在体骨髓原位造血细胞间纳米通道的扫描电子显微镜(SEM)证据
鲁姗姗 吴彩琴 李 华 徐 婷 葛均波 张红旗,4△
(1复旦大学基础医学院人体解剖与组织胚胎学系 上海 200032; 2上海市心血管病研究所 上海 200032;3中国科学院上海生物科学研究所生物化学与细胞生物研究所 上海 200031; 4复旦大学医学图像处理与计算机辅助手术重点实验室 上海 200032;5上海中医药大学护理学院 上海 201203)
在体骨髓原位造血细胞间纳米通道的扫描电子显微镜(SEM)证据
鲁姗姗1吴彩琴5李 华2,3徐 婷1葛均波2张红旗1,4△
(1复旦大学基础医学院人体解剖与组织胚胎学系 上海 200032;2上海市心血管病研究所 上海 200032;3中国科学院上海生物科学研究所生物化学与细胞生物研究所 上海 200031;4复旦大学医学图像处理与计算机辅助手术重点实验室 上海 200032;5上海中医药大学护理学院 上海 201203)
目的 探讨在体骨髓细胞之间是否存在细胞间纳米通道(intercellular nanotubes)。方法 利用扫描电子显微镜(scanning electron microscope,SEM)在体原位观察C57BL/6小鼠骨髓中细胞间纳米通道的分布、形态以及可能的形成机制。结果 骨髓造血细胞之间存在纳米通道结构。SEM显示该结构在骨髓组织中散在分布,位于骨髓内血窦内、外侧。骨髓造血细胞间纳米通道的管径粗细不均,平均长度为5.85 μm (1.58~18.54 μm),平均直径为364 nm (202~541 nm),还可见一些小颗粒状物质黏附在纳米通道表面。此外,小鼠骨髓造血细胞可能通过伸出突起的形式形成细胞间纳米通道。结论 本研究首次为小鼠骨髓造血细胞之间存在细胞间纳米通道提供了形态学证据。
细胞间纳米通道; 骨髓; 在体; 扫描电子显微镜(SEM)
Intercellular nanotubes,also known as tunneling nanotubes[1],have been recently described as long membranous tubes that directly interconnect among various types of cells.Different from secretion of extracellular signaling molecules followed by receptor-mediated signal transduction in target cells,and direct communication of adjacent cells via gap junctions,intercellular nanotubes are more like transient and direct intercellular communications over long distance through nanotubular structures[2].
Nanotubes were firstly described by Rustom,etal[1]in 2004 between cultured PC12 cells,followed by immune cells[3],macrophages[4],T cells[5],kidney cells[6],cardiac myoblasts[7],natural killer cells[8],neurons[9],retinal epithelial cells[10],lung cancer cells[11],and myeloid cells[12].Meanwhile,they were also found in mouse cornea[13]and zebrafish embryo[14]invivo.Based on these studies,the length of intercellular nanotubes ranged from tens of micrometers to hundreds of micrometers,which varied in different cell types.And intercellular nanotubes were mostly several hundred nanometers in width,classified as thick or thin according to the threshold of 700 nm[15].Moreover,cytoskeletons (F-actin and microtubule) were found to be the main components of nanotubes.Under the circumstance,actin-driven protrusions participated in the formation of intercellular nanotubes between two adjacent cells.To explain the mechanisms,two different hypotheses were proposed:one,a protrusion from one cell stretching out to connect to a neighboring cell; the other,a pair of connected cells moving apart and then forming nanotubes[2,16].By means of the characteristic structure,nanotubes act as highways for intercellular organelles and cytoplasmic molecules,as well as signals transport.Furthermore,it was also reported to help several pathogens to spread among cells[2,17].
To date,although the existence of long and thin intercellular nanotubes originated from various cells has been clearly demonstrated bothinvivoandinvitro,specific biological markers of intercellular nanotubes are still unknown.Therefore,morphological properties remain the main criteria for the identification of intercellular nanotubes.In the current research,scanning electron microscope (SEM) was utilized to confirm and describe the presence,location,morphological features and possible generation of intercellular nanotubes in mice bone marrowinsitu.
