Cardioprotective potential of hydro-alcoholic fruit extract of Ananas comosus against isoproterenol induced myocardial infraction in Wistar Albino rats.
Priya Saxena, Dharamveer Panjwani
Department of Pharmacology, School of Pharmacy, Babu Banarasi Das University Lucknow, UP, India
Cardioprotective potential of hydro-alcoholic fruit extract of Ananas comosus against isoproterenol induced myocardial infraction in Wistar Albino rats.
Priya Saxena*, Dharamveer Panjwani
Department of Pharmacology, School of Pharmacy, Babu Banarasi Das University Lucknow, UP, India
Objectives: To evaluate the cardioprotective effects of hydro alcoholic extract ofAnanas comosus(A. comosus) (HEAC), on Isoproterenol (ISO) induced myocardial infarction in Albino Wistar rats.
Methods: Myocardial infarction was induced by Isoproterenol (85 mg/kg,s.c.) for two consecutive days at an interval of 24 h. Rats were pretreated with HEAC (200- 400 mg/kg/day, oral) for a period of 30 days and Isoproterenol (ISO) was injected on 31st and 32nd day and after 24 h blood was collected through retro-orbital plexus for the estimation of biochemical parameters and histopathological studies were also performed. Results: In the present study, ISO administration significantly elevated the cholesterol, low density lipoprotein, very low density lipoprotein, triglycerides, alanine aminotransferase and aspartate aminotransferase levels while it decreases high density lipoprotein and total protein in plasma and administration of HEAC decreases the level of cholesterol, low density lipoprotein, very low density lipoprotein, triglycerides, alanine aminotransferase and aspartate aminotransferase levels while it increases high density lipoprotein and total protein levels. Pretreatment with the HEAC protected the cardiotoxicity induced by Isoproterenol. The histopathological findings of the ISO-induced myocardium showed infracted zone with inflammatory cells, lipid droplets, myocardial necrosis and vacuolization of myofibrils which were reduced by the pretreatment of HEAC. Conclusion: It can be concluded that HEAC possess cardioprotective activity against Isoproterenol induced myocardial infarction in rats.
ARTICLE INFO
Article history:
Received 31 July 2014
Received in revised form 15 September 2014
Accepted 17 September 2014
Available online 20 December 2014
Ananas comosus
1. Introduction
Cardiovascular disease (CVD) remains the principal cause of death in developed and developing countries, claiming 17.1 million lives a year. According to world health organisation it is predicted that CVD will be the most important cause of mortality in India by 2020[1]. Among several cardiovascular diseases particularly myocardial infarction (MI) has become a worldwide health problem affecting all economic groups of the society and it continues to be a major public health problem, not only in western and industrialized countries but also increasingly in developing countries, such as India and makes significant contribution to the mortality statistics[2,3]. Myocardial infarction (MI) is the commonly known as ischemic heart disease or heart attack. It occurs as the acute condition of necrosis of the myocardium that results due to the imbalance between coronary blood supply and myocardial demand[4]. MI symptoms include acute coronary syndrome, chest pain, sweating, palpitations and anxiety. Some of the important risk factors of MI are smoking, hypercholesterolemia, high low density lipoprotein and low high density lipoprotein, hyperlipoproteinemia, Diabetes, elevated blood pressure, older age and Obesity. Complications of myocardial infarction include Arrhythmias, Congestive Heart Failure, Cardiogenic Shock, Ventricular Aneurysm and Pericarditis[5]. It is well recognized that there is increasing generation of reactive oxygen species such as superoxide anion and hydroxyl radicals and other reactive oxygen species (ROS) in failing myocardium tissue, bringing about oxidative damage of membrane lipids, proteins, carbohydrates and DNA. Hence, therapeutic intervention with antioxidant may be useful in preventing thesedeleterious changes[6].
