microRNAs调控动物骨骼肌发育的研究进展
盛熙晖,邓桂馨,倪和民,刘云海,邢书涵,郭 勇
(北京农学院动物科技学院,北京 102206)
microRNAs调控动物骨骼肌发育的研究进展
盛熙晖,邓桂馨,倪和民,刘云海,邢书涵,郭 勇*
(北京农学院动物科技学院,北京 102206)
microRNAs(miRNAs)是一类重要的内源性非编码小分子RNA,参与调控机体生长、发育的各个环节。近年研究表明,miRNAs在动物骨骼肌发育过程中发挥着重要的调节作用。本文综述了目前已发现的调控动物骨骼肌发育的关键miRNAs。
microRNA;骨骼肌发育;动物;调控
MicroRNAs (miRNAs),是一类长度在22个核苷酸左右的内源性非编码小分子单链RNA,广泛存在于植物、线虫及人类的细胞中,具有高度的进化保守性[1]。miRNAs介导的基因转录后调控在生物体发育过程中有着重要的意义,其主要通过与靶基因3′UTR区的特异性结合,引起靶基因mRNA降解或转录后翻译受抑[2]。从第1个miRNA的发现至今,有关miRNAs的研究突飞猛进。截至目前,miRNA数据库中注册的成熟miRNAs的数量已达28 645条,分布于植物、动物、单细胞藻类、病毒等200多个物种中(数据来源于miRBase Release21.0:2014.06.at http://microRNA.sanger.ac.uk)。这些miRNAs参与调控生物体的生长、发育、分化、信号转导、疾病发生等各个方面[3-6]。其中,有研究表明miRNAs可以调控动物骨骼肌细胞的增殖与分化过程。
骨骼肌是脊椎动物身体中最丰富的组织。动物肌肉量的增加主要通过2种途径,肌纤维数目的增加和肌纤维直径或者横截面积的变大。其中,肌纤维数目的增加主要发生在胎儿期。当个体出生时,大部分动物的肌纤维数目已基本恒定,出生后则主要依靠增加原有肌纤维的直径或者横截面积使整个肌肉束变大,从而增加肌肉含量[7]。在骨骼肌的生成过程中,不同发育阶段会形成不同类型的成肌细胞(胚胎成肌细胞、胎儿成肌细胞和卫星细胞)[8],这些成肌细胞经过一系列的增殖、迁移、分化,最终形成多种类型的快、慢肌纤维[9]。该过程涉及了众多基因的表达、信号途径及网络式调控。尽管利用候选基因法、分子标记-QTL连锁分析等技术,已找到了一些与生长发育有关的基因或标记,例如,生肌调节因子家族(MRFs)[10]、肌细胞增强子因子2家族(MEF2)[11]、转化生长因子β超家族(TGF-β)[12]、成对框基因(Pax)[13]、胰岛素样生长因子(IGFs)[14]等,但仍然有相当数量的生肌调节因子和转录因子有待鉴定。有关肌肉生长的分子机制尚未彻底阐明。因此,对miRNAs调控骨骼肌细胞增殖分化的深入研究,有利于人们对动物骨骼肌生长发育机制的理解,并为动物育种工作提供理论基础。
1 miRNAs在骨骼肌中的表达模式
C2C12细胞是小鼠成肌细胞系,由D.Yaffe等于1977年建立[15]。在低血清条件下进行体外培养时,C2C12细胞可被诱导进行成肌分化,进而成熟为多核肌管,同时伴随myosin、myogenin以及其他分化基因的表达。目前,C2C12细胞作为体外模型,已广泛应用于骨骼肌的增殖与分化研究。研究表明,一些miRNAs在骨骼肌细胞的不同发育阶段呈现出不同的表达水平。例如,当C2C12细胞由增殖阶段转换到分化阶段时,分别有77和68个miRNAs发生了2倍以上的表达上调和下调[16]。其中,上调基因有miR-133b、miR-133a-1、miR-133a-2和miR-206,下调基因有miR-703、miR-122a、miR-9-2和miR-805。这表明,骨骼肌的发育同时受到miRNAs的正向和反向的共同调节。另有研究发现,在成肌细胞的分化过程中,miR-1、miR-133a、miR-133b和miR-206的表达呈现上升趋势[17]。