Materials and Methods
Animals Four male C57BL/6 mice of 8 weeks old with the weight between 15 g and 20 g (Department of Laboratory Animal Science,Fudan University),were used in accordance with the local ethical guidelines.These mice were housed at 22 ℃ under a 12 h light/12 h dark cycle,with free access to standard laboratory chow and tap water.This study was approved by the Institutional Ethics Board of Fudan University,according to the generally accepted international standards
SEM The mice were anesthetized by intraperitoneal injection of pentobarbital (50 mg/kg) (Sigma,USA).After mice skin was disinfected with 70% ethanol for 1 min,the femurs were harvested,and then the soft tissues attached to the femurs were removed.Subsequently,the femurs were cut into two halves along their longitudinal axis by microsurgical scissors in preparation for samples treatment.The specimens were handled as follows:(1) Incubation in 4% buffered glutaraldehyde for 3 h; (2) Washing 3 times in PBS (10 min each time) to remove all traces of glutaraldehyde; (3) Postfixation in 2% osmium tetroxide for 2 h; (4) Dehydration through a graded series of ethanols (50%,70%,80%,95%,100%,and 100%) for 30 min each time; and (5) Transference to a critical point dryer and sputter-coated with gold.Micrographs were taken with a Philips XL30E SEM.
Results
Intercellular nanotubes,also termed as tunneling nanotubes which presented long and thin supracellular membrane structures,were revealed between hematopoietic cells in mice bone marrowinvivo.
Distribution of intercellular nanotubes Under SEM,intercellular nanotubes were occasionally found between two different hematopoietic cells with similar shape and size in mice bone marrowinsitu.The intercellular membrane nanotubes which showed a seamless transition to the plasma membrane surface of both adjacent connected cells,were observed to be scattered among hematopoietic cells (Fig 1A).Especially,surrounding the sinusoid in bone marrow,the architecture of membrane bridges appeared frequently; on the lumen of sinusoid,the two cells connected by intercellular nanotubes were additionally observed to be located on the surface of endothelial cells of sinusoid (Fig 1B).

Fig 1 Architecture of intercellular nanotubes between hematopoietic cells in mice bone marrow in vivo
Dimension of intercellular nanotubes The different length of the intercellular nanotubes was shown in various linked hematopoietic cells (Fig 2).In the region of sinusoid where relatively rich cells with intercellular nanotubes existed,the shortest membrane bridge observed outside sinusoid was 1.58 μm in length (Fig 2A),which was far shorter than that inside sinusoid,more than 4.50 μm generally (Fig 2C).Subsequently,the average length of intercellular nanotubes identified was analyzed to be 5.85 μm (n=13,σ=4.83 μm),far shorter than the length of nanotubes observed in the previous study of cultured cells (tens of micrometers to hundreds of micrometers); and the diameter of intercellular nanotubes identified was 364 nm (n=18,σ=136 nm) in average (202~541 nm),in accordance with the criteria of thin intercellular nanotubes (below 700 nm).
Adhesive granules on the surface of intercellular nanotubes Clusters of small granules or particles adhered to the surface of intercellular nanotubes under SEM (Fig 3) which might be cell-surface proteins and/or factors.
Formation of intercellular nanotubes Membrane continuity between connected cells resulted in a direct junction consisted of two different protrusions extended from each connected cell (Fig 4A).Another possible process which intercellular nanotube could form was that a cell protruding a free-ended extension connected to an adjacent target cell to form a nanotube (Fig 4B).

Fig 2 Length of intercellular nanotubes between hematopoietic cells in mice bone marrow in vivo
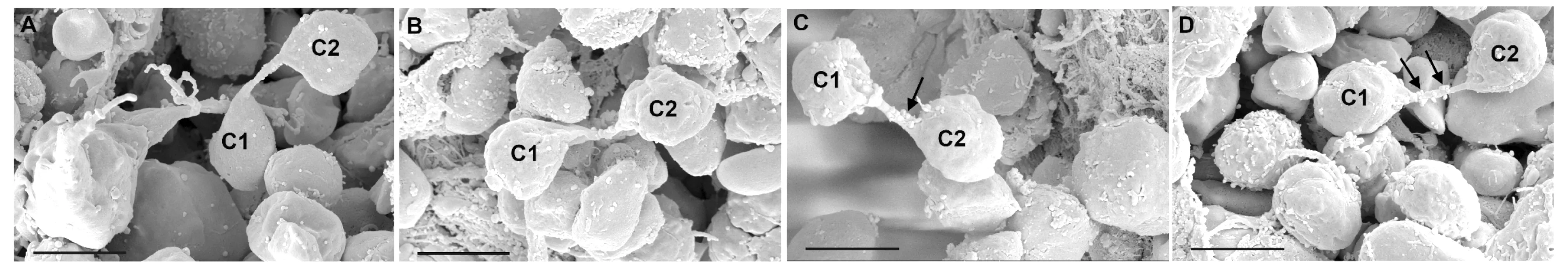
Fig 3 Adhesive substance on the surface of intercellular nanotubes in mice bone marrow in vivo
With various distribution of different connected cells, putative chemotactic granules (black arrows) were recruited and adhered on the surface of intercellular nanotubes in mice bone marrow under different physiological conditions (A&B: less; C&D: more), which might be cell-surface proteins and/or factors secrected by themselves or adjacent others to assist the migration of intercellular organelles (bar=5 μm).

