Preparation and electrochemical performance of MnO2 nanorods as electrode materials for supercapacitor
ZHANG Bowen, YU Bo, WANG Daoai, ZHOU Feng, LIU Weimin
(State Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, Gansu, China)
PreparationandelectrochemicalperformanceofMnO2nanorodsaselectrodematerialsforsupercapacitor
ZHANG Bowen, YU Bo, WANG Daoai, ZHOU Feng, LIU Weimin*
(StateKeyLaboratoryofSolidLubrication,LanzhouInstituteofChemicalPhysics,ChineseAcademyofSciences,Lanzhou730000,Gansu,China)
MnO2nanorods were successfully synthesized via simple hydrothermal method in the presence of onion carbon as the reducing agent, KMnO4as the oxidizing agent, and dilute sulphuric acid as the solvent. The phase composition, structure and morphology of as-obtained MnO2nanorods were characterized by powder X-ray diffraction and transmission electron microscopy. The electrochemical performance of MnO2nanorods as electrode materials for supercapacitor was evaluated with a CHI660B electrochemical workstation. Results show that as-obtained MnO2nanorods consist of pureα-MnO2and have a diameter of 5-10 nm and a length of 50-100 nm. As electrode materials for supercapacitor, MnO2nanorods exhibit good long-term stability and excellent charging and discharging capacities, showing potential application in supercapacitor.
MnO2; nanorods; supercapacitor; electrode material; preparation; electrochemical performance
As various fossil energy sources (oil, natural gas, coal, etc.) tend to be used up while concerns are increasingly drawn to the environmental pollution of the earth, the human society have no choices but searching clean energy sources especially cheap green energy sources as replacements. As a new type of clean energy sources, supercapacitor is extensively focused on because of its high energy power, long cycle life, low environmental pollution and high reliability, and it has been widely applied in electric cars, hybrid cars and many other facilities. Usually, a supercapacitor contains three major parts including electrode, electrolyte and membrane[1], and its electrochemical properties are mainly determined by the physical and chemical properties of the electrode material. At present, the most widely used electrode material for commercial supercapacitor is activated carbon material, but activated carbon material has a low theoretical capacity and cannot satisfy the demand for large capacity of supercapacitor. Therefore, transition metal oxides and conductive polymers as electrode materials for supercapacitor are increasingly drawing interest, since they exhibit high specific capacity[2]. MnO2, with low price and desired environmental acceptance, is a representative of the transition metal oxides that can be used as electrode material of supercapacitor[3-5]. Unfortunately, traditional hydrothermal route usually affords MnO2nanowires with a large size and involves bivalent manganese salt and seven valence manganese salt as the raw materials, and as-fabricated MnO2possesses low specific capacity and poor circulation performance owing to retarded cationic insertion[6].
In the present research, we use onion carbon as the reducing agent to prepare MnO2nanorods with a small size through a simple hydrothermal route. The electrochemical properties of the supercapacitor assembled with as-prepared MnO2nanorods as the electrode materials were tested.
1 Experimental
1.1 Materials
Onion carbon was prepared at our laboratory. Analytical grade reagents potassium permanganate and concentrated sulfuric acid were commercially obtained and used as-received.
1.2 Preparation of MnO2 nanorods
12 mg of onion carbon was put into a hydrothermal kettle containing 100 mL of deionized water and stirred ultrasonically for 20 min. Then 668 mg of anhydrous potassium permanganate and 0.2 mL of 98% sulfuric acid were added. After ultrasonic dispersion for 20 min, the mixed solution was heated for 18 h. Upon completion of the reaction, the kettle was cooled to room temperature, and the solution was centrifuged with deionized water at a speed of 2 000 rpm for 5 times, followed by drying under 80 ℃ for 2 h to afford spherical MnO2coated carbon nanocomposite.
1.3 Characterization of MnO2 nanorods
The phase and structure of MnO2nanorods were characterized by X-ray powder diffraction (XRD, Dutch Philips) and transmission electron microscopy (TEM; JEM-2010, JEOL, Japan). The electrochemical properties of MnO2nanorods as the electrode materials of supercapacitor were tested with a CHI660B-type electrochemical workstation (Huachen Instrument Co. Ltd., Shanghai, China).