依据组织表达特性,调节骨骼肌发育的miRNAs可分为2类:一类miRNAs仅在骨骼肌细胞中特异表达,例如miR-1、miR-206和miR-133;而另一类miRNAs除在骨骼肌中表达外,亦可在多种组织细胞中表达。研究表明,这2类miRNAs均在骨骼肌的生长、发育以及骨骼肌疾病方面发挥着重要的调控作用。其中,以肌肉特异表达miRNAs的研究最为深入。
同时,在骨骼肌发育过程中,miRNAs的表达具有阶段特异性。一些miRNAs在个体胚胎期的表达水平高于生后期[18-19],这些miRNAs参与调控胚胎发育和组织同一性的保持[20]。另有研究人员对猪不同发育阶段的肌肉组织进行miRNAs检测,结果显示miR-133在成年猪的肌肉中高度表达,但在新生仔猪和胚胎中表达水平较低。miR-24和miR-27在肌肉卫星细胞和成年个体中高度表达,而miR-368、miR-376和miR-423-5p在初生仔猪中表达量较高[21]。
2 miRNAs调控动物骨骼肌发育的研究现状
研究表明,miRNAs在动物骨骼肌发育过程中发挥着重要的调控作用。这些miRNAs可与生肌调节因子(如MyoD和myogenin)形成反馈回路来精确调控骨骼肌发育。例如,miR-1和miR-206通过抑制Pax7的表达来促进成肌细胞的分化[22-23],同时,miRNAs的表达又受控于MyoD、myogenin、MEF2和Pax7的调节[23-25]。
miRNAs调控骨骼肌的发育过程,主要作用于3个方面:成肌细胞的分化、增殖以及骨骼肌疾病。
2.1 miRNAs调节成肌细胞的分化
miR-1是在心肌和骨骼肌中特异表达的miRNAs之一,具有高度的进化保守性,可以调控成肌细胞的分化过程。在小鼠C2C12细胞中的研究表明,miR-1以组蛋白去乙酰化酶4(HDAC4)为靶点,促进成肌细胞的分化[26]。其中,HDAC4主要通过调控MEF2(一种重要的肌肉相关转录因子)来抑制肌肉分化和骨骼肌基因表达[27]。在猪上的研究表明,miR-1以calponin 3 (CNN3)基因作为靶基因来调控猪的骨骼肌发育过程。研究同时发现,CNN3基因的单核苷酸多态性(SNPs)与猪的生长性状显著相关[28]。另有报道,在成肌细胞中,YY1基因可以抑制肌肉miRNAs(miR-1、miR-206和miR-133)的表达,同时miR-1又可以反向作用于YY1基因,由此形成了一个调控骨骼肌成肌分化的负反馈环[16]。miR-1在心的分化发育中也扮演着重要的角色。研究表明,在果蝇发育过程中,miR-1的突变可导致心和肌肉前体细胞的分化受阻[29]。在小鼠心中,miR-1的过量表达可降低心肌细胞的扩张,减少增殖细胞的数量[30]。
miR-206是miR-1家族的成员之一,与miR-1具有相同的种子序列,在脊椎动物的骨骼肌中特异表达[31]。目前的研究表明,miR-206在肌肉发生过程中发挥着重要的调控作用,并且具有多个靶基因,调控方式复杂。例如,通过对Texel绵羊的研究发现,造成该品种肌肉异常发达的原因是由于肌肉生长抑制素基因(Myostatin)3′UTR内的1个点突变。该突变产生了1个可以被miR-1和miR-206同时作用的靶位点,从而引起了miRNAs介导的Myostatin蛋白浓度的转录后降低,而造成肌肉肥大[32]。研究人员应用体内和体外的试验证明,在调控胚胎体节的成肌分化和成体骨骼肌卫星细胞的分化过程中,配对盒基因3(Pax3)和配对盒基因7(Pax7)是miR-1和miR-206的关键靶点[22-23,33]。在小鼠C2C12细胞中的多项研究表明,miR-206可分别通过下调缝隙连接蛋白43(Cx43)、DNA聚合酶α1(Pola1)和组蛋白去乙酰化酶4(HDAC4)[34]的表达,促进成肌细胞的分化。此外,研究人员应用体外模型研究发现,MyoD通过诱导增加miR-206初级转录本的表达,引起其靶基因(Fstl1和Utrn)的表达下调,进而调控成肌细胞的分化过程[25]。