The animal model of MI plays an important role in the prevention, diagnosis and therapy of human MI. Experimental induction of MI by Isoproterenol (ISO) in animals is a well-established model to study the protective role of different cardioprotective agents[7]. Isoproterenol [1-(3, 4-dihydroxyphenyl)-2-isopropylamino ethanol hydrochloride] (ISO), a synthetic catecholamine and β -adrenergic agonist, is documented to causes severe oxidative stress in the myocardium and resulting in infarctlike necrosis of the heart muscle[8].
Ananas comosusL. (A. comosus) pineapple belongs to Family Bromeliaceae (pineapple) is a tropical to subtropical fruit native to Thailand, Philippines, China, Brazil and India[9]. In Thailand,A. comosuswas used as an indigenous medicinal plant for the treatment of dysuria[10]. The cortices ofA. ComosusL. served as alexipharmic, antitussive, and antidiarrhea agents in china and other countries. Its leaves were also used as antidyspepsia or antidiarrheal agents in Chinese traditional medicine[11].A. Comosusis also known to possess anti-fertility and abortifacient activities[12], hepato-protective[13], and anti-depressant[14].A. comosusleaves enriched with phenols has antidiabetic[15] and anti-hypolipidemic[16]. Pineapple contains several pharmacologically active phytochemicals such as ananasate, beta-sitosterol, campesterol, chlorogenic acid, rutin, naringenin, Bromelain, vitamin A, B and C, glycosides and flavonoids[14].
2. Materials and methods
2.1. Plant material
The fresh fruit ofA. comosuswere collected from Lucknow (U.P.) India, during the month of July 2013 and was authenticated by NISCAIR, Delhi, (Reference letter no. NISCAIR/RHMD/Consult/2013/2312/92).
2.2. Extraction of A. comosus
The fruits ofA. comosus(Pineapple) after drying were coarsely powdered, passed through sieve and extracted with hydro alcoholic solution (70% ethanol) using maceration process[15].
2.3. Drugs and chemicals
Isoproterenol hydrochloride (Sigma Aldrich, USA), Atorvastatin (Pfizer), ethanol (s d Fine-Chem Limited, Mumbai), diethyl ether (s d Fine-Chem Limited, Mumbai), ethyl diamine tetra acetic acid EDTA (s d Fine-Chem Limited, Mumbai). All chemicals and reagents used in this study were of analytical grade.
2.4. Experimental animals
Albino Wistar rats weighing 100-180 g of either sex were used in the present study. The animals were housed in clean polypropylene cages in an air conditioned room and were kept under standard conditions of humidity (50 ±5)%, temperature (25±2)℃ and light (12 h light/12 h dark cycle). The bedding material of the cages was changed every day. They were kept on standard pallet diet and water ad libitum. They were initially acclimatized to the laboratory environment for seven days prior to their use. The experimental protocol was approved by the Institutional Animal Ethics Committee [BBDNIIT/IAEC/031/2014].
2.5. Acute toxicity studies
The acute toxicity study is aimed to establish the therapeutic indexi.e., the ratio between the pharmacologically effective dose and the lethal dose, and also to perform the primary screening. The hydro alcoholic extract ofA. comosus(HEAC) was administered once orally. Immediately after dosing, the mice were observed continuously for 4 h for symptoms of toxicity like tremors, convulsions, tonic extension, muscle spasm, loss of righting reflex, ataxia, sedation, hypnosis, lacrimation, diarrhea, salivation and writhing. Mice were then kept under observation upto 72 h for any mortality[17].
2.6 Induction of myocardial infarction
Isoproterenol (ISO) 85 mg/kg was dissolved in physiological saline solution and was injected subcutaneously to rats daily for 2 consecutive days to induce experimental myocardial infarction[6].
2.7 Experimental design
Animals after acclimatization in the laboratory were randomly divided into five groups of six animals in each group.
Group 1 Control rats received Dist.water (1 mL/kg, p.o.)
Group 2 ISO Control rats received Isoproterenol (85 mg/kg, s.c.)
