Micellization Behavior of an Amphiphilic Drug Promethazine Hydrochloride-Surfactant System in an Aqueous Medium
KABIR-UD-DIN KHANAbbul Bashar NAQVIAndleeb Z.
(Department of Chemistry,Aligarh Muslim University,Aligarh-202002,India)
1 Introduction
Surfactant molecules,due to their amphiphilic nature,selfassociate in aqueous solution to form micelles above a certain concentration(known as critical micelle concentration,cmc). Below cmc,they accumulate at the air-solution interface.Both the cmc and the properties of aggregates(or micelles)are governed by several factors including temperature,type and concentration of additives,pH,etc.
Mixed micelles formed by two or more amphiphiles are often used in industrial,pharmaceutical,and medicinal formulations.Ionic-ionic or ionic-nonionic mixed systems1-3are important from fundamental as well as application point of view as the mixtures show nonideal behavior.If synergistic interactions among the mixing amphiphiles are present,they could lower the cmc values of the mixed systems.4,5
Nonionic surfactants are used in pharmaceuticals to increase their stability and to enhance the dissolution rate of active ingredients.6,7These are also used to facilitate solubilization and to increase the stability of drug-carrier emulsions.8-10Literature also reports the use of ionic surfactants in drug delivery.11,12
Many drugs,particularly those with the local anesthetic,tranquillizing,antidepressant and antibiotic actions,are amphiphilic in nature,and exert their activity by interaction with biological membranes.13Phenothiazine drugs belong to the family of amphiphilic drugs which share a general structure of planar tricyclic ring system with a short hydrocarbon chain carrying a terminal,charged nitrogen atom.It has been established that these drugs form aggregates of approximately 6-12 monomers in aqueous solution.13,14
Although these drugs are amphiphilic in nature,they are not hydrophilic enough to be used without a carrier.Among various compounds used as carrier,surfactants possess a number of unbeaten advantages.15,16Micelle size permits the extravasation and accumulation in a variety of pathological sites.Also, they are easy to be prepared on a large scale.
With this viewpoint we studied association behavior of a phenothiazine drug,promethazine hydrochloride(PMT),in presence of cationic and nonionic surfactants at 303.15 K. PMT is an antihistamine used for the symptomatic relief of hypersensitivity reactions.The pKavalue of PMT is 9.117and at low pH values it exists in protonated form while at high pH values it becomes neutral.
2 Experimental
2.1 Chemicals
The amphiphilic drug promethazine hydrochloride(PMT,≥ 98%,Sigma,USA),cationic and nonionic surfactants(decyltrimethyl ammonium bromide(DeTAB,≥98%,TCI,Japan),dodecyltrimethyl ammonium bromide(DTAB,≥98%,TCI,Japan),tetradecyltrimethyl ammonium bromide(TTAB,≥99%, Sigma,USA),cetyltrimethyl ammonium bromide(CTAB,≥99%,Merck,Germany),t-octyl phenoxypolyethoxyethanol(n= 9-10,TX-100,n=7-8,TX-114,Fluka,Switzerland),polyoxyethylenesorbitan monolaurate(Tween 20,LOBA Chemie,India),polyoxyethylenesorbitan monopalmitate(Tween 40,Koch-Light,England),polyoxyethylenesorbitan monostearate(Tween 60 LOBA Chemie,India),polyoxyethylenesorbitan monooleate (Tween 80,LOBAChemie,India))were used as received.Their aqueoussolutionswerepreparedindoubly-distilled water.
2.2 Surface tension measurements
The ring detachment method(Du Noüy Tensiometer)was used to measure surface tension(γ).The ring used in the measurement was cleaned by washing with doubly-distilled water followed by heating through alcohol flame.γ was measured after successive addition of concentrated stock solution in water at 303.15 K.The γ value decreased upto a certain value(i.e., cmc)with successive addition of solution of particular molarity in water,then it became constant.This break point corresponds to cmc value as shown in Fig.1.The accuracy on the individual surface tension reading is approximately±0.5 mN· m-1.
3 Results and discussion
For amphiphile mixtures,the two characteristic phenomena are the formation of mixed monolayers at the interface and mixed micelles in the bulk solution.
Representative plots for surface tension(γ)vs lgC for pure components are shown in Fig.1.The values of cmc for pure components agree well with the literature values.13,18-21Fig.2 depicts the variation of cmc values of PMT-surfactant mixed systems with different mole fractions of surfactants(α1).Fig.2(a) contains data for cationic surfactants while Fig.2(b)for nonionic ones.

