城市生活污水SNAD工艺的启动研究
郑照明,李泽兵,刘常敬,陈光辉,郑林雪,马 静,李 军,赵白航 (北京工业大学,水质科学与水环境恢复工程北京市重点实验室,北京 100124)
城市生活污水SNAD工艺的启动研究
郑照明,李泽兵,刘常敬,陈光辉,郑林雪,马 静,李 军*,赵白航 (北京工业大学,水质科学与水环境恢复工程北京市重点实验室,北京 100124)
采用SBR反应器,以城市生活污水为原水,进行同步亚硝化、厌氧氨氧化、反硝化(SNAD)工艺的启动研究.首先接种厌氧氨氧化(anammox)颗粒污泥,在高曝气量下(500L/h)培养得到亚硝化颗粒污泥,然后再次接种anammox颗粒污泥,在低曝气量下(40L/h)培养得到SNAD颗粒污泥.在亚硝化稳定期,氨氮平均去除率达到94%,亚硝态氮平均积累率达到95%.在SNAD稳定期,总氮平均去除率为85%.批试实验结果表明,亚硝化稳定期亚硝化颗粒污泥的好氧氨氮和亚硝态氮氧化活性分别为为0.234和0kgN/(kgVSS·d).SNAD颗粒污泥的厌氧氨氧化总氮去除、亚硝态氮反硝化、好氧氨氮氧化、好氧亚硝态氮氧化活性分别为0.158、0.104、0.281、0kg/(kgVSS·d),其中硝态氮反硝化活性在0~120min和120~360min内分别为0.061和0.104kg/(kgVSS·d).扫描电镜显示,SNAD颗粒污泥表面以短杆状菌和球状菌为主,可能为好氧氨氧化菌(AOB)和反硝化菌,颗粒污泥内部以火山口状的细菌为主,可能为anammox菌.
厌氧氨氧化;亚硝化;反硝化;生活污水;颗粒污泥
传统生物脱氮通常采用硝化和反硝化工艺,曝气能耗高,需要额外投加有机碳源.短程硝化反硝化可以减少25%的曝气量和40%的碳源[1].厌氧氨氧化菌在厌氧条件下将NH4+-N和NO2--N转化为氮气,无需消耗有机碳源[2-3].CANON工艺可以在一个反应器中实现总氮的去除,主要应用于高氨氮废水处理中,如污泥脱水液[4-5].CANON工艺的反应式如下[6]:

高氨氮废水容易产生高浓度的FA和FNA,有助于抑制NOB的生长,实现总氮的稳定去除[7-9].最近的研究表明,CANON工艺也可以在低温低氨氮废水中实现. Vazquez-Padin等[10]在SBR反应器中,控制氨氮负荷为 0.2kgN/(m3·d),温度为15℃,启动了以颗粒污泥为主体的CANON工艺.高浓度有机物对厌氧氨氧化菌的生长有一定的抑制作用[11],但是当有机物浓度较低时,厌氧氨氧化菌仍然可以表现出较好的厌氧氨氧化性能[12-15].有研究报道亚硝化菌,厌氧氨氧化菌,反硝化菌可以在一个反应器中共存[16-17].陈慧慧等[16]采用人工配水,温度控制为35℃,在生物转盘反应器内实现了SNAD工艺.徐峥勇等[17]采用间歇曝气的方式,控制温度为30℃,在SBR反应器中启动了处理垃圾渗滤液的SNAD工艺.城市污水水量大,处理费用高,目前对以城市生活污水为进水的SNAD工艺的研究较少.由于AOB 和anammox的生长速率缓慢[3,18],采用颗粒污泥和SBR组合工艺有助于提高反应器对微生物的持流能力,从而提高系统的稳定性.本实验研究了以城市污水为原水的SNAD颗粒污泥工艺的启动性能,以期为SNAD的工程应用提供技术支持.
1 材料与方法
1.1 实验装置
试验采用SBR反应器,材质为有机玻璃,反应器为圆柱形结构,高62cm,直径38cm,总体积为90L,有效容积为70.3L;在底部设置曝气盘,采用转子流量计调节曝气量;反应器中安装搅拌器(转速200r/min)来进行混合,以加强传质效果;采用温度控制箱在线监测并控制反应器内水温;排水口设置在底部以上20cm处,排水比为67.7%;SBR试验装置如图1所示.