Group 3 Standard group rats received Atorvastatin (10 mg/kg) Group 4 Test 1 rats received lower dose HEAC (200 mg/kg/ day)
Group 5 Test 2 rats received higher dose of HEAC (400 mg/ kg/day) for 30 days.
Isoproterenol (ISO) was injected on 31st and 32nd day and on the next day blood was collected through retro-orbital plexus under mild ether anaesthesia. Plasma was obtained by cold centrifugation of sample set at 3000 rpm for 10 min. Later, animals were sacrificed by cervical dislocation and heart tissues were excised immediately, rinsed in icechilled saline and stored at -80 ℃ till further use for the biochemical estimations and histopathological analysis.
2.8. Biochemical assays
The collected serum was used for the estimation of cardiac marker enzymes Alanine aminotransferase (ALT), Aspartate aminotransferase (AST) and serum was also estimated for the lipid profile including Cholesterol (C), Triglycerides (TG), High density lipoprotein-cholesterol (HDL-C), and total protein using commercially available standard enzymatic kits (Randox Laboratories Ltd., UK).Very low density lipoprotein-cholesterol (VLDL- C), Low density lipoproteincholesterol (LDL- C) was calculated using the following formulae[18].

2.9. Histopathological assessment of myocardial damage
After sacrifice, the cardiac apex was rapidly dissected out and washed immediately with ice-cold normal saline and fixed in 10% buffered formalin. The fixed tissues were embedded in paraffin, sectioned at 5 µm and stained with haematoxylin and eosin (H&E). The sections were examined under light microscope and photomicrographs were taken by a Zeiss Axioskop 40 photomicroscope at × 200 magnifications[6].
2.10. Statistical analysis
Results of all above estimations have been indicated in terms of means + SEM. Differences between the groups were statistically determined by analysis of variance, one way ANOVA with Tukey post-test using GraphPad Instat version 5.00, GraphPad Software. The level of significance was set atP<0.05.
3. Results
3.1. Effect of acute toxicity study of HEAC
The results indicated that the HEAC showed no change of behaviour upto 2 hrs and no. mortality was observed upto 24 hrs at the maximum dose level of 4000 mg/kg b.wt. Therefore further studies were carried out at the dose levels of 200 & 400 mg/kg b.wt. This shows no lethal or toxic effect upto 4000 mg/kg b.wt. dose.
3.2. Effect of HEAC on cholesterol and triglycerides levels
ISO induced myocardial infracted rats showed a significant increase in the levels of serum total cholesterol and triglycerides (Table 1). HEAC pretreatment significantly decreased the levels/concentrations of cholesterol and triglycerides in the serum of ISO induced myocardial infracted rats. As depicted in Figure 1 and Figure 2.
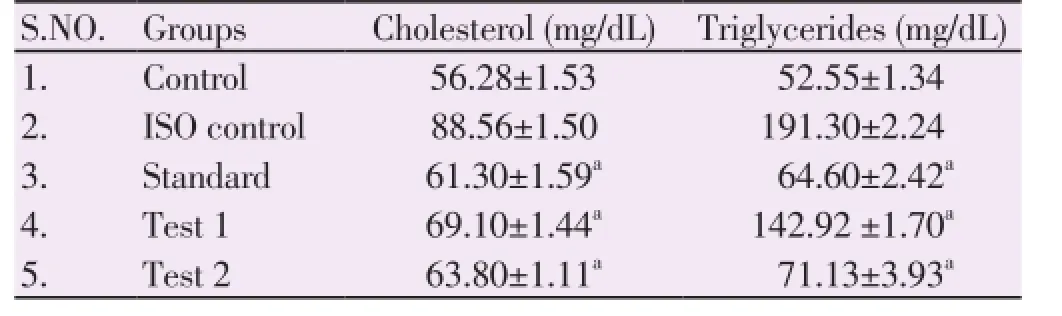
Table 1 Effect of HEAC fruit on cholesterol & triglycerides levels.