1.4 The assembly of supercapacitor
The electrochemical performance of MnO2nanorods as supercapacitor electrode materials was evaluated at room temperature using the two electrode methods. As-prepared MnO2nanorods (80%, mass fraction; the same hereafter), acetylene black (15%), and polytetrafluroethylene (PTFE) powder (5%) were mixed into a paste while a small amount of water was introduced. Then the paste was coated onto a nickel foam (an area of 2.0 cm × 2.0 cm was coated) while the quantity of the active substance was kept around 10 mg. Resultant MnO2-coated nickel foam as the electrode was dried under vacuum for 4 h. Finally, the electrode was immersed into 1 mol/L lithium sulfate solution overnight. The electrochemical performance of the MnO2-coated nickel foam as the working electrode was tested with unaltered nickel foil (2.0 cm × 2.0 cm) as the counter electrode.
2 Results and discussion
2.1 Characterization of the materials
Fig. 1 shows the XRD pattern of as-prepared MnO2nanorods. It is seen that the XRD peaks of as-prepared MnO2nanorods can be well indexed to tetragonal alpha MnO2(JCPDS No. 44-0141; space group: i2/m (87), lattice constants:a= 0.995 6 nm,c= 0.286 0 nm)[7], and no peaks of other impurities are observed. This indicates that as-prepared MnO2nanorods consist of highly pure crystalline ofα-MnO2[8].

Fig.1 XRD pattern of as-obtained MnO2 nanorods
Fig.2 shows the TEM image of as-prepared MnO2nanorods. It can be seen that as-prepared MnO2nanorods are solid rods and have a diameter of about 5-10 nm and a length of 50-100 nm. Besides, as-prepared MnO2nanorods have smooth surfaces and relatively uniform size, which is consistent with the performance of tunnel type MnO2. During charging and discharging processes, this kind of tunnel type MnO2as the electrode material of supercapacitor favors cationic insertion, and its nanoscale size (referring to large surface area) is helpful to promoting electrolyte insertion and improving specific capacity[9].

Fig.2 TEM images of as-obtained MnO2 nanorods
2.2 Electrochemical performance
Fig.3 shows the charge-discharge curves of MnO2nanorods as the electrode of supercapacitor. During the first lithium ion insertion, the electrode made of MnO2nanorods undergoes the following reactions:
Where C+is electrolyte cation (lithium ion in this article), MnOα(OC)βand MnOα-β(OC)β+δrefer to high oxidation state Mn and low oxidation state Mn, respectively. The reaction in equation (1) is irreversible, which means that as-obtained electrode material exhibits some capacity attenuation. Fig. 3 shows the initial discharge-charge curve of the MnO2nanorods electrode. It can be seen that the super capacitor based on MnO2has a discharge capacity of 316 mAh/g and a charge capacity of 279 mAh/g (corresponding first cycle charging efficiency is 88%). The relatively high charging efficiency is attributed to the stable crystal structure as well as small and uniform size of the MnO2nanorods[10].
2.3 The stability analysis
We conducted repeat charging and discharging experiments to study the stability of MnO2nanorods as the electrode of supercapacitor. Fig.4 shows the variation of specific capacity of MnO2nanorods electrode with the number of charging and discharging. It can be seen that MnO2nanorods electrode gives only a slight attenuation with increasing charging and discharging cycle, and the attenuation trend gradually declines while the electrode specific capacity tends to be stable. After 100 cycles of charging and discharging, the specific capacity of MnO2is stabilized, and it is retained at a rate of more than 90%. This demonstrates that as-prepared MnO2nanorods as electrode materials of supercapacitor may find promising applications.

Fig.3 Initial discharge-charge curve of the MnO2 nanorods electrode
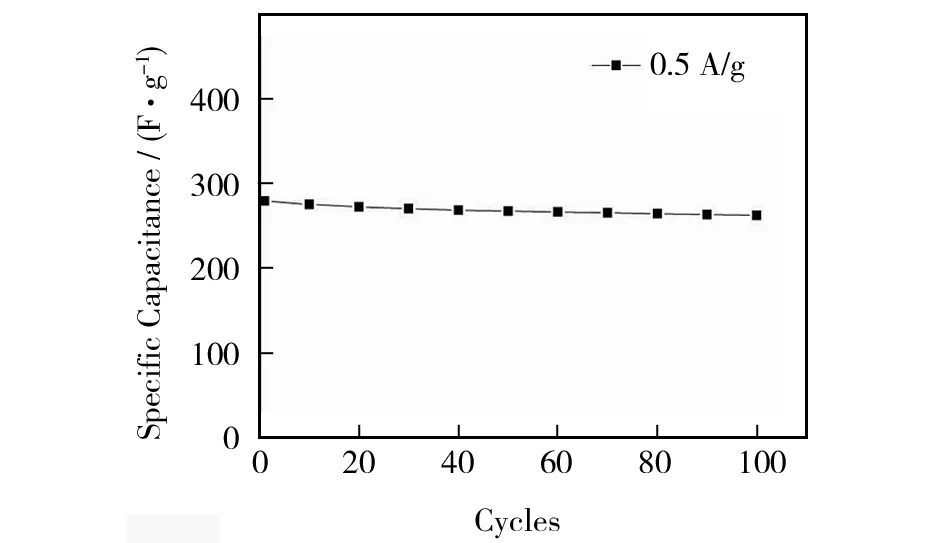
Fig.4 Charge-discharge cycle performance of MnO2 nanorods electrode
3 Conclusions
MnO2nanorods have been successfully synthesized by hydrothermal method with dilute sulphuric acid solution as the solvent, onion carbon as the reducing agent and potassium permanganate as the oxidant. The phase, structure and morphology of as-synthesized MnO2nanorods have been characterized. It has been found that as-prepared MnO2nanorods exhibit high charging and discharging capacities as well as good cycle stability, showing promising potential as the electrode materials of supercapacitor.