此外,一些非肌肉特异性的miRNAs在成肌细胞的分化过程中亦具有调控功能(表1)。例如,miR-181在成肌细胞分化过程中显著上调,并通过下调抑制分化因子Hox-A11基因的表达调控成肌分化过程[35];miR-148a在成肌细胞分化过程中被诱导表达,并通过下调Rho相关蛋白激酶1(ROCK1)的表达来促进细胞分化[36];miR-125b可以通过靶向胰岛素样生长因子2 (IGF-II),负调控骨骼肌分化过程[37];miR-23a通过抑制快肌肌动蛋白重链(MYH)的表达抑制成肌细胞分化[38];miR-199a-3p在骨骼肌中高度表达,并可通过调控IGF-1/AKT/mTOR信号通路中的若干基因来调控C2C12成肌细胞的分化[39];miR-186同样可以抑制成肌细胞的分化,通过抑制成肌调节因子Myogenin的表达来完成[40]。
表1 调控骨骼肌细胞增殖分化的miRNAs及其靶基因
Table 1 The miRNAs and their target genes regulating myoblast proliferation and differentiation
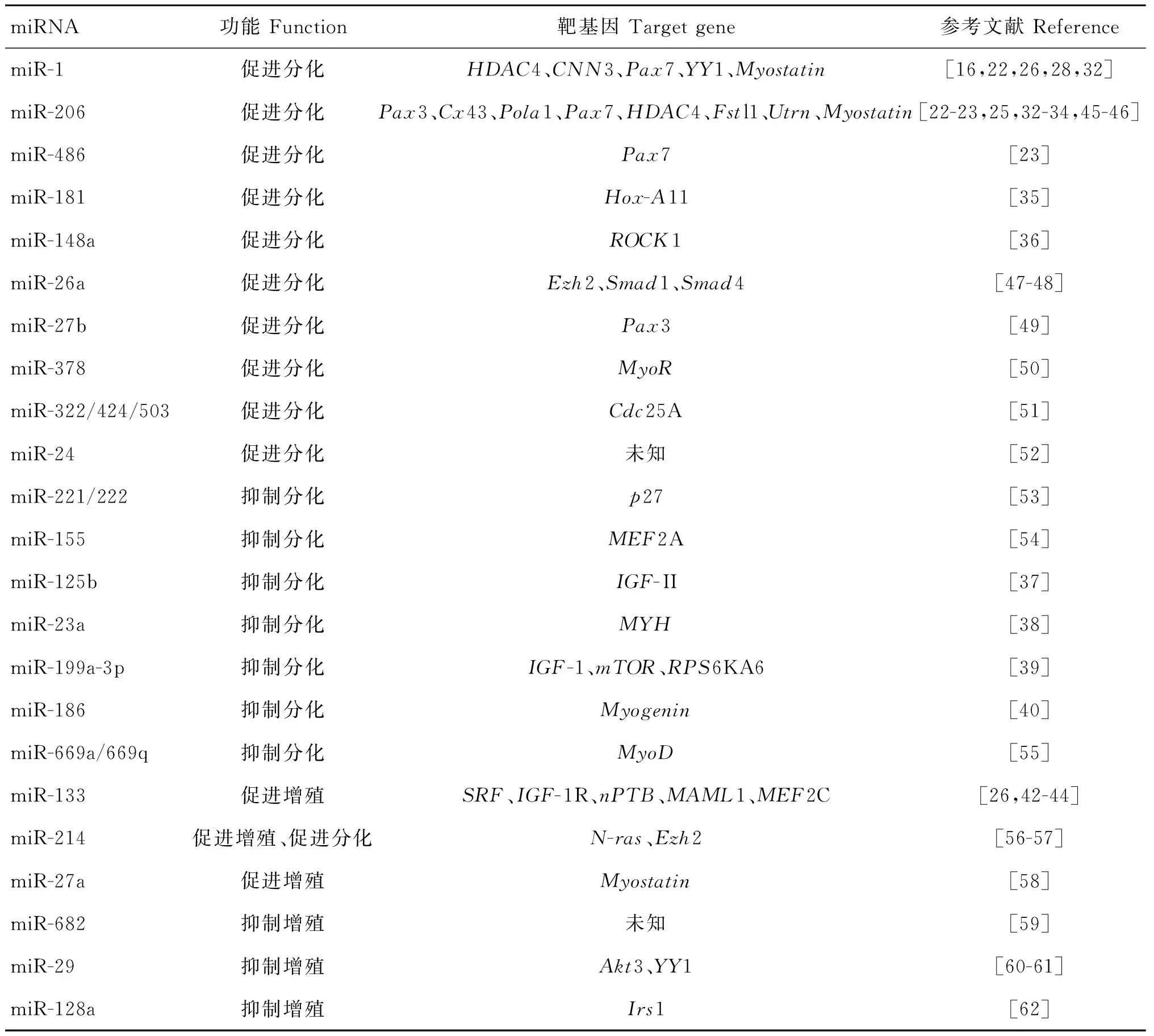
miRNA功能Function靶基因Targetgene参考文献ReferencemiR-1促进分化HDAC4、CNN3、Pax7、YY1、Myostatin[16,22,26,28,32]miR-206促进分化Pax3、Cx43、Pola1、Pax7、HDAC4、Fstl1、Utrn、Myostatin[22-23,25,32-34,45-46]miR-486促进分化Pax7[23]miR-181促进分化Hox-A11[35]miR-148a促进分化ROCK1[36]miR-26a促进分化Ezh2、Smad1、Smad4[47-48]miR-27b促进分化Pax3[49]miR-378促进分化MyoR[50]miR-322/424/503促进分化Cdc25A[51]miR-24促进分化未知[52]miR-221/222抑制分化p27[53]miR-155抑制分化MEF2A[54]miR-125b抑制分化IGF-II[37]miR-23a抑制分化MYH[38]miR-199a-3p抑制分化IGF-1、mTOR、RPS6KA6[39]miR-186抑制分化Myogenin[40]miR-669a/669q抑制分化MyoD[55]miR-133促进增殖SRF、IGF-1R、nPTB、MAML1、MEF2C[26,42-44]miR-214促进增殖、促进分化N-ras、Ezh2[56-57]miR-27a促进增殖Myostatin[58]miR-682抑制增殖未知[59]miR-29抑制增殖Akt3、YY1[60-61]miR-128a抑制增殖Irs1[62]
2.2 miRNAs调节成肌细胞的增殖