Fig 4 Proposed formation of intercellular nanotubes in mice bone marrow in vivo
Discussion
The present study firstly illustrated the characteristics of intercellular nanotubes between hematopoietic cells in mice bone marrowinvivounder SEM.And the morphology of intercellular connections basically accorded with previous descriptions of intercellular nanotubes bothinvivoandinvitro.While,they had some special features of their own,including relatively short (<20 μm,5.85 μm on average) and granules on the surface of intercellular nanotubes.
Published data revealed that in the central cornea the extremely long membrane nanotubes was more common than that in the peripheral cornea,enable more isolated cells to communicate with widely spaced dendritic cells,thereby forming a potential immunological “syncytium”[13].In our study,intercellular nanotubes were occasionally found between two different hematopoietic cells in mice whole bone marrowinsitu.Notly,surrounding the sinusoid in bone marrow,the architecture of membrane bridges appeared frequently; and on the lumen of sinusoid,they were also observed adhere to the surface of endothelial cells of sinusoid.The different distribution owed mainly to the variety of spatial structures of different tissues.
The previous studies showed that the intercellular nanotubes ranged from between 50 and 200 nm in diameter in cultured neural cells to 700 nm in macrophage cell lines,and in primary cultures of human NK cells the average length of nanotubes was about 30 μm.While,140 μm between natural killer (NK) cells and macrophages[1,3-4].In the current study,the diameter of intercellular nanotubes identified was 364 nm on average (202-541 nm); and the membrane bridge observed outside sinusoid was 1.58-4.52 μm in length,far shorter than that of the above data.Additionally,cell-surface proteins and/or factors in the pattern of clusters of small granules were observed to be adhered to the surface of intercellular nanotubes.These differences were due to the various cell growth environment and cell growth state.
In recent years,researchers put forward two different mechanisms of intercellular nanotubes:dislodgement of connected cells and outgrowth of a filopodium-like protrusion[17].According to the former,as cells move apart,intercellular nanotubes could form after intercellular contact between the same type of cells,such as T cells[5]and human macrophages[4],and different types of cells,such as human NK cells[8]; moreover,intercellular bridges between the epiblast cells were discovered in the developing zebrafish embryoinvivo,formed by the daughters of a dividing cell maintaining a membrane tether as they move apart after mitosis[14].As for the latter,a protrusion from one cell was driven out to link a neighboring cell,and then formed nanotubes.This mechanism was observed between the same type of cells,such as PC12 cells[1],and different types of cells,such as immature hippocampal neurons[9].Our study provided further evidence that the manners mentioned above might occur in mice bone marrowinvivoat the same time under different physiological conditions.
In sum,the current study provided the first evidence for the presence of intercellular nanotubes in mice bone marrowinvivounder SEM,and elaborated tissue distribution,morphological characteristics and potential integration of intercellular nanotubes of their own.However,the details about the composition,ultrastructure and biological functions of intercellular nanotubes in various tissues remain to be further investigated.
[1] Rustom A,Saffrich R,Markovic I,etal.Nanotubular highways for intercellular organelle transport[J].Science,2004,303(5660):1007-1010.
[2] Gerdes HH,Rustom A,Wang X.Tunneling nanotubes,an emerging intercellular communication route in development[J].MechDev,2013,130(6-8):381-387.
[3] Onfelt B,Nedvetzki S,Yanagi K,etal.Cutting edge:Membrane nanotubes connect immune cells[J].JImmunol,2004,173(3):1511-1513.
[4] Onfelt B,Nedvetzki S,Benninger RK,etal.Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria[J].JImmunol,2006,177(12):8476-883.
[5] Sowinski S,Jolly C,Berninghausen O,etal.Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission[J].NatCellBiol,2008,10(2):211-219.
[6] Gurke S,Barroso JF,Hodneland E,etal.Tunneling nanotube (TNT)-like structures facilitate a constitutive,actomyosin-dependent exchange of endocytic organelles between normal rat kidney cells[J].ExpCellRes,2008,314(20):3669-3683.
