To evaluate anti-anxiety activity of thymol
Sanjay Singh Bhandari, Mahaveer Prasad Kabra
1Department of Pharmacology, M.D.U. Rohtak, Haryana, India
2Department of Pharmacology, Kota College of Phramacy, Kota, Rajasthan, India
To evaluate anti-anxiety activity of thymol
Sanjay Singh Bhandari*1, Mahaveer Prasad Kabra2
1Department of Pharmacology, M.D.U. Rohtak, Haryana, India
2Department of Pharmacology, Kota College of Phramacy, Kota, Rajasthan, India
Objective: To evaluate anti-anxiety activity of thymol (5, 10, 20 mg/kg. i.p.) in Swiss albino mice. Methods: Six group (n=5) of mice were used in this study. Drug was given to each animal intraperitonealy, behavior testing was performed in animal models after 30 min of all treatment, time spent in light area/open space was observed for 5 min duration (300 s). Significant increase in percentage of time spent in open arms of EPM and significant increase in percentage of time spent in light compartment of LDT indicate anxiolytic-like effect respectively. Significant decreased in above parameters indicates anxiogenic effect. Results: Thymol 20 mg/kg significantly increased percentage of time spent in open arms of EPM and light compartment of LDT as compared to their vehicle treated group. Conclusions: Thymol (20 mg/kg) produced significant anti-anxiety effect as compared to vehicle (0.01% ethanol) treated mice in both EPM and LDT behavioral models.
ARTICLE INFO
Article history:
Received 8 August 2013
Received in revised form 15 September 2013
Accepted 24 September 2013
Available online 20 June 2014
Anxiety
Thymol
Diazepam
Neurotransmitters
Behavioural paradigms
Stress
1. Introduction
Anxiety disorders are common mental disorders that share extreme or pathological anxiety as the primary disturbance in mood or emotional tone[1]. Common denomination of all anxiety disorders is a state of increased fear and exaggerated version of the acute stress response[2]. Literature revealed a link between anxiety and problems with the regulation of various neurotransmitters. The large numbers of neurotransmitters, peptides, hormones, and other neuromodulators have been implicated in fear and anxiety[3]. 5-HT pathway originating from the dorsal raphe nucleus (DRN) and innervating the amygdala and frontal cortex facilitates conditioned fear. Selective 5-HT reuptake inhibitors (SSRIs) and 5-HT1Aor 5-HT3receptorselective drugs can have anti-anxiety effects in certain anxiety disorders and animal models[4]. Several preclinical studies have shown that stress and anxiety cause a marked increase in NA release in several rat brain regions, including the hypothalamus, the amygdala, and the LC. γ-Aminobutyric acid (GABA) is the most abundant inhibitory neurotransmitter in the brain. The GABAA-benzodiazepine receptor is an important target for several anxiolytic drugs and may therefore play an important role in anxiety-related disorders[5]. It is well reported that Diazepam, GABAA-benzodiazepine receptor agonist at the dose of 2 mg/kg, i.p. significantly showed antianxiety activity in various behavioural paradigms of animals[6]. Several studies have shown that positive allosteric modulators (which potentiate GABA action), such as progesterone and allopregnanolone, have anxiolytic effects in various animal models[7]. Several peptides, such as cholecystokinin (CCK), neuro-peptide Y (NPY), tachykinins (substance P, neuro-kinins A and B), and natriuretic peptides (atrial natriuretic peptide or C-type natriuretic peptide) may play important roles in fear and anxiety-related behaviors[8]. There is increasing evidence that NO may underlie anxiety in the elevated plus-maze test, an animal model of anxiety. NO analogues have been found to reduce GABA-gated current via cGMP-dependent pathways, leading to anxiety[9]. cGMP downregulates GABA receptor function in hippocampus, an important area involved in anxiety[10]. The elevated plus-maze (EPM) test, one of the most popular animal models for research on anxiety, is based on the natural aversion of rodents for open spaces and uses an elevated plus-maze with two open andtwo closed arms[11,12]. This test is rapid and was found to be sensitive to the effects of both anxiolytic and anxiogenic agents. The Light/Dark exploration test (LDT) is another commonly used model for anxiety[13], devised by Crawley[14], this test is based on the innate aversion of rodents to brightly illuminated areas and on the spontaneous exploratory behaviour