miR-133在心肌和骨骼肌中特异表达。该家族包括2个成员,miR-133a和miR-133b。miR-133b与miR-206成簇位于小鼠的1号染色体上,其转录和表达并不相同。miR-133a与miR-1成簇位于小鼠的2号和18号染色体上,并一起转录[41]。但是,miR-133 和miR-1在骨骼肌发育过程中发挥着相反的生物学功能,miR-1促进肌肉分化,而miR-133抑制肌肉分化、促进成肌细胞增殖。研究表明,miR-133通过调控SRF[26]、MAML1[42]、IGF-1R[43]与nPTB[44]的表达,促进成肌细胞的增殖。与miR-1相似,miR-133在心发育中也发挥着不可或缺的调控作用。在爪蟾中的研究表明,如果原核期时过表达miR-1,可导致心的完全缺失。而在胚胎中注射miR-133,心组织虽然可以形成,但是结构紊乱,不能形成腔室且不能环化[26]。
参与调控成肌细胞增殖过程的miRNAs中也包含了一些非肌肉特异性的miRNAs。例如,在小鼠成肌细胞中,miR-214既可以促进成肌细胞的增殖,又可以促进其分化[56];miR-27a通过抑制肌肉生成的重要抑制因子Myostatin,促进细胞的增殖[58];另有研究人员发现,miR-27a可以被亮氨酸诱导表达,并促进亮氨酸所诱导的成肌细胞的增殖[63]。同时,一些miRNAs可以抑制成肌细胞的增殖过程。例如,miR-682在生肌祖先细胞增殖过程中表达上调,并抑制其细胞增殖[59];miR-29通过转录后下调Akt3基因(丝氨酸/苏氨酸蛋白激酶家族成员之一)的表达,可以抑制小鼠成肌细胞的增殖并促进肌管形成[60];另有,在小鼠中的研究表明,miR-128a的过量表达可以抑制成肌细胞的增殖,其靶基因为Irs1 (胰岛素受体底物1),该基因是胰岛素信号通路中的重要基因之一。对小鼠进行连续4周的miR-128a表达抑制,显著诱导产生了小鼠的肌肉肥大[62]。
2.3 miRNAs与一些骨骼肌疾病密切相关
目前的研究表明,一些miRNAs与骨骼肌疾病密切相关。例如,I.Eisenberg等发现,185个miRNAs在10个人类主要的肌肉疾病中存在表达差异。其中,miR-146、miR-155、miR-221、miR-222和miR-214在全部样本中均有差异表达[64];miR-31参与调控肌肉萎缩症,研究发现抑制miR-31可望作为该疾病治疗的有效手段[65]。另有研究表明,miR-206以及其他肌肉特异的miRNAs可用来监测杜氏肌营养不良症的病理进展,作为该疾病的生物标志物[66]。miRNAs亦与骨骼肌肥大症有关。有报道指出,miR-1和miR-133a在骨骼肌肥大的小鼠肌肉中表达下调[67]。miRNA也与肌肉癌相关。研究显示,miR-29作为肿瘤抑制者,能靶向作用于YY1基因,抑制横纹肌肉瘤生长并刺激分化[68]。
3 展望
综上所述,miRNAs在动物骨骼肌发育过程中发挥着关键的调控作用,并且作用机制复杂。目前,针对miRNAs的研究主要集中于miRNAs的深度挖掘及其功能鉴定。尽管已鉴定出众多的miRNAs及其在骨骼肌中的作用靶点,但是其具体的调控机制仍需进一步研究,例如miRNAs介导的调控网络、miRNAs与重要信号通路的作用关系等。并且,目前miRNAs在骨骼肌中的功能研究主要应用的是体外细胞模型,未来人们应该采用体内研究技术,如转基因、基因敲除技术等,深入阐明miRNAs在骨骼肌发育过程中的调控机制,为今后的动物育种和疾病治疗奠定理论基础。
[1] BARTEL D P.MicroRNAs:genomics,biogenesis,mechanism,and function [J].Cell,2004,116(2):281-297.
