A New 2D Layer Manganese(Ⅱ)Complex Assembled by Flexible 1,4-Benzenebis(thioacetic acid)Ligand:Synthesis,Crystal Structure and Magnetic Property
WANG Xiao-Juan YIN Jian-Ling CHEN Jing FENG Yun-Long(Zhejiang Key Laboratory for Reactive Chemistry on Solid Surfaces,Institute of Physical Chemistry,Zhejiang Normal University,Jinhua,Zhejiang 321004,China)
A New 2D Layer Manganese(Ⅱ)Complex Assembled by Flexible 1,4-Benzenebis(thioacetic acid)Ligand:Synthesis,Crystal Structure and Magnetic Property
WANG Xiao-Juan YIN Jian-Ling CHEN Jing FENG Yun-Long*
(Zhejiang Key Laboratory for Reactive Chemistry on Solid Surfaces,Institute of Physical Chemistry,Zhejiang Normal University,Jinhua,Zhejiang 321004,China)
A new Mn(Ⅱ)complex based on 1,4-benzenebis(thioacetic acid)(H2L),[Mn3L3(phen)2]n(1,phen=1,10-phenanthroline),has been hydrothermally synthesized and characterized by single crystal X-ray diffraction,elemental analysis,IR spectrum,and TG analysis.The structure can be considered as a two-dimensional(2D)layered architecture which consists of linear trinuclear Mn(Ⅱ)units.The magnetic susceptibility data are interpreted with the linear trinuclear law,yielding J and g values of-1.95(5)cm-1and 1.98(1).The exchange integral(J)indicates a weak antiferromagnetic interactions in the linear trinuclear Mn(Ⅱ)unit.CCDC:734943.
Mn(Ⅱ)complex;hydrothermal synthesis;1,4-benzenebis(thioacetic acid);crystal structure;magnetic property
The design and synthesis of novel polymeric complexes has developed rapidly in recent years due to their potential applications in some fields,such as gas storage,size-and shape-selective catalysis,material science,medicineandmagnetochemistry,aswellastheir intriguing variety of architectures and topologies[1-9].In contrast to the great deal of work on hydrothermal synthesis with phenylene diacetate ligands[10-14],there havebeen few reportsofstudieson phenylene dithioacetate ligands[15-17].Hence,we designed a multicarboxylate ligand,1,4-benzenebis (thioacetic acid)(H2L,Scheme 1),on the basis of the 1,4-benzenebisoxyacetate[14,18]and phenylthioacetate[19].The-S-CH2-groups make H2L ligand more flexible in comparison with the corresponding benzene-dicarboxylate, while the existence of the benzene ring also provides a rigid element. Recently, we have synthesized some complexes that contain first row transition metal cationsbridged by H2L and bipyridine analogues ligands[20-21].Moreover,manganese ions not only have versatile coordination behavior but also with well magnetism.Herein, we report the synthesis, structural characterizations and magnetic property of a new Mn(Ⅱ)complex,[Mn3L3(phen)2]n(1).
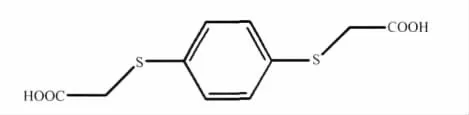
Scheme 1 Molecular structure of H2L ligand
1 Experimental
1.1 Materials and general methods
All solvents and starting materials for the synthesis were purchased commercially without further purification.H2L was obtained according to a rational procedure by 1,4-benzenebisthiol[17].The complex was obtained under hydrothermal reaction in a 25 mL Tefion-lined stainless steel Parr bomb.Data collection was performed on a Bruker SMART APEXⅡCCD diffractometer with Mo Kα radiation(λ=0.071 073 nm).Elementary analysis was performed on a EuroEA3000 element analyzer.IR spectrum was measured in KBr pellets on a Nicolet 5DX FTIR spectrometer.Thermogravimetric analysis(TGA)was performed at a rate of 10℃·min-1under oxygen stream using a Netzsch STA449C apparatus.The magnetic measurement was carriedoutwithaMPMSSQUID magnetometer Quantum Design SQUID MPMS XL-7 instruments working in the temperature range of 2~300 K at an applied field of 0.1 T.