1.2 运行工况
实验分为两个阶段,阶段一(0~29d)为亚硝化颗粒污泥培养阶段,阶段二(30~74d)为SNAD颗粒污泥培养阶段.阶段一的运行条件为:控制温度为30℃,曝气量为500L/h,pH不控制,控制反应周期为8h,每个周期包括进水(5min),曝气(468min),沉淀(6min),排水(6min),当反应器开始进水时,反应器内曝气系统就开始工作.阶段二运行条件为:曝气量为40L/h,控制反应周期为9h,每个周期包括进水(5min),曝气(528min),沉淀(6min),排水(6min),其他条件同阶段一.

图1 SBR反应器示意Fig.1 The schematic diagram of SBR reactor
1.3 实验用水和接种污泥
1.3.1 实验用水 试验原水采用北京工业大学家属区生活污水,试验阶段主要水质指标如下: CODCr200~300mg/L; N-N 60~80mg/L;N-N<1mg/L; N-N<1mg/L; TOC 50~60mg/L; TN 100~140mg/L; pH为7.5~8.0;碱度300~400mg/L.
1.3.2 接种污泥 接种污泥取自于实验室厌氧氨氧化固定床反应器下部的厌氧氨氧化颗粒污泥[19],厌氧氨氧化固定床采用人工配水,进水N-N为40~50mg/L,N-N为50~60mg/L,其他组分参照文献[20].温度为24~26℃,HRT=3h,总氮平均去除负荷为0.5kg/(m3·d).为了培养亚硝化颗粒污泥,在第0d向SBR反应器中加入anammox颗粒污泥4L,颗粒污泥浓度为45g/L.为了使反应器快速表现出SNAD效能,在第30d,再次向SBR反应器中加入anammox颗粒污泥4L,颗粒污泥浓度为45g/L.
1.4 分析方法
NH4+-N:纳氏试剂光度法;NO2--N:N-(1-萘基)-乙二胺分光光度法;NO3--N:麝香草酚分光光度法;MLSS、MLVSS:重量法;DO、温度:WTW/Multi 3420测定仪;扫描电镜:Hitachi S-4300扫描电子显微镜;数码照片:iPhone5;CODCr:按中国国家环保局和美国环境总署发布的标准方法测定,考虑NO2--N对COD测定的影响,取COD=COD检测-8/7[NO2--N][21];采用vario TOC测定仪测定TN和TOC.
扫描电镜(SEM)样品制备:固定、冲洗、脱水、置换、干燥、粘样、镀膜.取出少量颗粒污泥,清洗2~3次后,经2.5%戊二醛固定1.5h,使用PBS清洗3遍,随后经体积分数分别为50%,70%,80%,90%和100%的乙醇进行梯度脱水,每次脱水10~15min,然后用乙酸异戊酯置换,置换后的样品于37℃干燥.干燥后,在样品表面镀上一层厚度为1500nm的金属膜,使用Hitachi S-4300型扫描电镜对样品进行观察.
FA和FNA的计算公式参照文献[7].

式中:T为温度,℃.
1.5 批式试验
1.5.1 批式试验水质 试验采用人工配水, 主要氮素成分为NH4Cl, NaNO2, KNO3, 碳源为乙酸钠, 碱度采用NaHCO3调节.各脱氮活性测定时的配水组分见表1.
1.5.2 批式试验装置和程序 批式试验采用500mL血清瓶.污泥浓度的确定:用分析天平称取20g左右湿污泥,将污泥和模拟配水一起放入有效容积为500mL血清瓶中.同时取5g左右湿污泥用滤纸包好,经烘箱和马弗炉处理,烘干时间及温度同常规污泥浓度测量条件相同,得到干物质/湿泥、挥发性物质/湿泥的比值,然后反算血清瓶中相应的MLSS,MLVSS.