[2] LAI E C.Micro RNAs are complementary to 3′ UTR sequence motifs that mediate negative post-transcriptional regulation [J].NatGenet,2002,30(4):363-364.[3] LEE R C,FEINBAUM R L,AMBROS V.The C.elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14 [J].Cell,1993,75(5):843-854.
[4] CHEN C Z,LI L,LODISH H F,et al.MicroRNAs modulate hematopoietic lineage differentiation [J].Science,2004,303(5654):83-86.
[5] WANG J,HUANG S K,ZHAO M,et al.Identification of a circulating microRNA signature for colorectal cancer detection [J].PLoSONE,2014,9(4):e87451.
[6] JOSSE C,BOUZNAD N,GEURTS P,et al.Identification of a microRNA landscape targeting the PI3K/Akt signaling pathway in inflammation-induced colorectal carcinogenesis [J].AmJPhysiolGastrointestLiverPhysiol,2014,306(3):G229-243.
[7] SWATLAND H J.Muscle growth in the fetal and neonatal pig [J].JAnimSci,1973,37(2):536-545.
[8] BIRESSI S,MOLINARO M,COSSU G.Cellular heterogeneity during vertebrate skeletal muscle development [J].DevBiol,2007,308(2):281-293.
[9] MILLER J B,EVERITT E A,SMITH T H,et al.Cellular and molecular diversity in skeletal muscle development:news frominvitroandinvivo[J].Bioessays,1993,15(3):191-196.
[10] RUDNICKI M A,SCHNEGELSBERG P N,STEAD R H,et al.MyoD or Myf-5 is required for the formation of skeletal muscle [J].Cell,1993,75(7):1351-1359.
[11] LUDOLPH D C,KONIECZNY S F.Transcription factor families:muscling in on the myogenic program [J].FASEBJ,1995,9(15):1595-1604.
[12] MANCEAU M,GROS J,SAVAGE K,et al.Myostatin promotes the terminal differentiation of embryonic muscle progenitors [J].GenesDev,2008,22(5):668-681.