1.2 Synthesis of[Mn3L3(phen)2]n
A mixture of H2L (0.103 g,0.4 mmol),MnCl2·4H2O (0.079 g,0.4 mmol),1,10-phen (0.040 g,0.2 mmol),and NaOH(0.032 g,0.8 mmol)in H2O(18 mL)was placed in a Tefion-lined stainless steel vessel and heated at 160℃for 72 h,then cooled to room temperature over 3 d.Block pink crystals of 1 were obtained and washed with water,dried in air(yield 48%).Anal.Calcd.for C54H40Mn3N4O12S6(%):C,50.12;H,3.12;N,4.33;S,14.86.Found(%):C,50.08;H,3.08;N,4.45;S,14.69.IR (KBr,cm-1):3 445,2930,1 601,1385,1209,1113,847,736,680,599,488.
1.3 X-ray crystallography
The diffraction data were collected on a Bruker SMART APEXⅡCCD diffractometer equipped with a graphite-monochromatized Mo Kα radiation (λ=0.071 073 nm)at 296(2)K.Intensity data were corrected by Lorentz-polarization factors and empirical absorption.The structure wassolved with direct methods and expanded with difference Fourier techniques.Except the hydrogen atoms on oxygen atoms were located from the difference Fourier maps,the other hydrogen atoms were generated geometrically.All calculations were performed using SHELXS-97[22]and SHELXL-97[23]program package.In the title complex,part of L2-ligands are disordered over two positions in 0.60∶0.40 ratio.Further details for structural analyses are summarized in Table 1,selected bond lengths and angles are listed in Table 2.
CCDC:734943.

Table 1 Crystal data and structure refinement for the complex
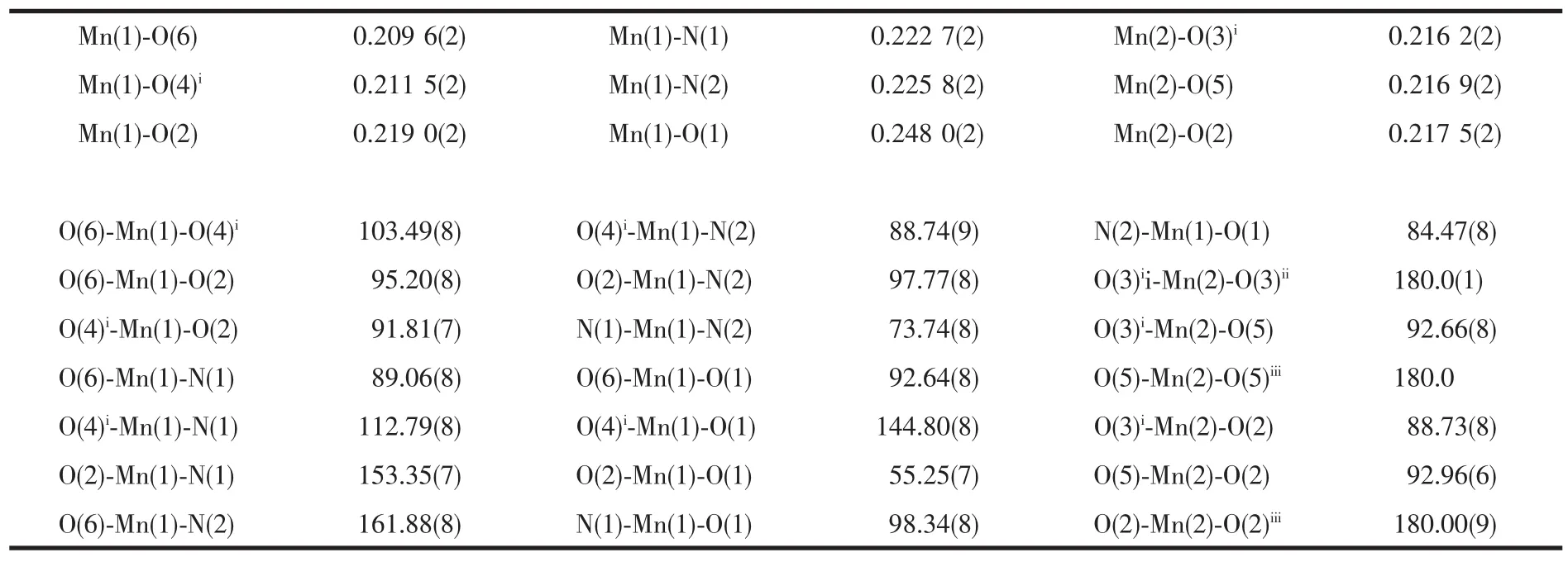
Table 2 Selected bond lengths(nm)and angles(°)for the complex