Fig.1 Variation of surface tension(γ)with concentration(C)for pure amphiphiles
For homologous series of surfactants,the cmc value increases with the increase of number of carbon atoms in the hydrophobic chain.In general,with the addition of one-CH2group the cmc value decreases to half.Increase in chain length increases the hydrophobic forces and micelle formation becomes easier.Among caionic and nonionic surfactants,nonionic ones have lower cmc values.This is due to the lack of electrical work.
The hydrophobic part of the drug molecule is short and rigid.Therefore,drug forms micelles at high concentration.The cmc values for the drug-surfactant mixed systems decrease sharply with the increase of mole fraction of surfactants and then become almost constant except for drug-DeTAB system. This indicates that the drug forms mixed micelles with the surfactants.For PMT-DeTAB system,cmc values show a minimum.At α1=0.1,it decreases sharply and then starts increasing again.The decrease is more pronounced with nonionic ones (Fig.2(b))than with cationic ones(Fig.2(a)).Presence of similar charge on both the drug and surfactants causes repulsion among head groups.Hence mixed micelle formation becomes difficult as compared to that with nonionic ones.
The ideal cmc,cmc*,is related to the mole fractions of mixing components and their cmc values in pure state by the following equation22:

where α1is mole fraction of the first component(i.e.,pure surfactant),while cmc1and cmc2are critical micelle concentrations for the first(i.e.,pure surfactant)and second(i.e.,pure drug)components,respectively.

Fig.2 Variation of the cmc values with the mole fraction(α1)of cationic(a)and nonionic(b)surfactants

Table 1 Surface properties(cmc,cmc*,Γmax,Amin,ΔG m,ΔG ads,Gmin)afor mixed PMT-surfactant systems at 303.15 K
The values of cmc*along with other parameters for micellization are given in Table 1.For PMT-cationic surfactant systems,cmc*values are higher than the experimental cmc values.For PMT-CPB and PMT-CPC,cmc values are close to cmc*values,which means that these surfactants mix ideally with PMT.For PMT-nonionic surfactant systems,except for Tween 40&Tween 80,cmc*values are lower than the cmc values.The cmc*values give an idea about the mixing behavior of the two components.Negative deviation means synergism in mixing while positive deviation means antagonism.Nonionic surfactants contain large hydrophobic portion which creates steric repulsions in the mixed micelles and,inspite of a reduction in electrical repulsions in their presence,the cmc values come out to be higher than the cmc*values.
In an amphiphile mixture,the mixing of hydrophobic chains can be considered as an ideal process while mixing of head groups is considered as a nonideal process.In order to have knowledge of nature and strength of interactions between two mixing components,Rubinghʹs model based on Regular Solution Theory(RST)23was used to calculate different parameters. This model not only characterizes interaction parameter,βm,but also explains the deviation from ideality.Using this model,micellar mole fraction of surfactants,,can be calculated by solving the following equation iteratively:23

The Xm1values so obtained are used to calculate βmby the following equation:

Also,activity coefficients,fm1and fm2,can be calculated by these values:

The programme for equation(2)was nonconvergent for PMT-nonionic surfactant systems.For PMT-cationic surfactant systems,the values of Xm1increase with the increase in α1as well as with the chain length of surfactants.As the surfactant changes from DeTAB to CTAB(or the number of methylene groups from 9 to 15),hydrophobicity of the surfactant molecules increases and its contribution in mixed micelles also increases. For all systems and at all mole fractions,Xm1(Table 2)values are greater than the stoichiometric mole fraction.This also indicates that almost all the added surfactants take part in mixed micelle formation.
Mole fraction of surfactants in ideal mixing conditions is given by24