[13] LAGHA M,KORMISH J D,ROCANCOURT D,et al.Pax3 regulation of FGF signaling affects the progression of embryonic progenitor cells into the myogenic program [J].GenesDev,2008,22(13):1828-1837.
[14] MUSARO A,MCCULLAGH K J,NAYA F J,et al.IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1 [J].Nature,1999,400(6744):581-585.
[15] YAFFE D,SAXEL O.Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle [J].Nature,1977,270(5639):725-727.
[16] LU L,ZHOU L,CHEN E Z,et al.A Novel YY1-miR-1 regulatory circuit in skeletal myogenesis revealed by genome-wide prediction of YY1-miRNA network [J].PLoSONE,2012,7(2):e27596.
[17] KOUTSOULIDOU A,MASTROYIANNOPOULOS N P,FURLING D,et al.Expression of miR-1,miR-133a,miR-133b and miR-206 increases during development of human skeletal muscle [J].BMCDevBiol,2011,11:34.
[18] HUANG T H,ZHU M J,LI X Y,et al.Discovery of porcine microRNAs and profiling from skeletal muscle tissues during development [J].PLoSONE,2008,3(9):e3225.
[19] LI T,WU R,ZHANG Y,et al.A systematic analysis of the skeletal muscle miRNA transcriptome of chicken varieties with divergent skeletal muscle growth identifies novel miRNAs and differentially expressed miRNAs [J].BMCGenomics,2011,12:186.
[20] YU Z,JIAN Z,SHEN S H,et al.Global analysis of microRNA target gene expression reveals that miRNA targets are lower expressed in mature mouse and Drosophila tissues than in the embryos [J].NucleicAcidsRes,2007,35(1):152-164.
[21] MCDANELD T G,SMITH T P,DOUMIT M E,et al.MicroRNA transcriptome profiles during swine skeletal muscle development [J].BMCGenomics,2009,10:77.
[22] CHEN J F,TAO Y,LI J,et al.microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7 [J].JCellBiol,2010,190(5):867-879.
[23] DEY B K,GAGAN J,DUTTA A.miR-206 and -486 induce myoblast differentiation by downregulating Pax7 [J].MolCellBiol,2011,31(1):203-214.
[24] RAO P K,KUMAR R M,FARKHONDEH M,et al.Myogenic factors that regulate expression of muscle-specific microRNAs [J].ProcNatlAcadSciUSA,2006,103(23):8721-8726.
[25] ROSENBERG M I,GEORGES S A,ASAWACHAICHARN A,et al.MyoD inhibits Fstl1 and Utrn expression by inducing transcription of miR-206 [J].JCellBiol,2006,175(1):77-85.
[26] CHEN J F,MANDEL E M,THOMSON J M,et al.The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation [J].NatGenet,2006,38(2):228-233.
[27] LU J,MCKINSEY T A,ZHANG C L,et al.Regulation of skeletal myogenesis by association of the MEF2 transcription factor with class II histone deacetylases [J].MolCell,2000,6(2):233-244.
[28] TANG Z,LIANG R,ZHAO S,et al.CNN3 is regulated by microRNA-1 during muscle development in pigs [J].IntJBiolSci,2014,10(4):377-385.
[29] KWON C,HAN Z,OLSON E N,et al.MicroRNA1 influences cardiac differentiation in Drosophila and regulates Notch signaling [J].ProcNatlAcadSciUSA,2005,102(52):18986-18991.
[30] ZHAO Y,SAMAL E,SRIVASTAVA D.Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis [J].Nature,2005,436(7048):214-220.
[31] BASKERVILLE S,BARTEL D P.Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes [J].RNA,2005,11(3):241-247.
[32] CLOP A,MARCQ F,TAKEDA H,et al.A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep [J].NatGenet,2006,38(7):813-818.
[33] GOLJANEK-WHYSALL K,SWEETMAN D,ABU-ELMAGD M,et al.MicroRNA regulation of the paired-box transcription factor Pax3 confers robustness to developmental timing of myogenesis [J].ProcNatlAcadSciUSA,2011,108(29):11936-11941.
[34] WINBANKS C E,WANG B,BEYER C,et al.TGF-beta regulates miR-206 and miR-29 to control myogenic differentiation through regulation of HDAC4 [J].JBiolChem,2011,286(16):13805-13814.