of rodents in response to mild stressors, i.e. novel environment and light. The LDT has been widely adopted as an anxiolytic screening test in mice[15]. Thymol (2-isopropyl-5-methylphenol) is naturally occurring phenolic monoterpene derivative of cymene, which is found in the oils of thyme and plants such asThymus vulgaris[16],Thymus glandulosus[17],Thymus hyemalis[18],Thymus zygis[19]. It exhibits multiple biological activities: antibacterial[20], antifungal[21], anti-oxidant[22], free radical scavenging[23] and anti-lipid peroxidative[24] properties. Thymol was also shown to have strong anti-inflammatory action by decreasing the release of inflammaroty metabolites like prostanoids, interleukins and leukotrienes[25,26]. Thymol acts as a GABAAreceptor agonist/modulator[27].
2. Materials and methods
2.1. Animals
Swiss albino mice of either sex (20-30 g) were employed in the present study. Animals were procured from Disease Free Small Animal House, LLRUVAS, Hisar, Haryana, India. Animals were provided normal diet and water ad libitum and were exposed to natural light and dark cycle at controlled room temperature of 20-25 ℃. The animals were acclimatized to the laboratory condition before experiments. The animals were kept fasted 2 h before drug administration, all the behavioral paradigm ware performed during day time between 9 a.m. and 2 p.m.[28]. Experimental protocol was approved by Institutional Animal Ethics Committee (IAEC). Care of the animals was taken as per guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forests, Government of India.
2.2. Drugs and chemicals
Thymol, Diazepam, Ethanol and Sodium chloride were used in this study. Thymol, was procured from Central Drug House (CDH) Ltd. India. Diazepam (Calmpose®); Ranbaxy Laboratories, Ltd., Gurgaon, India. Normal saline (0.9% NaCl) was used as vehicle for Diazepam while absolute ethanol solution (0.01%) was used as vehicle for Thymol. Volume of injection for mouse was 10 mL/kg.
2.3. Selection of doses
Doses were selected on the basis of literature,i.e., Thymol (5, 10, 20 mg/kg, i.p.), Diazepam (2 mg/kg, i.p.)[29].
2.4. Behavioral paradigms
2.4.1. Elevated plus maze
The elevated plus maze (EPM) was first proposed as an animal model of anxiety by Handley and Mithani[30]. Elevated plus maze consisted of two close arms, 16 cm×5 cm×12 cm, and two open arm, 16 cm×5 cm, connected to a central platform (5 cm×5 cm). The maze was elevated to a height of 25 cm above the floor. During the experiment each mouse was placed in the central compartment face towards either of open arm, observed for 5 min to record the time spent in open arms, the total observation of experiment was 5 min (300 s). An entry was counted when all four paws of the mouse entered an open or closed arm. Arm entry was defined as all four paws having crossed the dividing line between and arm and the central area[28]. Maze was washed thoroughly with 5% ethanol after each observation to remove odor[31]. Percent of time spent by mice in open arms was calculated as follows:

2.4.2. Light/ dark test
The light/dark test (LDT) model was first described by the Crawely and Goodwin[32]. The apparatus consisted of rectangular shaped box (45 cm×27 cm×27 cm) partition into two compartments connected by a 7.5 cm×7.5 cm opening in the wall between the compartments. An animal was placed in the centre of the light compartment face toward opening of the wall and observed for 5 min, for time spent in open (white/light) compartment. Apparatus was thoroughly washed with 5% ethanol after each observation to remove odor[31]. Percent of time spent by mice in light area was calculated as follows:

2.5. Experimental protocol
There were six group of mice used in this study. Each group consisted of five mice. Behavior testing was performed carefully in stepwise manneri.e. mice in each group were subjected to maximum two behavioral tests of anxiety, one followed by another to minimize the stress, that may arise from continuous exposure to these paradigms. Doses and routes of administration of drugs were selected according to previous studies as reported in the literature. All treatments (vehicle, 10 mL/kg; Thymol 5, 10 and 20 mg/kg; Daizepam 2 mg/kg) were administered intraperitoneally (i.p.) in a fixed volume of 1 mL/100 g body weight in separate groups of mice. Behavior testing was performedi.e.drug was given toeach animal, after 30 min behavior testing was performed in EPM and LDT.