[7] He K,Luo W,Zhang Y,etal.Intercellular transportation of quantum dots mediated by membrane nanotubes[J].ACSNano,2010,4(6):3015-3022.
[8] Chauveau A,Aucher A,Eissmann P,etal.Membrane nanotubes facilitate long-distance interactions between natural killer cells and target cells[J].ProcNatlAcadSciUSA,2010,107(12):5545-5550.
[9] Wang X,Bukoreshtliev NV,Gerdes HH.Developing neurons form transient nanotubes facilitating electrical coupling and calcium signaling with distant astrocytes[J].PLoSOne,2012,7(10):e47429.
[10] Domhan S,Ma L,Tai A,etal.Intercellular communication by exchange of cytoplasmic material via tunneling nano-tube like structures in primary human renal epithelial cells[J].PLoSOne,2011,6(6):e21283.
[11] Wang ZG,Liu SL,Tian ZQ,etal.Myosin-driven intercellular transportation of wheat germ agglutinin mediated by membrane nanotubes between human lung cancer cells[J].ACSNano,2012,6(11):10033-10041.
[12] Seyed-Razavi Y,Hickey MJ,Kuffova L,etal.Membrane nanotubes in myeloid cells in the adult mouse cornea represent a novel mode of immune cell interaction[J].ImmunolCellBiol,2013,91(1):89-95.
[13] Chinnery HR,Pearlman E,McMenamin PG.Cutting edge:Membrane nanotubes in vivo:a feature of MHC class Ⅱ+ cells in the mouse cornea[J].JImmunol,2008,180(9):5779-5783.
[14] Caneparo L,Pantazis P,Dempsey W,etal.Intercellular bridges in vertebrate gastrulation[J].PLoSOne,2011,6(5):e20230.
[15] Austefjord MW,Gerdes HH,Wang X.Tunneling nanotubes:Diversity in morphology and structure[J].CommunIntegrBiol,2014,7(1):e27934.
[16] Davis DM,Sowinski S.Membrane nanotubes:dynamic long-distance connections between animal cells[J].NatRevMolCellBiol,2008,9(6):431-436.
[17] Zhang J,Zhang Y.Membrane nanotubes:novel communication between distant cells[J].SciChinaLifeSci,2013,56(11):994-999.
Intercellular nanotube between hematopoietic cells in bone marrow:scanning electron microscope (SEM) evidenceinvivo
LU Shan-shan1, WU Cai-qin5,LI Hua2,3, XU Ting1, GE Jun-bo2, ZHANG Hong-qi1,4△
(1DepartmentofAnatomy,Histology&Embryology,SchoolofBasicMedicalSciences,FudanUniversity,Shanghai200032,China;2ShanghaiInstituteofCardiovascularDiseases,Shanghai200032,China;3InstituteofBiochemistryandCellBiology,ShanghaiInstitutesofBiologicalSciences,ChineseAcademyofSciences,Shanghai200031,China;4KeyLaboratoryofMedicalImagingComputingandComputerAssistedInterventionofShanghai,FudanUniversity,Shanghai200032,China;5SchoolofNursing,ShanghaiUniversityofTraditionalChineseMedicine,Shanghai201203,China)
Objective To investigate whether intercellular nanotubes are present in bone marrowinvivo. Methods Scanning electron microscopy (SEM) was applied to observe the distribution and morphology and proposed formed mechanism of intercellular membrane nanotubes in C57BL/6 mice bone marrowinsitu. Results The results revealed that intercellular nanotubes emerged in a pattern of scattered state in the entire bone marrow,especially inside and/or outside sinusoid.Moreover,the intercellular nanotubes with uneven diameters were shown 5.85 μm in average length (1.58-18.54 μm) and 364 nm in average width (202-541 nm),as well as clusters of small granules adhered to the surface of intercellular nanotubes.In addition,proposed mechanisms of the formation of intercellular nanotubes in mice bone marrow were elucidated that membrane continuity was formed by free-ended protrusions. Conclusions The present study for the first time captured the visual evidence for the existence of intercellular nanotubes in mice bone marrow invivo.
intercellular nanotubes; bone marrow;invivo; scanning electron microscope (SEM)
国家自然科学基金青年项目(81300232)
R 329.2+4
A
10.3969/j.issn.1672-8467.2015.02.006
2014-09-26;编辑:段佳)
△Corresponding author E-mail:zhanghq58@126.com
*This study was supported by the Young Project of the National Natural Science Foundation of China (81300232).