Table 2 Various physicochemical parameters(X,X,βm,f,ΔGex,X,βσ,f)afor mixed PMT-surfactant systems at 303.15 K

Table 2 Various physicochemical parameters(X,X,βm,f,ΔGex,X,βσ,f)afor mixed PMT-surfactant systems at 303.15 K
micellar mole fraction of surfactants,ideal micellar mole fraction of surfactants,βm:interaction parameter in mixed micelle, activity coefficients in mixed micelle,ΔGex:excess free energy of mixing,:mole fraction of surfactants in mixed monolayer, βσ:interaction parameter in mixed monolayer,:activity coefficients in mixed monolayer
System PMT-DeTAB α1X1m X1ideal βm f1m f2m X1σ βσf1σf2σ PMT-DTAB PMT-TTAB PMT-CTAB 0.2699 0.5067 0.6111 0.7792 0.4100 0.6282 0.8932 0.9437 0.5872 0.6568 0.7949 0.8339 0.6741 0.8507 0.8674 -2.52 -1.29 -1.98 -1.76 -2.14 -3.60 -0.22 -0.93 -4.00 -8.29 -4.64 -5.72 -4.49 -3.92 -4.71 0.26 0.73 0.74 0.92 0.47 0.61 0.99 0.99 0.51 0.38 0.82 0.85 0.62 0.91 0.92 0.83 0.72 0.48 0.34 0.69 0.24 0.83 0.44 0.25 0.03 0.05 0.02 0.13 0.06 0.03 PMT-CPB 0.8495-1.340.970.38 PMT-CPC 0.1 0.5 0.7 0.9 0.1 0.5 0.7 0.9 0.1 0.5 0.7 0.9 0.1 0.5 0.7 0.9 0.1 0.5 0.7 0.9 0.1 0.5 0.7 0.9 0.3639 0.4719 0.5739 0.7414 0.3913 0.6706 0.7982 0.8579 0.5661 0.7441 0.8041 0.8808 0.6367 0.8287 0.9079 0.9115 0.7506 0.8679 0.9209 0.987 0.7079 0.9217 0.9139 0.0768 0.4282 0.6361 0.8708 0.2558 0.7557 0.8783 0.9653 0.6373 0.9405 0.9736 0.9930 0.8450 0.9800 0.9913 0.9977 0.9012 0.9879 0.9948 0.9986 0.8961 0.9872 0.9945 0.9985 -7.08 -3.14 -1.77 -1.77 -2.88 -1.23 -1.01 -2.13 -2.25 -3.48 -3.61 -3.90 -4.16 -3.53 -3.00 -4.59 -2.22 -3.44 -3.33 -2.77 -3.05 -2.25 -3.44 0.06 0.42 0.73 0.89 0.34 0.87 0.96 0.96 0.65 0.79 0.87 0.95 0.58 0.90 0.97 0.96 0.87 0.94 0.98 0.99 0.77 0.98 0.97 0.39 0.49 0.56 0.38 0.64 0.57 0.52 0.21 0.48 0.15 0.097 0.049 0.185 0.088 0.084 0.022 0.286 0.075 0.059 0.067 0.216 0.148 0.057 ΔGex/(kJ·mol-1) -4.13 -1.97 -1.09 -0.86 -1.73 -0.68 -0.41 -0.65 -1.39 -1.67 -1.43 -1.03 -2.42 -1.26 -0.63 -0.93 -1.05 -0.99 -0.61 -0.09 -1.60 -0.41 -0.68
The nature and strength of the interactions among mixed micelles can be evaluated with the interaction parameter,βm.The βm,according to RST,is zero for ideal mixing;negative for synergistically formed mixed micelles;positive for antagonism and is assumed to be constant for a particular system.But in actual conditions,βmvaries with the composition of mixed system.The βmvalues(Table 2)are negative indicating mixed micelle formation through attractive interactions.Barring βmvalues for DeTAB at α1=0.1,average taken for a particular system,shows slight decrease and then an increase with the increase in chain length from C10to C16.With the increase in chain length,surfactantsʹhydrophobicity increase and surfactants interact more strongly with the drug causing more stable mixed micelle formation.For CPB and CPC,values are almost same.