[35] NAGUIBNEVA I,AMEYAR-ZAZOUA M,POLESSKAYA A,et al.The microRNA miR-181 targets the homeobox protein Hox-A11 during mammalian myoblast differentiation [J].NatCellBiol,2006,8(3):278-284.
[36] ZHANG J,YING Z Z,TANG Z L,et al.MicroRNA-148a promotes myogenic differentiation by targeting the ROCK1 gene [J].JBiolChem,2012,287(25):21093-21101.
[37] GE Y,SUN Y,CHEN J.IGF-II is regulated by microRNA-125b in skeletal myogenesis [J].JCellBiol,2011,192(1):69-81.
[38] WANG L,CHEN X,ZHENG Y,et al.MiR-23a inhibits myogenic differentiation through down regulation of fast myosin heavy chain isoforms [J].ExpCellRes,2012,318(18):2324-2334.
[39] JIA L,LI Y F,WU G F,et al.MiRNA-199a-3p regulates C2C12 myoblast differentiation through IGF-1/AKT/mTOR signal pathway [J].IntJMolSci,2014,15(1):296-308.
[40] ANTONIOU A,MASTROYIANNOPOULOS N P,UNEY J B,et al.miR-186 inhibits muscle cell differentiation through myogenin regulation[J].JBiolChem,2014,289(7):3923-3935.
[41] LAGOS-QUINTANA M,RAUHUT R,YALCIN A,et al.Identification of tissue-specific microRNAs from mouse [J].CurrBiol,2002,12(9):735-739.
[42] CESANA M,CACCHIARELLI D,LEGNINI I,et al.A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA [J].Cell,2011,147(2):358-369.
[43] HUANG M B,XU H,XIE S J,et al.Insulin-like growth factor-1 receptor is regulated by microRNA-133 during skeletal myogenesis [J].PLoSONE,2011,6(12):e29173.
[44] BOUTZ P L,CHAWLA G,STOILOV P,et al.MicroRNAs regulate the expression of the alternative splicing factor nPTB during muscle development [J].GenesDev,2007,21(1):71-84.
[45] ANDERSON C,CATOE H,WERNER R.MiR-206 regulates connexin43 expression during skeletal muscle development [J].NucleicAcidsRes,2006,34(20):5863-5871.
[46] KIM H K,LEE Y S,SIVAPRASAD U,et al.Muscle-specific microRNA miR-206 promotes muscle differentiation [J].JCellBiol,2006,174(5):677-687.
[47] WONG C F,TELLAM,R L.MicroRNA-26a targets the histone methyltransferase enhancer of Zeste homolog 2 during myogenesis [J].JBiolChem,2008,283:9836-9843.
[48] DEY B K,GAGAN J,YAN Z,et al.miR-26a is required for skeletal muscle differentiation and regeneration in mice.[J].GenesDev,2012,26:2180-2191.
[49] CRIST C G,MONTARRAS D,PALLAFACCHINA G,et al.Muscle stem cell behavior is modified by microRNA-27 regulation of Pax3 expression.[J].ProcNatlAcadSci,2009,106:13383-13387.
[50] GAGAN J,DEY B K,LAYER R,et al.MicroRNA-378 targets the myogenic repressor MyoR during myoblast differentiation.[J].JBiolChem,2011,286:19431-19438.
[51] SARKAR S,DEY B K,DUTTA A.MiR-322/424 and -503 are induced during muscle differentiation and promote cell cycle quiescence and differentiation by down-regulation of Cdc25A [J].MolBiolCell,2010,21(13):2138-2149.
[52] SUN Q,ZHANG Y,YANG G,et al.Transforming growth factorbeta-regulated miR-24 promotes skeletal muscle differentiation [J].NucleicAcidsRes,2008,36:2690-2699.
[53] CARDINALI B,CASTELLANI L,FASANARO P,et al.Microrna-221 and microrna-222 modulate differentiation and maturation of skeletal muscle cells [J].PLoSONE,2009,4:e7607.
[54] SEOK H Y,TATSUGUCHI M,CALLIS T E,et al.miR-155 inhibits expression of the MEF2A protein to repress skeletal muscle differentiation [J].JBiolChem,2011,286(41):35339-35346.