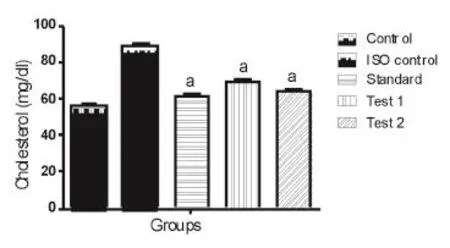
Figure 1. Effect of HEAC on cholesterol.

Figure 2. Effect of HEAC on triglycerides.

Table 2 Effect of HEAC fruit on LDL-C, VLDL-C & HDL-C levels.
3.3. Effect of HEAC on Low Density Lipoprotein-Cholesterol,Very Low Density Lipoprotein-Cholesterol and High Density Lipoprotein- Cholesterol levels
ISO induced myocardial infracted rats revealed a significant increase in the levels of serum LDL-C and VLDL-C with a significant decrease in the levels of serum HDL-C (Table 2). Pretreatment with HEAC significantly decreased the levels of serum LDL-C, VLDL-C and significantly increased the levels of serum HDL-C in ISO induced myocardial infracted rats. As depicted in Figure 3, 4 and 5.

Figure 3. Effect of HEAC on low-density lipoprotein.

Figure 4. Effect of HEAC on very low - density lipoprotein.
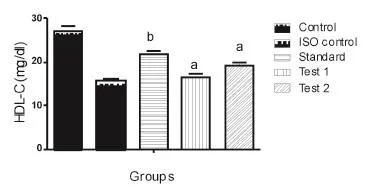
Figure 5. Effect of HEAC on high- density lipoprotein.
3.4. Effect of HEAC on Alanine aminotransferase & Aspartate aminotransferase levels
Isoproterenol induction caused significant increase in the activities of serum in cardiac marker enzymes (AST and ALT) when compared with control rats (Table 3). Oral treatment with HEAC restored all the Isoproterenol-induced alterations of serum to normal levels. As depicted in Figure 6 and 7.

Table 3 Effect of HEAC fruit on alanine aminotransferase & aspartate aminotransferase levels.

Figure 6. Effect of HEAC on Aspartate aminotransferase.

Figure 7. Effect of HEAC on Alanine aminotransferase.
3.5. Effect of HEAC on Total protein level
ISO injected rats showed a significant decrease in total protein as compared to control rats (Table 4). Administration of HEAC to ISO injected rats showed a significant increase in total protein level as compared to ISO injected rats due to presence of antioxidant. As depicted in Figure 8.
3.6. Histopathological Studies
Group 1- Control (A) heart showing normal cardiac muscle fibres without any fraying of infarction. Group 2- Negative Control (B) (ISO, 85 mg/kg) heart showing infracted zone with edema, inflammatory cells, lipid droplets, myocardialnecrosis and vacuolization of myofibrils. Atorvastatintreated (C) heart showing edema with mild inflammatory cells infiltration at the myocardium. HEAC (D) (200 mg/ kg + 85 mg/kg) treated group shows moderate edema and inflammatory cells with decreased area of coagulative necrosis of myocardial fibres. HEAC (E) (400 mg/kg + 85 mg/ kg) treated group shows mild edema but no infarction and inflammatory. As depicted in Fig. 9.

Table 4 Effect of HEAC fruit on Total protein level.

Figure 8. Effect of HEAC on total protein.
4. Discussion
Myocardial infarction (MI) is one of the main causes of death from cardiovascular disease (CVD)[19]. Myocardial infarction, a highly prevalent ischemic condition characterized by tissue necrosis develops essentially due to an imbalance between oxygen need and actual supply[20].
The present study was designed to investigate the protective effect onA. comosusIsoproterenol-induced myocardial functional and structural damage through reduction of lipid peroxidation.