[1] ARICO A S, BRUCE P, SSROSATI B, et al. Nanostructured materials for advanced energy conversion and storage devices [J]. Nat Mater, 2005, 4: 366-377.
[2] SIMON P, GOGOTISI Y. Materials for electrochemical capacitors [J]. Nat Mater, 2008, 7: 845-854.
[3] SUGIMOTO W, IWATA H, YASUNAGA Y, et al. Preparation of ruthenic acid nanosheets and utilization of its interlayer surface for electrochemical energy storage [J]. Angew Chem Int Ed, 2003, 42(34): 4092-4096.
[4] LIU Haipeng, YE Tao, MAO Chengde. Fluorescent carbon nanoparticles derived from candle soot[J]. Angew Chem Int Ed, 2007, 46(34): 6473-6475.
[5] WANG Dawei, LI Feng, LIU Min, et al. 3D Aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage [J]. Angew Chem Int Ed, 2008, 47(2): 373-376.
[6] KOU Yan, XU Yanhong, GUO Zhaoqi, et al. Supercapacitive energy storage and electric power supply using an aza-fused π-conjugated microporous framework[J]. Angew Chem Int Ed, 2011, 50(37): 8753-8757.
[7] REDDY R N, REDDY R G. Chemical transformation of the electrode surface of lithium-ion battery after storing at high temperature [J]. J Power Sources, 2003, 124(1): 330-337.
[8] SUBRAMANIAN V, ZHU H W, WEI B Q. Nanostructured MnO2: hydrothermal synthesis and electrochemical properties as a supercapacitor electrode material [J]. J Power Sources, 2006, 159(1): 361-364.
[9] AMADE R, JOVER E, CAGLAR B, et al. Optimization of MnO2/vertically aligned carbon nanotube composite for supercapacitor application [J]. J Power Sources, 2011, 196(13): 5779-5783.
[10] LIU Chang, LI Feng, MA Laipeng, et al. Advanced materials for energy storage [J]. Adv Mater, 2010, 22(8): E28-62.
[责任编辑:毛立群]
超级电容器电极材料MnO2纳米棒的制备及其电化学性能
张博文,于 波,王道爱,周 峰,刘维民*
(中国科学院兰州化学物理研究所 固体润滑国家重点实验室,甘肃 兰州 730000)
以洋葱碳为还原剂,KMnO4为氧化剂,稀硫酸溶液为溶剂,采用水热法一步制备MnO2纳米棒. 利用X射线衍射仪和透射电子显微镜分析了MnO2纳米棒的物相、结构、形貌;将MnO2纳米棒作为电极材料组装了超级电容器,采用电池测试系统测定了超级电容器的电化学性能. 结果表明,所得到的产物为α-MnO2,其直径为5~10 nm,长度为50~100 nm;以MnO2纳米棒作为电极材料组装的超级电容器具有较高的比容量和稳定性,有望在超级电容器的研究和应用中得到推广.
MnO2;纳米棒;超级电容器;电极材料;制备;电化学性能
date:2014-02-20.
Supported by the Natural Science Foundation of China (21101162, 21125316) and the Ministry of Science and Technology of China (“973” project, 2013CB632300).
Biography:ZHANG Bowen (1983-), male, PhD student, majoring in material chemistry.*
, E-mail: zhaoyb902@henu.edu.cn.
O 613.5DocumentcodeAArticleID1008-1011(2014)05-0441-04
10.14002/j.hxya.2014.05.001