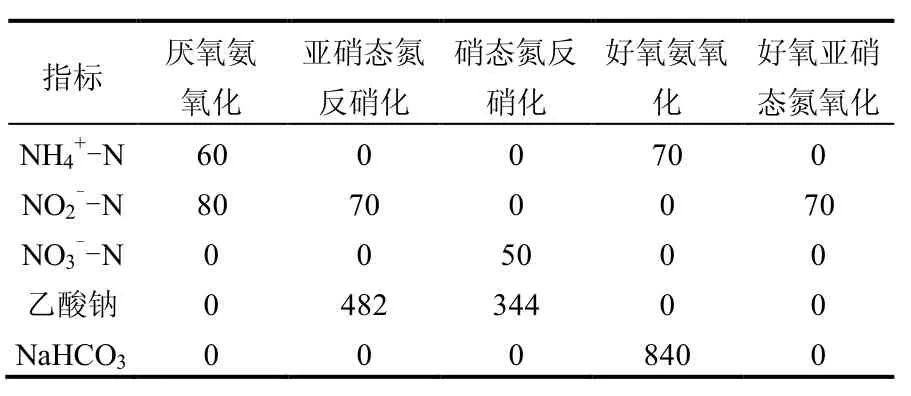
表1 脱氮活性测定时的主要配水组分(mg/L)Table 1 The synthetic wastewater used for measuring nitrogen removal performance (mg/L)
厌氧氨氧化活性的测定方法参照文献[22-23],为了保证颗粒污泥的厌氧氨氧化活性,进行如下操作:(1)配置泥水混合液;(2)启动恒温磁力搅拌器,转速为200r/min,盖紧瓶塞,通氮气30min(氮气纯度99.999%);(3)停止通氮气,将血清瓶将连同磁力搅拌器放入30 ℃的恒温培养箱中,每隔1h取样测定主要组分浓度,每次取样体积5mL.亚硝态氮和硝态氮反硝化活性测定:操作方法同厌氧氨氧化活性的测定.好氧氨氮氧化活性和亚硝态氮氧化活性测定:(1)配置泥水混合液;(2)往血清瓶中鼓入空气,曝气量控制为250mL/min(周期内DO大于6mg/L),启动恒温磁力搅拌器,转速为200r/min,将血清瓶将连同磁力搅拌器放入30℃的恒温培养箱中,每隔1h取样测定主要组分浓度,每次取样体积5mL.
2 结果与讨论
2.1 亚硝化颗粒污泥培养
2.1.1 亚硝化启动特性 培养亚硝化颗粒污泥的目的是为了在SNAD脱氮阶段中给anammox菌提供NO2--N.经过20d的驯化,成功培养出高效的亚硝化颗粒污泥.系统进出水氮素变化规律如图2所示.进水NH4+-N浓度为70~80mg/L,颗粒污泥的好氧氨氮氧化活性逐渐增强,到第20d时,出水NH4+-N降低为3mg/L,NH4+-N去除率达到93%,NO2--N积累率达到95%,表明亚硝化颗粒污泥培养成功.Blackburne等[24]的研究表明为了防止亚硝化失稳,应该将SBR的曝气时间控制在N-N降解完之前,从第21~29d,虽然出水N-N在5mg/L以下,但是亚硝化颗粒污泥表现出良好的亚硝化稳定性,N-N平均积累率为95%.系统进出水COD变化规律如图3所示.系统对COD去除能力逐渐增强. 本实验阶段进水COD为210~260mg/L,从15d起,出水COD低于50mg/L,COD去除率保持在80%以上.