Isoproterenol or Isoprenaline is a synthetic catecholamine and beta-adrenergic agonist that causes severe oxidative stress in the myocardium resulting in infarct-like necrosis of the heart muscles which leads to development of MI. ISO-induced myocardial necrosis is the most authenticated model of MI in rats[21]. On auto-oxidation, ISO generates highly cytotoxic free radicals known to stimulate peroxidation of membrane phospholipids and cause severe damage to the myocardial membrane[22]. Some of the mechanisms proposed to explain ISO-induced damage to cardiac myocytes include hypoxia due to myocardial hyperactivity and coronary hypotension, increased intracellular calcium overload, depletion of energy and excessive production highly cytotoxic free radicals as a result of catecholamine autoxidation which can cause loss of function and integrity of myocardial membranes[23].
Catecholamines are important regulators of myocardial contractility and metabolism. Catecholamines are responsible for cellular damage, observed in clinical conditions like angina, transient myocardial hypoxia, acute coronary insufficiency and subendocardial infarct. Animals develop infarct like lesions when injected with ISO, a potent catecholamine. These lesions are morphologically similar to those of ‘coagulative myocytolysis’ or myofibrillar degeneration, one of the finding described in acute myocardial infarction[18].
Administration of large amount of catecholamines, particularly Isoproterenol to experimental animals constitutes a rapid and reproducible means of provoking myocardial ischemia[24].
ISO metabolism produces quinines, which react with oxygen to produce superoxide anion and hydrogen peroxide, leading to oxidative stress thereby damaging the myocardial cells. The excessive formation of free radicals as well as lipid peroxidation has been recognized as one of the possible mechanism for myocardial damage caused by ISO. Lipid peroxidation is one of the main manifestations of oxidative damage initiated by reactive oxygen species (ROS) and has been linked to altered membrane structure and enzyme inactivation[7].
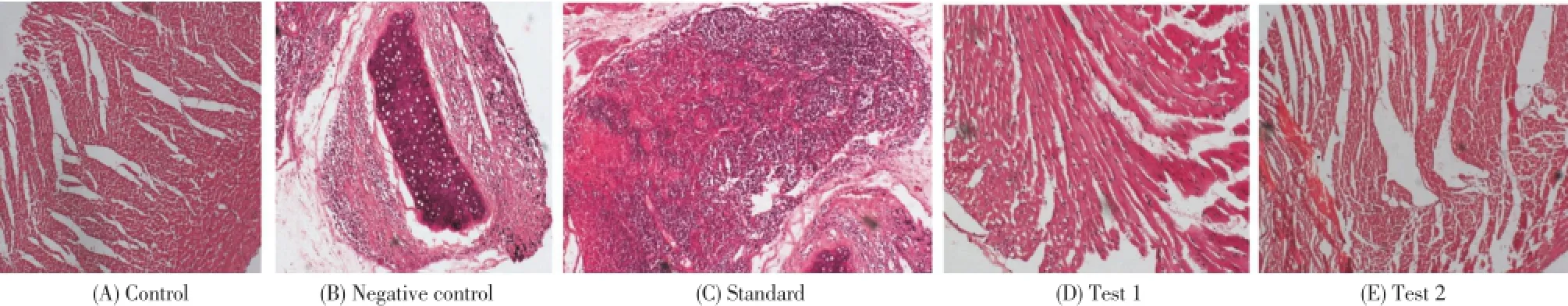
Figure 9. Effect of A. comosus on the histological morphology of rat heart shown by hematoxylin and eosin staining (×200): (A) control; (B) ISO control; (C) standard; (D) test 1; (E) test 2.
Antioxidants constitute the foremost defence system thatlimits the toxicity related to free radicals. The balance between antioxidants and free radicals is an important process for the effective removal of oxidative stress in intracellular organelles. In pathological conditions like MI, in which the generation of ROS can dramatically upset this balance with an increased demand on the antioxidant defence system[25]. Hydrophilic antioxidants (like Vitamin C) appears to be the first line antioxidant defences against reperfusion damage during the return of blood flow play an important role in protecting the integrity of cellular membranes from oxidative damage at later times. Decrease in the level of vitamin c could be due to increased utilization of vitamin C excess increased ROS [4]. Vitamin E is a lipid soluble antioxidant that protects membrane unsaturated fatty acids and other components from oxidation by free radicals[20].