2 Results and discussion
2.1 Crystal structure
Single-crystal X-ray diffraction analysis revealed that the title complex is a 2D layer structure containing linear trinuclear Mn(Ⅱ)units.As shown in Fig.1,it consists of the trinuclear[Mn3(μ2-COO)6]unit,in which Mn(2)atom lies a crystallographic center at(0.25,0.25,0),and is six-coordinated by carboxylic O atoms from six different L2-ligands(Mn(2)-O 0.217 5(2),0.216 2(2),0.216 9(2)nm)to form an octahedral geometry.The Mn(1)is six-coordinated by four O atoms from three L2-ligands(Mn(1)-O 0.248 0(2),0.219 0(2),0.211 5(2),0.209 6(2)nm),and two N atomsfrom one phen molecule(Mn(1)-N 0.222 7(2)and 0.225 8(2)nm)to form a distorted octahedral geometry.Each pair of Mn(Ⅱ) atoms is μ-linked by three carboxylic groups of the individual L2-ligands with Mn(1)…Mn(2)distances of 0.3626(1)nm.The structure is similar to those found in [Mn3(ppei)2(OAc)6][24], [Zn3L3(2,2′-bipy)2][20], and[Co3(OAc)8][25].
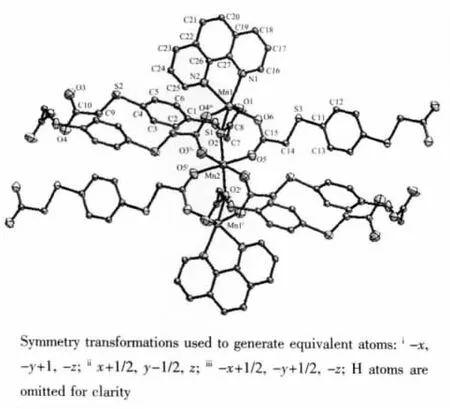
Fig.1 Coordination environment of trinuclear Mn(Ⅱ)unit
As illustrated in Fig.2,the trinuclear[Mn3(μ2-COO)6]units are joined by flexible carboxyl O atoms to generate 2D networks.Notably,in this 2D layer,it consists of two kinds of link modes:single-stranded along b axis and double-stranded mode along c axis.To better understand this structure,the linear trinuclear Mn(Ⅱ)units serve as nodes,and the nodes are linked by carboxylic O atoms of L2-ligands,resulting in a 2D(4,4)net in the ab plane.