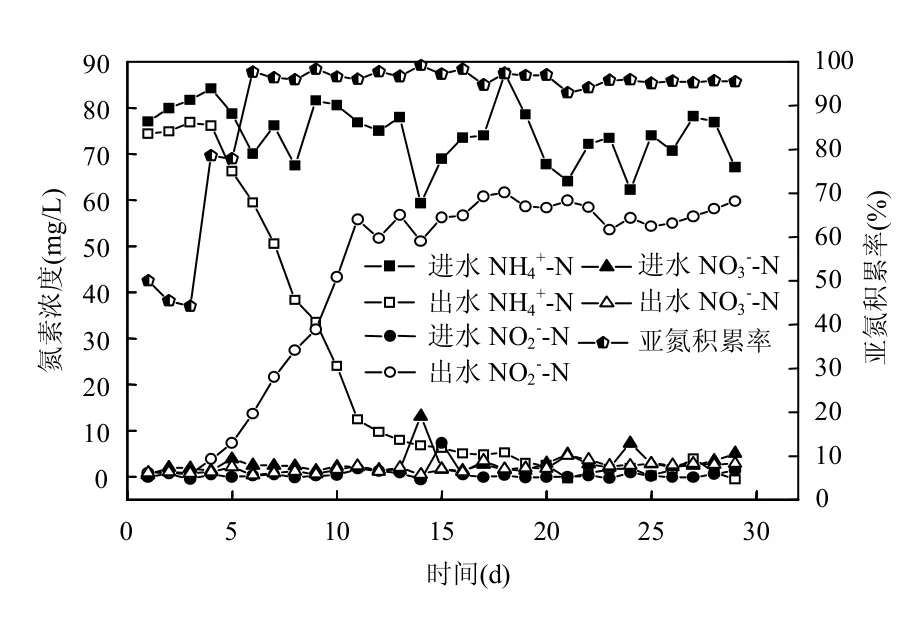
图2 亚硝化启动过程氮素去除特性Fig.2 The nitrogen removal performance of partial nitrification during start-up stage

图3 亚硝化启动过程COD去除特性Fig.3 The COD removal performance of partial nitrification during start-up stage
2.1.2 亚硝化稳定阶段沿程取样分析 亚硝化稳定阶段(第29d),SBR在一个周期内的pH值, DO和各基质浓度变化如图4所示. 0~30min,溶液中的pH值上升到7.9, DO保持在4mg/L这个平台;30~480min,溶液pH值下降到7.0, DO缓慢上升至7.2mg/L.经过480min,N-N浓度从52mg/L降低到2mg/L, N-N浓度从19.2mg/L上升到56.6mg/L,N-N浓度变化较小,保持在2mg/L左右,N-N去除率为96.1%,N-N积累率为96.6%.

图4 亚硝化稳定阶段周期内主要组分浓度变化Fig.4 The variations of main constituents of partial nitrification during stable stage in one cycle
2.1.3 亚硝化稳定阶段颗粒污泥脱氮活性 亚硝化稳定阶段(第29d)亚硝化颗粒污泥的脱氮活性结果如图5所示.亚硝化颗粒污泥的好氧氨氮氧化和亚硝态氮氧化速率分别为0.234(图5A)和0kgN/(kgVSS·d)(图5B).进一步证明本阶段培养的亚硝化颗粒污泥颗粒具有良好的亚硝化性能. 2.1.4 亚硝化颗粒污泥培养成功的关键因素 FNA和FA对NOB的联合抑制作用:FA和FNA对硝化过程有抑制作用,但是AOB对FA和FNA的耐受力要比NOB强.Anthonisen等[7]发现FA为0.1~1.0mgN/L时,NOB 的活性受到抑制,但是AOB的活性在FA为 10~150mgN/L时才受到抑制.Park等[8]对1个实际工程,2个小试污泥的研究表明FA对NOB的抑制浓度0.7mg/L,对AOB的抑制浓度4~22.4mg/L,FNA为 0.17mg N/L时, AOB的活性将被抑制50%,而FNA为0.02~0.10mgN/L时,NOB的活性将被抑制50%. Vadivelu等[9]报道FNA浓度低于0.08mg/L时,nitrosomonas的合成代谢和分解代谢仍未受到抑制,但是当FNA浓度为0.023mg/L时,nitrobacter合成代谢被完全抑制.本实验亚硝化启动后期(第29d),SBR周期内FA、FNA的变化情况如图6所示.从0到120min, FA大于1mg/L,有助于抑制NOB的活性,随着NN的氧化,FA逐渐变为零,但是随着pH值的下降和N-N浓度的积累,FNA的浓度不断增加,到420min时,FNA的浓度达到0.033mg/L,处于文献报道抑制N-N氧化的FNA浓度范围[8-9].在亚硝化启动前期,出水N-N浓度较低,周期内FA 对NOB的抑制起主要作用.因此启动初期FA对NOB的抑制,启动后期FNA和FA对NOB的联合抑制作用是本实验亚硝化启动的关键因素.韩晓宇等[25]在连续流A/O反应器中,采用FA和FNA联合抑制方式处理消化污泥脱水液,实现了稳定的短程硝化.研究表明,NOB对FA具有适应性.Villaverde等[26]研究发现经过4个月的运行,FA对NOB的临界抑制浓度从0.2mg NH3-N/ gVSS上升到0.7mg NH3-N/gVSS. Fux等[27]的研究表明,在MBBR反应器中,经过一段时间,NOB能够适应20mg/L的FA浓度.由于本实验的目的是为了培养亚硝化颗粒污泥,为后续阶段的厌氧氨氧化菌提供亚硝酸盐,所以没有对FA和FNA联合抑制NOB实现亚硝化的稳定性进行深入的研究.