2.6. Statistical analysis
All the results were expressed as mean±SEM. All statistical analysis was done using one way analysis of variance (ANOVA) followed by the Tukey’s post hoc test. P<0.05 was considered as significant when compared to their respective control group.
3. Results
In elevated plus maze and light/dark test, significant increase in percentage of time spent in open arms and significant increase in percentage of time spent in light compartment indicate anxiolytic-like effect respectively. On the other hand, significant decreased in above parameters indicates anxiogenic effect.
3.1. Effect of different drug treatments on mice behavior in elevated plus maze
Diazepam significantly increased percentage of time spent in open arms of elevated plus maze. Thymol 20 mg/kg significantly increased percentage of time spent in open arms as compared to vehicle (0.01% ethanol) treated mice. Therefore thymol (20 mg/kg) produced significant antianxiety effect as compared to vehicle (0.01% ethanol) treated mice (Table 1; Figure 1).
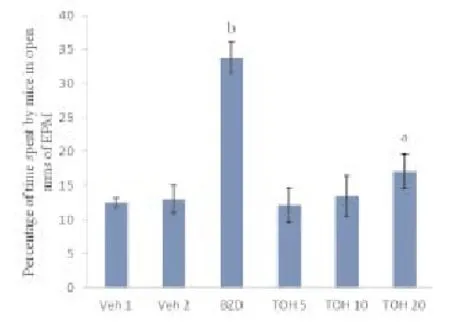
Figure 1. Values are expressed as Mean ± SEM, n=5 in each group. Data was analyzed by one-way ANOVA followed by Tukey’s Posthoc test.aP<0.05 significant difference from vehicle 1 treated group,bP<0.001 significant difference from vehicle 2 treated group.
3.2. Effect of different drug treatments on mice behavior in light/dark test
Diazepam significantly increased percentage of time spent in light compartment of light/dark test. Thymol (20 mg/kg) significantly increased percentage of time spent in light compartment of light/dark test as compared to vehicle (0.01% ethanol) treated mice. Therefore thymol (20 mg/kg) produced significant anti-anxiety effect as compared to vehicle (0.01% ethanol) treated mice (Table 2; Figure 2).

Table 1 Effect of different treatments on percentage of time spent by mice in open arms of elevated plus maze.

Table 2 Effect of different treatments on percentage of time spent by mice in light compartment of light/dark test.
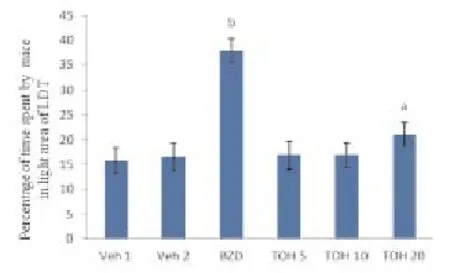
Figure 2. Values are expressed as Mean ± SEM, n=5 in each group. Data was analyzed by one-way ANOVA followed by Tukey’s Posthoc test.aP<0.05 significant difference from vehicle 1 treated group,bP<0.001 significant difference from its vehicle 2 treated group.