The values of ΔGex(Table 2)are all negative and the average values for all the systems are in the range of 1-1.5 kJ·mol-1except PMT-CPB and PMT-CPC.The negative ΔGexvalues indicate that the process of mixing is favorable.
Fig.3(a,b)show the variation of surface excess(Γmax)with the mole fraction of surfactants.Γmaxis a useful measure of the adsorption effectiveness of the amphiphiles at the air-solution interface,as it is the maximum value adsorption can attain.On the basis of adsorption isotherms,Γmax(in mol·m-2)at the air-solution interface was obtained from the Gibbs adsorption equation24:

and minimum area per head group(Aminin nm2)at the interface was obtained by

where NAis the Avogadro number.The Γmaxvalues(Table 1)for both cationic and nonionic surfactants decrease as the mole fraction of surfactants in the solution increases,(except for DeTAB).Cationic surfactants form mixed micelles with the drug molecules and because of the similar charge,the mixed micelles as well as the mixed monolayers will experience more repulsions.Hence,the molecules will try to be as far apart as possible and Γmaxdecreases.
Nonionic surfactants also form mixed micelles with the drug.These surfactants reduce the repulsion among head groups and Γmaxshould increase in their presence.However,for PMT-nonionic surfactant systems Γmaxdecreases for TX-100 and TX-114,while it increases for Tweens,with the increase in concentration of surfactants.The hydrophobic part of both the drug and the nonionic surfactants are bulky and the drugʹs part is rigid also.Therefore,the mixed micelles and mixed monolayers will experience more steric hindrance and the molecules will be far from each other.Therefore,Γmaxdecreases.
The Aminvalues(Table 1,Fig.4(a,b)),calculated from equation(9),follow trend opposite to that of Γmax.The results are self-explanatory in the light of above discussion.As the molecules lie farther from each other,Aminvalues increase.
Analogous to equations(2-5),Rosenʹs theory24,25can be used to calculate mole fraction of surfactant at the interface,X,interaction parameters for monolayer,βσ,and activity coefficients,fand f(Table 2).Xvalue increases with the increase in mole fraction and chain length of surfactants.For CPB and CPC,the equation for Xwas nonconvergent.Xvalues in almost all cases were lower than Xvalues.This indicates that contribution of surfactants is more in mixed micelles than in the mixed monolayers.The rigid hydrophobic structure of the drug makes it easier for the drug to adsorb at the interface than to adjust in curved micellar interface.Hence,more drug and less surfactants are present at the air-solution interface.