颗粒污泥粒径对溶解氧传质的阻碍:大多数研究表明AOB对氧气的亲和力比NOB强,因此在DO受限时,NOB受到的影响大于AOB[28-29]. Wiesmann等[28]的研究表明AOB和NOB的氧饱和动力学常数分别为0.3和1.1mg/L.Bae等[29]研究发现DO由0.5mg/L增至2.5mg/L时,比氨氧化速率kA的增加量大于比亚硝态氮氧化速率kN的增加量.Tokutomi等[30]发现DO低于1.0mg/L时,AOB的增殖速率是NOB的2.6倍.颗粒污泥粒径对溶解氧的传质具有很大影响.Philips等[31]的研究结果表明,当溶液中的DO为3.3mg/L时,在生物膜表面以内30μm处,DO降低为0mg/L. Rathnayake等[32]的研究表明,当溶液中的DO为2mg/L时,在颗粒污泥表面以内300μm处,DO降低为0mg/L.因此当溶液中的DO很高时,在颗粒污泥内部存在很大的低DO区域,有助于抑制NOB的活性,形成以AOB为优势菌的亚硝化颗粒污泥. Vazquez-Padin等[10]采用SBR反应器,控制溶液中的DO为8mg/L,在室温为18~24℃时,成功培养出亚硝化颗粒污泥,亚硝化颗粒污泥的平均粒径为3mm, N-N积累率在95%以上.杨洋等[33]采用曝气上流式污泥床,在DO为3mg/L的条件下也成功培养出亚硝化颗粒污泥.由图4可知,本实验周期内的DO为4.0~7.2mg/L,亚硝态氮积累率大于90%,其中颗粒污泥平均粒径为2mm.

图5 亚硝化稳定阶段颗粒污泥批试脱氮特性Fig.5 The nitrogen removal performance of partial nitrification granule during stable stage in batch test
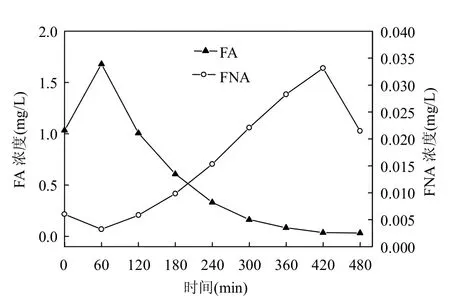
图6 亚硝化稳定阶段周期内FA和FNA浓度变化Fig.6 The evolution of FA and FNA of partial nitrification during stable stage in one cycle
2.2 SNAD颗粒污泥的培养
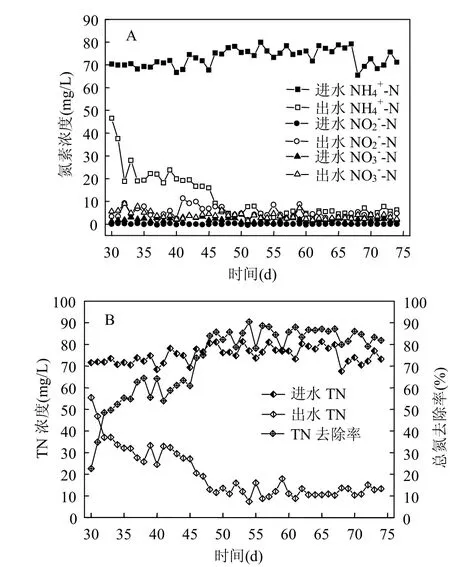
图7 SNAD启动过程氮素去除特性Fig.7 The nitrogen removal performance of SNAD during start-up stage
2.2.1 SNAD启动特性 SNAD颗粒培养阶段出水氮素浓度变化情况如图7所示.进水N-N浓度为70~80mg/L,经过18d的培养(30~48d),总氮去除率从22.6%上升到83.9%,在之后25d(49~74d),系统出水总氮平均值为10mg/L,总氮平均去除率为85%,其中出水N-N平均值为5mg/L,出水N-N和N-N浓度平均值在5mg/L以下.系统进出水COD变化规律如图8所示.进水COD为210~260mg/L,出水COD平均值为50mg/L,COD平均去除率为80%.有机物浓度对厌氧氨氧化菌和反硝化菌的共存具有重要影响.因为反硝化菌能够利用有机物将N-N还原为氮气,和anammox菌竞争N-N. Mollinue等[34]研究表明,以短程硝化废水为反应基质,当anammox反应器的进水COD为121mg/L时,anammox菌脱氮性能良好,但是当进水COD高于290mg/L时,anammox菌脱氮性能恶化.杨洋等[33]研究表明,加入20mg/L葡萄糖对anammox菌影响不大,但是加入200mg/L葡萄糖可明显抑制anammox菌.本实验进水COD浓度为210~260mg/L,系统连续25d(49~74d)脱氮效果稳定,有机物对系统的长期脱氮的影响有待于进一步研究.