[55] CRIPPA S,CASSANO M,MESSINA G,et al.miR669a and miR669q prevent skeletal muscle differentiation in postnatal cardiac progenitors.[J].JCellBiol,2011,193:1197-1212.
[56] FENG Y,CAO J H,LI X Y,et al.Inhibition of miR-214 expression represses proliferation and differentiation of C2C12 myoblasts [J].CellBiochemFunct,2011,29(5):378-383.
[57] JUAN A H,KUMAR R M,MARX J G,et al.Mir-214-dependent regulation of the polycomb protein Ezh2 in skeletal muscle and embryonic stem cells [J].MolCell,2009,36:61-74.
[58] HUANG Z,CHEN X,YU B,et al.MicroRNA-27a promotes myoblast proliferation by targeting myostatin [J].BiochemBiophysResCommun,2012,423(2):265-269.
[59] CHEN Y,GELFOND J,MCMANUS L M,et al.Temporal microRNA expression duringinvitromyogenic progenitor cell proliferation and differentiation:regulation of proliferation by miR-682 [J].PhysiolGenomics,2011,43(10):621-630.
[60] WEI W,HE H B,ZHANG W Y,et al.miR-29 targets Akt3 to reduce proliferation and facilitate differentiation of myoblasts in skeletal muscle development [J].CellDeathDis,2013,4:e668.
[61] WANG X H,HU Z,KLEIN J D,et al.Decreased miR-29 suppresses myogenesis in CKD [J].JAmSocNephrol,2011,22:2068-2076.
[62] MOTOHASHI N,ALEXANDER M S,SHIMIZU-MOTOHASHI Y,et al.Regulation of IRS1/Akt insulin signaling by microRNA-128a during myogenesis [J].JCellSci,2013,126(Pt 12):2678-2691.
[63] CHEN X,HUANG Z,CHEN D,et al.MicroRNA-27a is induced by leucine and contributes to leucine-induced proliferation promotion in C2C12 cells [J].IntJMolSci,2013,14(7):14076-14084.
[64] EISENBERG I,ERAN A,NISHINO I,et al.Distinctive patterns of microRNA expression in primary muscular disorders [J].ProcNatlAcadSciUSA,2007,104(43):17016-17021.
[65] CACCHIARELLI D,INCITTI T,MARTONE J,et al.miR-31 modulates dystrophin expression:new implications for Duchenne muscular dystrophy therapy [J].EMBORep,2011,12(2):136-141.
[66] HU J,KONG M,YE Y,et al.Serum miR-206 and other muscle-specific microRNAs as non-invasive biomarkers for Duchenne muscular dystrophy [J].JNeurochem,2014,129(5):577-883.
[67] MCCARTHY J J,ESSER K A.MicroRNA-1 and microRNA-133a expression are decreased during skeletal muscle hypertrophy [J].JApplPhysiol,2007,102(1):306-313.
[68] WANG H,GARZON R,SUN H,et al.NF-kappaB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma [J].CancerCell,2008,14(5):369-381.
(编辑 郭云雁)
Research Progress on MicroRNAs Regulating Animal Skeletal Muscle Development
SHENG Xi-hui,DENG Gui-xin,NI He-min,LIU Yun-hai,XING Shu-han,GUO Yong*
(CollegeofAnimalScienceandTechnology,BeijingUniversityofAgriculture,Beijing102206,China)
MicroRNAs (miRNAs) are small noncoding RNA molecules that play important roles in the regulation of animal growth and development.Recent studies have demonstrated that miRNAs are required in the process of animal skeletal muscle development.Here,the recent advances of the roles of miRNAs in the skeletal muscle development were reviewed.
microRNA;skeletal muscle development;animal;regulation
10.11843/j.issn.0366-6964.2015.02.001
2014-05-06
北京市自然科学基金(6132003);北京市教委科技计划项目(PXM 2014_014207_000001)
盛熙晖(1983-),女,内蒙古人,讲师,博士,主要从事动物分子育种研究,Tel:010-80795592,E-mail:shengxh03@163.com
*通信作者:郭 勇,教授,E-mail: y63guo@126.com
Q52
A
0366-6964(2015)02-0179-07