Fig.3 Variation of Γmaxvalues with the mole fraction(α1)of cationic(a)and nonionic(b)surfactants
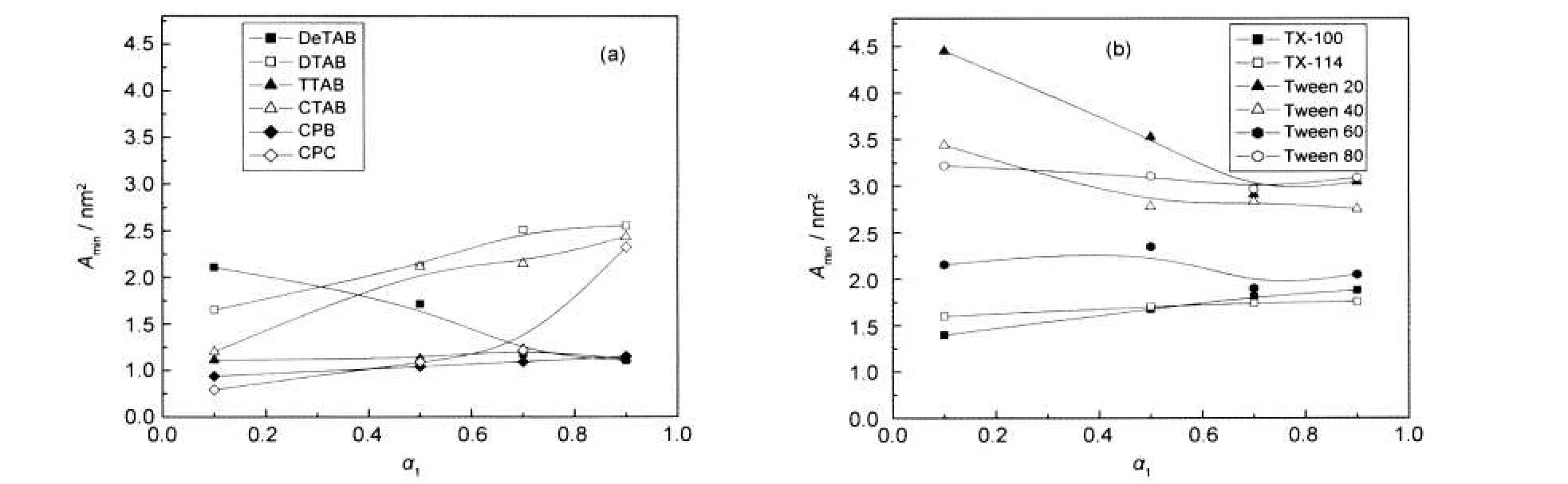
Fig.4 Variation ofAminvalues with the mole fraction(α1)of cationic(a)and nonionic(b)surfactants
The βσvalues,although negative,are smaller in magnitude then βmvalues.For PMT-DeTAB and PMT-DTAB,the average βσvalues,β,are close to 2 whereas for CTAB and TTAB the values are close to 5.The fand fvalues are less than unity and are also less than fand fvalues(fvalues for DeTAB and DTAB are more than fvalues).These fractional values ofandindicate nonideality.
The values of different parameters obtained are used to calculate the different types of energies,viz.standard Gibbʹs free energy of micellization(ΔGm),standard energy change of adsorption at interface(ΔGads),and molar free energy of maximum adsorption attained at the cmc(Gmin).

where Xcmcis the cmc in mole fraction units.
All the values of ΔGm(Table 1)are negative which indicates that micelle formation is spontaneous.The magnitude of ΔGmfor pure drug is always less than that for the pure surfactants as well as for drug-surfactant mixtures.In the drug-surfactant systems,the spontaneity is in the order:PMT-DeTAB>PMT-TTAB>PMT-DTAB>PMT-Tween80>PMT-Tween 60>PMT-Tween40>PMT-Tween20>PMT-TX-114>PMT-TX-100>PMT-CPC>PMT-CPB>PMT-CTAB.

where πcmcis the surface pressure at the cmc(=γ0-γcmc).
We can see(Table 1)that the process of interfacial adsorption is the most spontaneous for Tween 20 and CPC and the least for DeTAB.DeTAB prefers to form aggregates than to adsorb at the surface.
The Gminvalues are evaluated by the equation27