Lipids play an important role in cardiovascular diseases, not only in hyperlipidemia and the development of atherosclerosis, but by modifying the composition, structure and stability of the cellular membranes[21]. Hypertriglyceridemic patients are also at a risk for cardiovascular disease often develops a lipoprotein profile characterized by elevated triglyceride, LDL, and low HDL cholesterol, which causes myocardial membrane damage[26]. Cholesterol is a major component of the atherosclerotic plaque that is associated with MI. The increased myocardial cholesterol content observed in ISO induced myocardial infarcted rats is because of increased uptake of LDL-C from the blood by myocardial membranes[2]. LDL formation occurs primarily by the catabolism of VLDL[27]. Further, elevated flux of fatty acids and impaired removal of VLDL from the plasma is the reason for the increased levels of triglycerides observed in ISO-induced myocardial infracted rats[2]. Whereas HDL inhibits the uptake of LDL from arterial wall and facilitates the transport of cholesterol from peripheral tissues to the liver, where it’s catabolised and excreted from the body[27].
Prior treatment with HEAC significantly decreased the levels of cholesterol, triglycerides, LDL and VLDL in the serum and increased the levels of HDL in ISO induced myocardial infarcted rats.
Myocardium contains plentiful concentrations of diagnostic markers of myocardial infarction and once metabolically damaged, it releases its contents into the extra cellular fluid. Enzymes, the macromolecules that leak from damaged tissues because of their tissue specificity and catalytic activity, are the best markers of tissue damage. Cytosolic enzymes which serve as the diagnostic markers like AST and ALT leak out from the damaged tissue to blood stream when cell membrane becomes permeable or rupture. The amount of these cellular enzymes in serum reflects the alterations in plasma membrane integrity and/or permeability[23]. In the present study ISO injected rats showed significant elevation in the levels of these marker enzymes in serum, which were in line with the previous reports and indication of ISO induced necrotic damage of the myocardium and leakiness of the plasma membrane and prior treatment with HEAC significantly decreased the levels of AST and ALT.
A decrease in total protein levels were observed in ISO injected rats and this could be due to increased free radical production by ISO. Administration of HEAC showed improvement in serum protein levels due to the presence of antioxidant activity as compared to ISO injected rats.
Atorvastatin is a cholesterol-lowering agent that acts by competitively inhibiting the rate limiting enzymes of cholesterol biosynthesis, 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase. Statins known prevent hypercholesterolemia, a major risk factor in the development of coronary heart disease and stroke. Pretreatment with Atorvastatin (10mg/kg) attenuated the cardiac damage caused by Isoprenaline[28].
The histopathological findings of myocardial tissue in control illustrated clear integrity of the myocardial cell membrane and no inflammatory cell infiltration was observed. The ISO-induced myocardium showed degeneration and disruption of cardiac myofibers, marked necrosis in the ventricular region, infracted zone with oedema, inflammatory cells and separation of cardiac muscle fibers. Pretreatment of HEAC (200 mg/kg) showed decreased area of infarction with coagulative necrosis and inflammatory cells with moderate oedema in myocardium. There was mild oedema but no infarction and inflammatory cells and the cardiac fibers were within the normal limits in myocardium of rats pretreated with HEAC (400 mg/kg). The protection might have been mediated throughA. comosusinduced increase in basal myocardial antioxidant enzyme activities.
In conclusion, the study reveals thatA. comosushas strong antioxidant and Bromelain activity and it can maintain cell membrane integrity and improve cardiac systolic/diastolic dysfunction induced by Isoproterenol. The findings proved to be more effective in reducing the extent of myocardial damage and significantly counteracted the oxidative stress during Isoproterenol-induced myocardial infarction in rats.
Conflict of interest statement
We declare that have no conflict of interest.