4. Conclusion
Elevated plus maze (EPM) and Light/dark test box (LDT) are two best models of anxiety. These both come under ethologically based animal models of fear and anxiety and involves the animal’s spontaneous or natural reactions (e.g.flight, avoidance and freezing) to stress stimuli that do not explicitly involve pain or discomfort[33]. In the present study, only thymol 20 mg/kg dose produced significant anti-anxiety like activity among its three doses. Diazepam binds to a specific subunit on the GABAAreceptor at a site distinct from the binding site of the endogenous GABA molecule, known as an allosteric site. The GABAAreceptor is an inhibitory channel which, when activated, decreases neuronal activity. Benzodiazepines cause an increased opening of the chloride ion channel when GABA binds to its site on the GABAAreceptor, leading to more chloride ions entering the neuron, which in turn leads to enhanced central nervous system depressant effects[34]. Thymol is a positive allosteric modulator of the GABAAreceptor[35]. The GABA-modulating and GABA-mimetic activities of thymol on human GABAAand fruitfly (Drosophila melanogaster Meig.) homomeric RDLac GABAAreceptors expressed in Xenopu oocytes. Thymol enhanced the GABA-dependent chloride currents in oocytes expressing various human GABAAreceptor isoforms as well as the insect GABAAreceptor[36]. It has been observed that NO donors produce 5-HT release in a biphasic way, with low concentrations of NO donors decreasing 5-HT release in the hypothalamus and high concentrations increasing it. Both effects are mediated by cGMP[37]. There is evidence suggesting the role of NO/cGMP signaling pathway in effect of NO on anxiety[38]. Inhibition of NO/cGMP signaling pathway by inhibiting of NOS has been reported to produce anti-anxiety effect[39]. Thymus vulgaris oil showed a significant decrease in aflatoxin-induced increased production of NO in liver and kidney proved its NO modulating anti-oxidant activity[40]. Thymus vulgaris extract significantly inhibit the enhanced production of NO, induced by LPS and INF-γ in a dose dependent manner[41]. Final conclusion of this study is that, the anti-anxiety like activity of thymol may through possible modulation of GABA pathway or/and NO-cGMP pathway or/and 5-HT pathway. Further study is needed to explore the precise mechanism in anxiety by the Thymol.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgement
I would like to thanks to Department of Pharmaceutical Sciences, M.D.U. Rohtak, Haryana for their financial support.
[1] Ninan PT. Recent perspectives on the diagnosis and treatment of generalized anxiety disorder. Am J Managed Care 2001; 7: S367-S376.
[2] Barbee JG. Mixed symptoms and syndromes of anxiety and depression: diagnostic, prognostic, and etiologic issues. Ann Clin Psychiatry 1998; 10: 15-29.
[3] Steimer T. The biology of fear and anxiety related behaviors. Dialogues Clin Neurosci 2002; 4: 231-249.
[4] Bagdy G. Serotonin, anxiety, and stress hormones. Focus on 5-HT receptor subtypes, species and gender differences. Ann NY Acad Sci 1998; 851: 357-363.
[5] Nutt DJ, Malizia AL. New insights into the role of the GABAA benzodiazepine receptor in psychiatric disorder. Br J Psychiatry 2001; 179: 390-396.
[6] Sharma V, Gilhotra R, Dhingra D, Gilhotra N. Possible underlying influence of p38MAPK and NF-κB in the diminished antianxiety effect of diazepam in stressed mice. J Pharmacol Sci 2011; 116: 257-263.
[7] Rupprecht R, Michele Di F, Hermann B. Neuroactive steroids: molecular mechanisms of action and implications for neuropsychopharmacology. Brain Res Rev 2001; 37: 59-67.
[8] Griebel G. Is there a future for neuropeptide receptor ligands in the treatment of anxiety disorders? Pharmacol Ther 1999; 82: 1-61.
[9] Wexler EM, Stanton PK, Nawy S. Nitric oxide depresses GABA receptor function via coactivation of cGMP-dependent kinase and phosphodiesterase. J Neurosci 1998; 18: 2342-2349.