Gminis the minimum energy of the given surface with fully adsorbed amphiphile molecules.The lower the value of the free energy,the more thermodynamically stable surface formed. Among pure components,TX-100 and TX-114 make the most stable surfaces while surface formed by PMT is the least stable (Table 1).For PMT-surfactant mixed systems the surface formed by PMT-TTAB is the most stable and PMT-Tween 80 mixed systems form the least stable surface.
4 Conclusions
The measurement of the surface tension and calculation of different parameters in the mixed micelles and mixed monolayers formed by an amphiphilic drug(PMT)and surfactants(nonionic and cationic)indicate the following:
(1)cmc values of PMT-surfactant systems decrease sharply and then become almost constant as the mole fraction of surfactant increases in the system.This means that PMT forms mixed micelles with the surfactants.
(2)Interaction parameters for mixed micelles and mixed monolayers,βmand βσ,are negative for all the systems,which indicate attractive interactions among mixing components.
(3)In general,Γmaxdecreases and Aminincreases with the increase in mole fraction of surfactants.Rigid structure of drug makes adsorption easier.
(4)ΔGmand ΔGadsvalues are negative and ΔGmis maximum for Tween 80 and minimum for DeTAB mixed systems whileis the highest for Tween 20 and lowest for DeTAB systems.
(1) Zhu,D.;Zhao,G.Colloids Surf.1990,49,269.
(2) Li,X.;Zhao,G.Colloids Surf.1992,64,185.
(3)Yu,Z.;Zhang,X.;Xu,G.;Zhao,G.J.Phys.Chem.1990,94, 3675.
(4) Hines,J.D.;Thomas,R.K.;Garrett,P.R.;Rennie,G.K.; Penfold,J.J.Phys.Chem.B 1997,101,9215.
(5) Shilaoch,A.;Blankschtein,D.Langmuir 1998,14,7166.
(6) Fontan,J.E.;Arnaud,P.;Chaumel,J.C.Int.J.Pharm.1991, 73,17.
(7) Sjokvist,E.;Nystorm,C.;Alden,M.;Carram-Lelham,N.Int.J. Pharm.1992,79,123.
(8) Florence,A.T.Techniques of Solubulization of Drugs; Yalkowsky,S.H.Ed.;Marcel Dekker Inc.:New York,1981.
(9) Fahelebom,K.M.S.;Timoney,R.F.;Carrigan,O.I.Pharm. Res.1993,10,631.
(10) Lundberg,B.J.Pharm.Sci.1994,83,72.
(11) Paulsson,M.;Edsman,K.Pharm.Res.2001,18,1586.
(12) Bhatt,P.A.;Dar,A.A.;Rather,G.M.J.Chem.Eng.Data 2008, 53,1271.
(13)Attwood,D.;Florence,A.T.Surfactant Systems;Chapman and Hall:New York,1983.
(14) Cheema,M.A.;Siddiq,M.;Barbosa,S.;Castro,E.;Egea,J.A.; Antelo,L.T.;Taboada,P.;Mosquera,V.Chemical Physics 2007,336,157.
(15) Cid,E.Pharma.Acta Helv.1971,46,377.
(16)Taboada,P.;Atwood,D.;Ruso,J.M.;Garcia,M.;Mosquera,V. Phys.Chem.Chem.Phys.2000,2,5175.
(17) Katsung,B.G.Basic and Chemical Pharmacology,9th ed.; McGraw Hill:New York,2004.
(18)Yeom,I.T.;Ghosh,M.M.;Cox,C.D.;Robinson,K.G. Environ.Sci.Technol.1995,29,3015.
(19) Traguer,D.;Csordas,A.Biochem.J.,1987,244,605.
(20)Acharya,K.R.;Bhattacharyya,S.C.;Moulik,S.P. J.Photochem.Photobiol.A:Chem.1999,122,47.
(21) Mukherjee,P.;Mysels,K.J.Critical Micelle Concentration of Aqueous Surfactant Systems;NSRDS-NBS 36:Washington,D. C.,1971.
(22) Mukherjee,P.Adv.Colloid Interface Sci.1967,1,242.
(23) Rubingh,D.N.Solution Chemistry of Surfactants;Mittal,K. L.Ed.;Plenum:New York,1979.
(24) Rosen,M.J.Surfactants and Interfacial Phenomena;Wiley-Interscience:New York,2004.
(25) Hua,X.Y.;Rosen,M.J.J.Colloid Interface Sci.1982,87,469.
(26) Rosen,M.J.;Aronson,S.Colloids Surf.1981,3,201.
(27) Sugihara,G.;Miyazono,A.M.;Nagadome,S.;Oida,T.; Hayashi,Y.;Ko,J.S.J.Oleo Sci.2003,52,449.