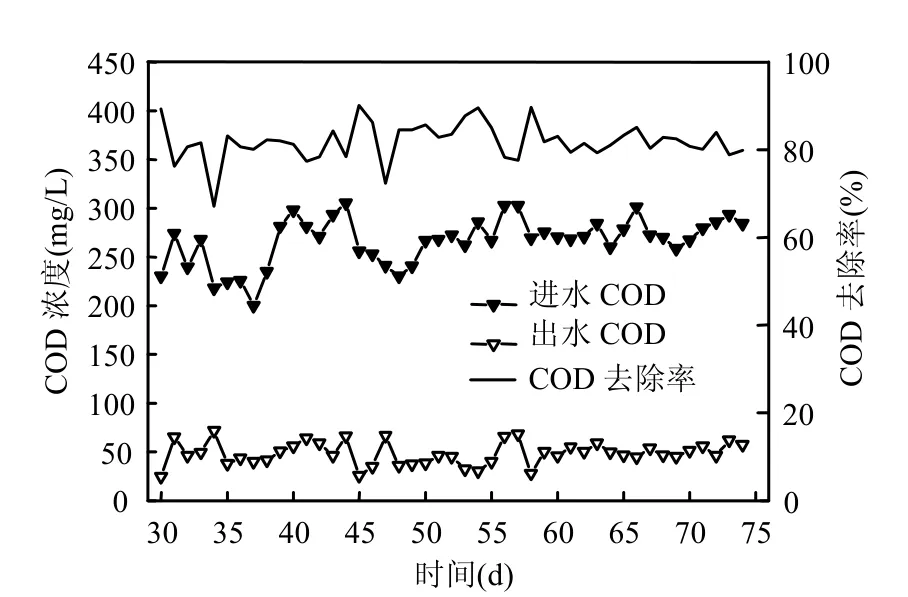
图8 SNAD启动过程COD去除特性Fig.8 The COD removal performance of SNAD during start-up stage

图9 SNAD稳定阶段周期内主要组分浓度变化Fig.9 The evolution of main constituents of SNAD during stable stage in one cycle
2.2.2 SNAD稳定阶段沿程取样分析 SNAD稳定阶段(第74d),SBR在一个周期内的pH值,DO和各基质浓度变化如图9所示.0~30min,溶液pH值上升到7.9,30~480min,溶液pH值下降到7.0. 0~60min, DO保持在0.15mg/L这个平台,60~540min, DO缓慢上升至1.4mg/L.在反应周期内,N-N浓度从55.3mg/L降低到2.6mg/L,N-N和N-N浓度变化不大,周期末N-N和N-N浓度分别为5.4mg/L和4.5mg/L,反应器表现出良好的总氮去除能力,TN浓度从72.4mg/L降低为12.6mg/L,总氮去除率达到82.6%.TOC表现为先降低后保持不变, 0~300min,TOC从45.7mg/L降低为14.5mg/L, 300min之后TOC浓度基本保持不变.
2.2.3 SNAD稳定阶段颗粒污泥脱氮活性SNAD稳定阶段(第74d)批试得到的脱氮活性如图10所示.颗粒污泥的好氧氨氮氧化和亚硝态氮氧化速率分别为0.281(图10A)和0kgN/ (kgVSS·d)(图10B).厌氧氨氧化,亚硝态氮反硝化总氮去除速率分别为0.158(图10C)和0.104kgN/(kgVSS·d)(图10D).硝态氮反硝化总氮去除速率在0~120min为0.061kgN/(kgVSS·d),在120~360min为0.104kgN/(kgVSS·d)(图10E).进一步证明本阶段培养的SNAD颗粒污泥具有良好的同步亚硝化、厌氧氨氧化、反硝化耦合脱氮的能力.