[10] Robello M, Amico C, Bucossi G, Cupello A, Rapallino MV, Thellung S. Nitric oxide and GABA receptor function in the rat cerebral cortex and cerebellar granule cells. Neuroscience 1996; 74: 99-105.
[11] Pellow S, Chopin P, File SE, Briley M. Validation of open closed arm entries in an elevated plus maze as a measure of anxiety in the rat. J Neurosci Methods 1985; 14: 149-167.
[12] Lister RG. NG-monomethyl-L-arginine, an inhibitor of nitric oxide synthase, increases extracellular GABA in the striatum of the freely moving rat. Neuroreport 1995; 6: 1426-1428.
[13] Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol 2003; 463: 55-65.
[14] Crawley JN. Neuropharmacologic specificity of a simple animalmodel for the behavioral actions of benzodiazepines. Pharmacol Biochem Behav 1981; 20: 15695-15699.
[15] Costall B, Jones BJ, Kelly ME, Naylor RJ, Tomkins DM. Exploration of mice in a black and white test box: validation as a model of anxiety. Pharmacol Biochem Behav 1989; 2: 777-785.
[16] Lee SJ, Umano K, Shibamoto T, Lee K. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chemistry 2005; 1: 131-137.
[17] Bouchra C, Achouri M, Idrissi HLM, Hmamouchi M. Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers: Fr. J Ethnopharmacology 2003; 89: 165-169.
[18] Goodner KL, Mahattanatawee K, Plotto A, Sotomayor J, Jordan M. Aromatic profiles of Thymus hyemalis and Spanish T. vulgaris essential oils by GC-MS/GC-O. Industrial Crops Prod 2006; 24: 264-268.
[19] Moldão-Martins M, Palavra A, Beiraodacosta M, Bernardogil M. Supercritical CO2extraction of Thymus zygis L. subsp. sylvestris aroma. J Supercritical Fluids 2000; 18: 25-34.
[20] Didry N, Dubreuil L, Pinkas M. Activity of thymol, carvacrol, cinnamaldehyde and eugenol on oral bacteria. Pharm Acta Helv 1994; 69: 25-28.
[21] Mahmoud AL. Antifungal action and antiaflatoxigenic properties of some essential oil constituents. Lett Appl Microbiol 1994; 19: 110-113.
[22] Aeschbach A, Loliger J, Scott BC, Murcia A, Butler J, Halliwell B, et al. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxyl-tyrosol. Food Chem Toxicol 1994; 32: 31-36.
[23] Kruk I, Michalska T, Lichszteld K, Kladna A, Aboul-Enein HY. The effect of thymol and its derivatives on reactions generating reactive oxygen species. Chemosphere 2000; 4: 1059-1064.
[24] Alam K, Nagi MN, Badary OA, Al-Shabanah OA, Al-Rikabi AC, Al-Bekairi AM. The protective action of thymol against carbon tetrachloride hepatotoxicity in mice. Pharmacol Res 1999; 40: 159-163.
[25] Sköld K, Twetman S, Hallgren A, Yucel-Lindberg T, Modéer T. Effect of a chlorhexidine/thymol containing varnish on prostaglandin E2levels in gingival crevicular fluid. Eur J Oral Sci 1998; 6: 571-575.
[26] Yucel-Lindberg T, Twetman S, Sköld-Larsson K, Modéer T. Effect of an antibacterial dental varnish on the levels of prostanoids, leukotriene B4, and interleukin-1 beta in gingival crevicular fluid. Acta Odontol Scand 1999; 57: 23-27.
[27] Sánchez ME, Turina AV, García DA, Nolan MV, Perillo MA. Surface activity of thymol: implications for an eventual pharmacological activity. Colloids Surf B Biointerfaces 2004; 4: 77-86.