[1] Upaganlawar A, Patel V, Balaraman R. Tomato lycopene attenuates myocardial infarction induced by Isoproterenol electrocardiographic, biochemical and anti-apopitotic study.Asian Pac J Trop Biomed 2012; 2(5): 345-351.
[2] Roy JA, Prince MSP. Preventive effects of p-coumaric acid on cardiac hypertrophy and alterations in electrocardiogram, lipids, and lipoproteins in experimentally induced myocardial infarcted rats. Food Chem Toxicol 2013; 60: 348-354.
[3] Saravanan G, Ponmurugan P, Sathiyavathi M, Vadivukkarasi S, Sengottuvelu S. Cardioprotective activity of Amaranthus viridis L. effect on serum marker enzymes, cardiac troponin and antioxidant system in experimental myocardial infarcted rats. Int J Cardiol 2013; 165: 494-498.
[4] Radhiga T, Rajamanickan C, Sundaresan A, Ezhumalai M, Pugalendi VK. Effect of ursolic acid treatment on apoptosis and DNA damage in Isoproterenol-induced myocardial infarction. Biochimie 2012; 94: 1135-1142.
[5] Radhika S, Smila KH, Muthezhilan R. Cardioprotective activity of Hybanthus Enneaspermus (Linn.) on isoproterenol induced rats. Indian J Fundam Appl Life Sci, 2011; 1(3): 90-97.
[6] Wang BS, Tian S, Yang GH, Yang YX, Du HG. Cardioprotective effect of salvianolic acid A on Isoproterenol-induced myocardial infarction in rats. Eur J Pharmacol 2009; 615(1-3): 125-132.
[7] Roy JS, Prince MSP. Protective effects of sinapic acid on lysosomal dysfunction in Isoproterenol induced myocardial infarcted rats. Food Chem Toxicol, 2012; 50(11): 3984-3989.
[8] Murugesan M, Revathi R, Manju V. Cardioprotective effect of Fenugreek on Isoproterenol-induced myocardial infarctions in rats. Indian J Pharmacol 2011; 43(5): 516-519.
[9] Haripyaree A, Guneshwor K, Damayant M. Evaluation of An tioxidant Properties of Phenolics Extracted from Ananas comosus L. Not Sci Biol 2010; 2(2): 68-71.
[10] Xie W, Wang W, Su H, Xing D, Pan Y, Du L. Effect of ethanolic extracts of Ananas comosus L. leaves on insulin sensitivity in rats and HepG2. Comp Biochem Physiol 2006; 143: 429-435.
[11] Kataki SM. Antibacterial activity, in vitro antioxidant activity and anthelmintic activity of ethanolic extract of Ananas comosus L. tender leaves. Pharmacolonline 2010; 2: 308-319.
[12] Satyavati VG. Indian plants and plant products with antifertility effect. Ancient Sci Life 1984; 3(4): 193-202.
[13] Dougnon TJ, Kpodékon TM, Lalèyè A, Ahissou H, Loko F. Effect of pineapple (Ananas comosus) on haematological and biochemical parameters in albinos Wistar rats intoxicated with Doliprane. Afr J Biotechnol 2011; 10(28): 5418-5422.
[14] Parle M, Goel P. Eat pineapple a day to keep depression at bay. Int J Res Ayur Pharm 2010; 1(2): 439-448.
[15] Vuyyuru BA, Govindarao M, Reddy DSCR, Harish B, Vishwanath J, Reddy GA. Antidiabetic activity of hydro alcoholic extract of Ananas comosus L. leaves in streptozotocin induced diabetic rats. Int J Pharm 2012; 2(1): 142-147.
[16] Xie W, Wang W, Su H, Xing D, Cai G, Du L. Hypolipidemic mechanisms of Ananas comosus L. leaves in mice: different from fibrates but similar to statins. J pharmacol Sci 2007; 103(3): 267-274.
[17] Khan S, Ravikumar V, Neelima K. Pharmacological intervention of the fruit of plant Ananas comosus acting as Wound healing agent in various animal models. Int J Pharm Techno 2011; 3(1): 1807-1824.