图10 SNAD稳定阶段颗粒污泥批试脱氮特性Fig.10 The nitrogen removal performance of SNAD granule during stable stage in batch test
2.2.4 SNAD颗粒污泥培养成功的关键因素颗粒污泥内部的低DO,厌氧氨氧化菌和反硝化菌对N-N的竞争是SNAD颗粒培养成功的关键因素.
颗粒污泥内部的低DO对NOB的抑制:实验控制曝气量为40L/h,图9表明在一个周期内,溶液中的DO处于较低的水平,由于颗粒污泥粒径对DO传质的阻碍,颗粒污泥内部的DO将更低,由于AOB对DO的亲和力比NOB强,因此颗粒污泥内部NOB的活性将受到抑制.
2.3 微生物形态观察

图11 亚硝化颗粒污泥照片Fig.11 The photo of partial nitrification granule
2.3.1 颗粒污泥照片 亚硝化颗粒污泥的演变 如图11所示,接种污泥为鲜红色anammox颗粒污泥(0d),粒径为2~3mm.经过一段时间的运行,anammox颗粒污泥表面逐渐被被白色絮体所包围,结合反应器的亚硝化特性和批试结果,这部分白色絮体可能为好氧氨氧化菌.图12为SNAD颗粒污泥稳定运行阶段(第74d)的照片,粒径为1~3mm,表面被灰色和白色的絮体包围,结合反应器的脱氮特性和批试结果,这部分絮体可能为好氧氨氧化菌和反硝化菌的复合体.SNAD颗粒内部为鲜红色,这些红菌应为anammox菌.

图12 SNAD颗粒污泥Fig.12 The photo of SNAD granule

图13 SNAD颗粒污泥表面和内部扫描电镜Fig.13 The SEM images of the external and the inner part of the SNAD granule
2.3.2 SNAD颗粒污泥扫描电镜图片 SNAD颗粒污泥的扫描电镜(第74d)如图13所示,在SNAD颗粒污泥的表面(图13A)主要是一些短杆状的细菌和球状菌,短杆菌大小约为0.5μm×2μm,球状菌直径为0.8~1.0μm, 结合反应器的脱氮特性和批试结果,这部分絮体可能为好氧氨氧化菌和反硝化菌的复合体,其种属特性有待于进一步验证.郭建华等[36]的研究得到的好氧氨氧化菌形态主要为短杆菌.彭安等[37]的研究表明anammox颗粒污泥表面的好氧氨氧化菌主要为球状菌. Zhong等[38]的研究表明厌氧氨氧化反硝化耦合脱氮颗粒污泥表面的反硝化菌主要为短杆状.在SNAD颗粒污泥内部(图13B)主要为火山口状细菌,直径为0.8~1.2μm,应为anammox菌,和Kartal等[39]的研究一致.
3 结论
3.1 在SBR中,接种anammox颗粒污泥,以生活污水为进水,在高曝气量(500L/h)下成功培养出亚硝化颗粒污泥,然后再次接种anammox颗粒污泥,在低曝气量下(40L/h)成功培养出SNAD颗粒污泥.批试表明亚硝化颗粒污泥的好氧氨氮氧化和亚硝态氮氧化速率分别为0.234和0kgN/ (kgVSS·d).SNAD颗粒污泥的好氧氨氮氧化和亚硝态氮氧化速率分别为0.281和0kgN/(kgVSS·d).厌氧氨氧化,亚硝态氮反硝化总氮去除速率分别为0.158和0.104kgN/(kgVSS·d).硝态氮反硝化总氮去除速率在0~120min和120~360min分别为0.061 和0.104 kgN/(kgVSS·d).
3.2 在亚硝化颗粒污泥的培养过程中,FA和FNA的联合抑制,颗粒污泥粒径对溶解氧传质的阻碍是本实验实现亚硝化的关键因素.在SNAD颗粒污泥的培养过程中,低DO,厌氧氨氧化菌和反硝化菌对NO2--N的竞争是SNAD颗粒培养成功的关键因素.