[28] Nishino T, Takeuchi T, Takechi K, Kamei C. Evaluation of anxiolytic-like effects of some short acting benzodiazepine hypnotics in mice. J Pharmacol Sci 2008; 107: 349-354.
[29] Sharma V, Gilhotra R, Dhingra D, Gilhotra N. Possible underlying influence of p38MAPK and NF-κB in the diminished antianxiety effect of diazepam in stressed mice. J Pharmacol Sci 2011; 116: 257-263.
[30] Handley SL, Mithani S. Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear’ motivated behavior. Arch Pharmacol 1984; 327: 1-5.
[31] Volke V, Soosar A, Koks S, Bourin M, Mannisto PT, Vasar E. 7-Nitroindazole, a nitric oxide synthase inhibitor, has anxiolytic-like properties in exploratory models of anxiety. Psychopharmacology (Berl) 1997; 131: 399-405.
[32] Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav 1980; 13: 167-170.
[33] Bourin M, Petit-Demoulière B, Dhonnchadha BN, Hascöet M. Animal models of anxiety in mice. Fundam Clin Pharmacol 2007; 21: 567-574.
[34] Riss J, Cloyd J, Gates J, Collins S. Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand 2008; 118: 69-86
[35] García DA, Bujons J, Vale C, Suñol C. Allosteric positive interaction of thymol with the GABAAreceptor in primary cultures of mouse cortical neurons. Neuropsychopharmacology 2006; 50: 25-35.
[36] Priestley CM, Williamson EM, Wafford KA, Sattelle DB. Thymol, a constituent of thyme essential oil, is a positive allosteric modulator of human GABAAreceptors and a homo-oligomeric GABA receptor from Drosophila melanogaster. Br J Pharmacol 2003; 140: 1363-72.
[37] Kaehler ST, Singewald N, Sinner C, Philippu A. Nitric oxide modulates the release of serotonin in the rat hypothalamus. Brain Res 1999; 835: 346 -349.
[38] Eroglu L, Cagalyan B. Anxiolytic and antidepressant properties of methylene blue in animal models. Pharmacol Res 1997; 36: 381-385.
[39] Spolidório PC, Echeverry MB, Iyomasa M, Guimarães FS, Del Bel EA. Anxiolytic effects induced by inhibition of the nitric oxide-cGMP pathway in the rat dorsal hippocampus. Psychopharmacology (Berl) 2007; 195: 183-192.
[40] El-Nekeety AA, Mohamed SR, Hathout AS, Hassan NS, Aly SE, Abdel-Wahhab MA. Antioxidant properties of Thymus vulgaris oil against aflatoxin induce oxidative stress in male rats. Toxicon 2011; 57: 984-991.
[41] Vigo E, Cepeda A, Gualillo O, Perez-Fernandez R. In-vitro antiinflammatory effect of Eucalyptus globulus and Thymus vulgaris: nitric oxideinhibition in J774A.1 murine macrophages. J Pharm Pharmacol 2004; 56: 257-263.
ment heading
10.1016/S2221-6189(14)60030-5
*Corresponding author: Sanjay Singh Bhandari, Department of Pharmacology, M.D.U. Rohtak, Haryana, India.
Tel: +919672625112
E-mail: bhandarisanjay001@gmail.com
 Journal of Acute Disease2014年2期
Journal of Acute Disease2014年2期
- Journal of Acute Disease的其它文章
- Submersion and acute respiratory failure
- A review on some poisonous plants and their medicinal values
- Laboratory diagnosis, clinical manifestations, epidemiological situation and public health importance of cutaneous leishmaniasis in Shushtar County, Southwestern Iran
- Antihyperglycemic and antihyperlipidemic properties of aqueous root extract of Icacina senegalensis in alloxan induced diabetic rats
- Neuroprotective and antioxidant role of Phoenix dactylifera in permanent bilateral common carotid occlusion in rats
- The ameliorative effects of Averroha carambola on humoral response to sheep erythrocytes in non-treated and cyclophosphamideimmunocompromised mice