[18] Patel V, Upaganlawar A, Zalawadia R, Balaraman R. Cardioprotective effect of melatonin against Isoproterenol induced myocardial infarction in rats: A biochemical, electrocardiographic and histoarchitectural evaluation. Eur J Pharmacol 2010; 644(1-3): 160-168.
[19] Li C, Gao Y, Tian J, Xing Y, Zhu H. Long-term oral asperosaponin VI attenuates cardiac dysfunction, myocardial fibrosis in a rat model of chronic myocardial infarction. Food Chem Toxicol 2012; 50: 1432-1438.
[20] Patel KD, Desai NS, Gandhi PH, Devkar VR, Ramachandran VA. Cardio protective effect of Coriandrum sativum L. on Isoproterenol induced myocardial necrosis in rats. Food Chem Toxicol 2012; 50: 3120-3125.
[21] Shaik H A, Rasool SN, Reedy KVA, Kareem AM, Krushna SG. Cardioprotective effect of HPLC standardized ethanolic extract of Terminalia pallida fruits against Isoproterenol- induced myocardial infarction in albino rats. J Ethnopharmacol 2012; 141: 33-40.
[22] Panda SV and Naik RS. Evaluation of cardioprotective activity of Ginkgo biloba and Ocimum sanctum in rodents. Altern Med Rev 2009; 14(2): 161- 171.
[23] Upaganlawar A, Balaraman R. Cardioprotective effects of Lagenaria siceraria fruit juice on Isoproterenol-induce myocardial infarction in wistar rats: a biomedical and histoarchitecture study. J Young Pharmacists 2011; 3(4): 297-303.
[24] Saiprasanna B, Babu MS, Ramani RY, Kumar PC, Rajeshree P. Cardioprotective effect of Pongamia pinnata hydro-alcoholic leaf extract against Isoproterenol induced myocardial infarction in wistar rats. Int J Med Pharmaceut Sci 2012; 2(3): 1-15.
[25] Priscilla HD, Prince MSP. Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem Bio Interact 2009; 179: 118-124.
[26] Thomes P, Rajendran M, Pasanban B, Rengasamy R. Cardioprotective activity of Cladosiphon okamuranus fucoidan against Isoproterenol induced myocardial infarction in rats. Phytomed 2010; 18(1): 52-57.
[27] Saravanan R, Shanmugam A, Rajkumar D. Preventive effect of glycosaminoglycans from Amussium pleuronectus (L.) on biomolecules, lactate dehydrogenase-isoenzyme and electrocardiographic pattern in Isoproterenol-induced myocardial infarction in wistar rats. Indian J Pharmacol 2012; 44(5): 602-606.
[28] Zaafan AM, Zaki FH, El-Brairy IA, Kenawy AS. Protective effects of Atorvastatin and quercetin on Isoprenaline-induced myocardial infarction in rats. Bulletin Faculty Pharm Cairo Univ 2013; 51: 35-41.
ment heading
10.1016/S2221-6189(14)60051-2
*Corresponding author: Dharamveer Panjwani, Department of Pharmacology, School of Pharmacy, Babu Banarasi Das University Lucknow, UP, India.
Tel: +91-9336010647
E-mail: dharamveerlko@gmail.com
Isoproterenol
Cardiotoxicity
Myocardial infarction
 Journal of Acute Disease2014年3期
Journal of Acute Disease2014年3期
- Journal of Acute Disease的其它文章
- Posterior reversible leukoencephalopathy syndrome presenting in a post-partum, 25-year-old-female with concomitant subarachnoid hemorrhage
- The epidemiology of tick-borne relapsing fever in Bijar County, North-Western Iran
- Patients with the tako-tsubo cardiomyopathy-clinical evaluation and outcome
- Acute brain hemorrhage in dengue
- Functional results of osteosynthesis with mini-plate and screws in metacarpal fractures
- Heart, tracheo-bronchial and thoracic spine trauma. Succesful multidisciplinary management: a challenging thoracic politrauma