Fig.2 2D(4,4)net in ab plane
2.2 Magnetic property
From the magnetic point of view,the title complex can be considered as a linear trinuclear Mn(Ⅱ)system.The magnetic behaviourofthe title complex is illustrated in Fig.3 by meansofthe magnetic susceptibility(χmT)vs the temperature(T).The χmT value per Mn3gradually decreases from 12.18 cm3·mol-1·K at 300 K to 4.25 cm3·mol-1·K at 0.85 K.The μeffvalue (9.87μB)at room temperature is smaller thanthat of 10.25μBexpected for three independent Mn(Ⅱ)ions withS=5/2.This result reveals the overall antiferroma-gnetic character of the system.The Heisenberg spin Hamiltonian model for the isotropic magnetic exchange interaction in the linear trinuclear Mn(Ⅱ)unit is given inH=-2Jwhereand the numbering has been employed in Fig.1.Take into account the fact that the magnetic coupling except Mn3unit is weak,using the contributions of intermole-cular force in the fit.Then the temperature dependence of the magnetic susceptibility is given by the equation:

A=35+84e5x+35e-2x+10e-7x+165e10x+84ex+35e-6x+10e-11x+e-14x+286e15x+165e4x+84e-5x+35e-12x+10e-17x+e-20x+455e20x+286e7x+165e-4x+84e-13x+35e-20x+10e-25x+680e25x+455e10x+286e-3x+165e-14x+84e-23x+35e-30x;B=6+8e5x+6e-2x+4e-7x+10e10x+8ex+6e-6x+4e-11x+2e-14x+12e15x+10e4x+8e-5x+6e-12x+4e-17x+2e-20x+14e20x+12e7x+10e-4x+8e-13x+6e-20x+4e-25x+16e25x+14e10x+12e-3x+10e-14x+8e-23x+6e-30x.x=J/(kT).The best-fit parameters obtained:J=-1.95(5)cm-1,g=1.98(1),zJ′=-0.41(1)cm-1,andR=9.8×10-6.The variation of χm-1also is well described by the Curie-Weiss law in the experimental temperature range 2~300 K withC=12.97 cm3·mol-1·K and θ=-25.056 K.The negativeJand θ values further indicate a weak antiferromagnetic interaction in the linear trinuclear Mn3unit.The spin-exchange interaction is comparable to that of the other with the similar structure[26-28].

Fig.3 Plots of the χmTand χm-1(inset)vsTfor the title complex
2.3 Thermogravimetric analysis
To study the stability of the title complex,thermogramimetric analytical (TGA)was performed.The TGA curve exhibits two steps of weight losses(Fig.4).It displays a high thermal stability,as there is no significant weight loss up to 315℃.An initial weight loss of 30.41%occurred between the temperature range of 315~420℃ corresponds to the removal of two phen molecules(calcd 30.63%),The second weight loss of 58.95% (calcd.59.81%)in the range of 480~575 ℃ is consistent with the removal of three L2-ligands.The remaining products may be MnO.

Fig.4 TGA curve of the title complex
In summary,we have successfully synthesized a new Mn(Ⅱ)complex based on 1,4-benzenebis(thioacetic acid)ligand under hydrothermal conditions.It is a 2D layered architecture which consists of linear trinuclear[Mn3(μ2-COO)6]units.Variable temperature magnetic susceptibility indicates the existence of a weak antiferromagnetic interactions in the linear trinuclear unit.
