferrin are crucial in maintaining systemic iron homeostasis.When the body's iron requirements decrease,the release of hepcidin from the liver is promoted,enhancing the degradation of transferrin,decreasing iron absorption and maintaining good iron homeostasis.[65]In people with HF,the heart usually undergoes pathological remodeling,such as LV hypertrophy and dilation.These ventricular remodelings increase heart load,increasing the heart's demand for energy and oxygen;if the demand is not met,HF symptoms are exacerbated.Iron demand is high because it is an essential raw material for energy metabolism in cardiac muscle cells and hemoglobin synthesis.
This study included non-HF individuals with LVEF > 50%.LVEF is a vital index for evaluating cardiac function,with higher LVEF reflecting better cardiac function.Iron demand is relatively low in individuals with better cardiac function,and the body regulates circulating iron levels through the hepcidin-ferroportin pathway based on actual iron requirements,reducing transferrin saturation.[65]Our conclusions are consistent with the abovementioned notion on iron homeostasis.However,the physiological mechanisms underlying iron homeostasis are complex and remain partially understood.Hence,high-quality studies are required to further validate the association between LVEF and circulating iron.
Our findings have important clinical and research implications for treating ID.Maintaining iron homeostasis may help improve adverse changes in cardiac function and structure in patients with ID and preserved EF;however,iron overload can also adversely affect cardiovascular disease.Therefore,interventions should be implemented under monitoring in such cases.[66,67]Furthermore,our two-sample bidirectional MR analysis had several advantages.(1) compared with observational studies,it reduces confounding and reverse causality;(2) we used the largest and most recent aggregate-level data known,and all data are based on European ethnic groups,effectively reducing bias owing to population stratification;and (3) we carefully chose the IVs to ensure result reliability,and the MR-Egger intercept test and MR-PRESSO analysis revealed no evidence of pleiotropy.However,there were some restrictions to our study: (1) we were unable to further explore causality regarding sex and age,among others;(2) because we only included participants of European ancestry in our study,the results cannot be applied to populations other than those of European descent;and (3) the current study assumes that systemic iron parameters affect LV structure and function in preserved EF populations over time because it considers the lifelong effects of SNPs,which is not entirely consistent with clinical practice.
In conclusion,the findings of this MR study indicate a causal relationship between TSAT and modifications in LV structure and function in a population with preserved EF.Similarly,our study may aid in improving the understanding of the critical role of ID in cardiac remodeling.Iron homeostasis may be a viable and promising new target for improving diastolic function and myocardial remodeling in HFpEF.Nevertheless,further validation in future large randomized controlled trials remains necessary.
DISCLOSURE
Acknowledgments
We thank PMIDs 33536631 and 31554410 for supplying information for this study.
Author Contributions
MA XB was responsible for the overall design of this study and primary analysis.MA XB,LV YL,and QIAN L wrote the manuscript and organized the figures.LIU YM reviewed and revised the manuscript.All the authors have read and agreed to the submission of the manuscript.
Funding Sources
This study was funded by the Key Research and Development of the Gansu Province (No.20YF8FA 079) and the Construction Project of the Gansu Clinical Medical Research Center (No.18JR2FA003).
Conflict of Interests
None
 Journal of Geriatric Cardiology2024年1期
Journal of Geriatric Cardiology2024年1期
- Journal of Geriatric Cardiology的其它文章
- Influencing Factors on Cardiovascular Health in China
- Development and validation of a model integrating clinical and coronary lesion-based functional assessment for longterm risk prediction in PCI patients
- Role of intravascular ultrasound and optical coherence tomography in intracoronary imaging for coronary artery disease:a systematic review