[1]Phan A,Doonan C,Uribe-Romo F J,et al.Acc.Chem.Res.,2009,43:58-67
[2]Lee J Y,Farha O K,Roberts J,et al.Chem.Soc.Rev.,2009,38:1450-1459
[3]Chen M S,Bai Z S,Okamura T,et al.CrystEngComm,2010,12:1935-1944
[4]Eddaoudi M,Kim J,Rosi N,et al.Science,2002,295:469-472
[5]Huh J O,Lee M H,Jang H,et al.Inorg.Chem.,2008,47:6566-6568
[6]García-Giménez J L,Alzuet G,González-álvarez M,et al.J.Inorg.Biochem.,2009,103:243-245
[7]Zhao X J,Zhu G S,Fang Q R,et al.Cryst.Growth Des.,2009,9:737-742
[8]Zhang Z J,Xiang S C,Zheng Q,et al.Cryst.Growth Des.,2010,10:2372-2375
[9]Moulton B,Lu J J,Zaworotko M J.J.Am.Chem.Soc.,2001,123:9224-9225
[10]Pan L,Adams K M,Hernandez H E,et al.J.Am.Chem.Soc.,2003,125:3062-3063
[11]CottonFA,LinC,MurilloCA.Inorg.Chem.,2001,40:472-477[12]Ren P,Xu N,Chen C,et al.Inorg.Chem.Commun.,2008,11:730-732
[13]Zhou J,Sun C Y,Jin L P.J.Mol.Struct.,2007,832:55-62
[14]Li X F,Han Z B,Cheng X N,et al.Inorg.Chem.Commun.,2006,9:1091-1095
[15]Grirrane A,Pastor A,Galindo A,et al.Angew.Chem.Int.Ed.,2005,44:3429-3432
[16]Deivaraj T C,Lai G X,Vittal J J.Inorg.Chem.,2000,39:1028-1034
[17]Su H,Feng Y L,Wen Y H.Acta Cryst.,2006,C62:m208-m210
[18]Hong X L,Li Y Z,Hu H M,et al.Cryst.Growth Des.,2006,6:1221-1226
[19]Sandhu G K,Sharma N,Tiekink E R T.J.Organomet.Chem.,1991,403:119-131
[20]Yin J L,Feng Y L,Lan Y Z.Inorg.Chim.Acta,2009,362:3769-3776
[21]CHEN Jing(陈静),YIN Jing-Ling(尹建玲),WANG Xiao-Juan(王晓娟),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26:1311-1314
[22]Sheldrick G M.SHELXS-97,Program for X-ray Crystal Structure Solution,Germany,University of Göttingen,1997.
[23]Sheldrick G M.SHELXL-97,Program for X-ray Crystal Structure Refinement,Germany,University of Göttingen,1997.
[24]Kang B,Kim M,Lee J,et al.J.Org.Chem.,2006,71:6721-6727
[25]ReynoldsⅢR A,Dunham W R,Coucouvanis D.Inorg.Chem.,1998,37:1232-1241
[26]Hsu K F,Wang S L.Inorg.Chem.,2000,39:1773-1778
[27]Lu X M,Li P Z,Wang X T,et al.Polyhedron,2008,27:3669-2673
[28]Wang M,Ma C B,Wang H S,et al.J.Mol.Struct.,2008,873:94-100
基于柔性1,4-苯二硫乙酸配体构筑的二维锰配合物的合成、晶体结构及磁性
王晓娟 尹建玲 陈 静 冯云龙*
(浙江师范大学物理化学研究所,浙江省固体表面反应化学重点实验室,金华 321004)
水热法合成了一个基于1,4-苯二硫乙酸(H2L)柔性配体的Mn(Ⅱ)配合物[Mn3L3(phen)2]n,并通过元素分析,红外光谱、热失重和X-射线单晶衍射实验对其结构进行了表征。结构分析表明,配合物是一个包含三核锰结构单元的二维层面结构。对配合物的变温磁化率研究表明,在三核锰离子间存在弱的反铁磁交换作用。
Mn(Ⅱ)配合物;水热合成;1,4-苯二硫乙酸;晶体结构;磁性
O614.7+11
:A
:1001-4861(2011)02-0367-05
2010-09-14。收修改稿日期:2010-10-11。
*通讯联系人。 E-mail:sky37@zjnu.cn
- 无机化学学报的其它文章
- Solvothermal Synthesis,Crystal Structure and Photoluminescence Property of a Coordination Polymer Based on 1,1′-Ethynebenzene-3,3′,5,5′-tetracarboxylate
- Synthesis,Structure and Fungicidal Activity of Organotin 1H-Tetrazolyl-1-acetates
- Synthesis and Characterization of Tungsten Oxide Nanostructures
- Syntheses,Crystal Structure and Optical Property of Two Bis-ligand Silver(Ⅰ)Complexes Containing Diphenic Acid and Bidentate N-donor Ligands
- Syntheses and Structures of Two Copper(Ⅱ)Complexes with Salicyl Mono-oxime Ligands
- Graphene-RuO2Nanocomposites:Hydrothermal Synthesis and Electrochemical Capacitance Properties