3.3 扫描电镜显示,SNAD颗粒污泥表面以短杆状菌和为主,主要是一些短杆状的细菌和球状菌,短杆菌大小约为0.5μm×2μm,球状菌直径为0.8~1.0μm,结合反应器的脱氮特性和批式试验结果,这部分絮体可能为好氧氨氧化菌和反硝化菌的复合体,其种属特性有待于进一步验证.SNAD颗粒污泥内部主要为火山口状细菌,直径为0.8~1.2μm,应为anammox菌.
[1]Fux C, Velten S, Carozzi V, et al. Efficient and stable nitritation and denitritation of ammonium-rich sludge dewatering liquor using an SBR with continuous loading [J]. Water Research,2006,40(14):2765-2775.
[2]Mulder A, Vandegraaf A A, Robertson L A, et al. Anaerobic ammonium oxidation discovered in a denitrifying fluidized-bed reactor [J]. Fems Microbiology Ecology, 1995,16(3):177-183.
[3]Strous M, Heijnen J J, Kuenen J G, et al. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms [J]. Applied Microbiology and Biotechnology, 1998,50(5):589-596.
[4]Third K A, Sliekers A O, Kuenen J G, et al. The CANON system (completely autotrophic nitrogen-removal over nitrite) under ammonium limitation: Interaction and competition between three groups of bacteria [J]. Systematic and Applied Microbiology,2001,24(4):588-596.
[5]Vazquez-Padin J R, Pozo M J, Jarpa M, et al. Treatment of anaerobic sludge digester effluents by the CANON process in an air pulsing SBR [J]. Journal of Hazardous Materials, 2009,166(1): 336-341.
[6]Sliekers A O, Derwort N, Campos-Gomez J L, et al. Completely autotrophic nitrogen removal over nitrite in one single reactor [J]. Water Research, 2002,36(10):2475-2482.
[7]Anthonisen A C, Loehr R C, Prakasam T, et al. Inhibition of nitrification by ammonia and nitrous-acid [J]. Journal Water Pollution Control Fedration, 1976, 48(5): 835-852.
[8]Park S, Bae W. Modeling kinetics of ammonium oxidation and nitrite oxidation under simultaneous inhibition by free ammonia and free nitrous acid [J]. Ppocess Biochemistry, 2009,44(6):631-640.
[9]Vadivelu V M, Keller J, Yuan Z. Free ammonia and free nitrous acid inhibition on the anabolic and catabolic processes of Nitrosomonas and Nitrobacter [J]. Water Science and Technology,2007,56(7):89-97.
[10]Vazquez-Padin J R, Figueroa M, Campos J L, et al. Nitrifying granular systems: A suitable technology to obtain stable partial nitrification at room temperature [J]. Separation and Purification Technology, 2010,74(2):178-186.
[11]Tang C J, Zheng P, Wang C H, et al. Suppression of anaerobic ammonium oxidizers under high organic content in high-rate Anammox UASB reactor [J]. Bioresource Technology, 2010,101(6):1762-1768.
[12]刘常敬,李泽兵,郑照明,等.苯酚对厌氧氨氧化工艺耦合反硝化的启动及脱氮性能的影响 [J]. 中国环境科学, 2014,34(5): 1145-1151.
[13]林 琳,李玉平,曹宏斌,等.焦化废水厌氧氨氧化生物脱氮的研究 [J]. 中国环境科学, 2010,30(9):1201-1206.
[14]陈婷婷,唐崇俭,郑 平.制药废水厌氧氨氧化脱氮性能与毒性机理的研究 [J]. 中国环境科学, 2010,30(4):504-509.
[15]刘常敬,李泽兵,郑照明,等.不同有机物对厌氧氨氧化耦合反硝化的影响 [J]. 中国环境科学, 2015,35(1):87-94.
[16]Chen H H, Liu S T, Yang F L, et al. The development of simultaneous partial nitrification,anammox and denitrification (SNAD) process in a single reactor for nitrogen removal [J]. Bioresource Technology, 2009,100(4):1548-1554.
[17]Xu Z Y, Zeng G M, Yang Z H, et al. Biological treatment of landfill leachate with the integration of partial nitrification,anaerobic ammonium oxidation and heterotrophic denitrification [J]. Bioresource Technology, 2010,101(1):79-86.
[18]Blackburne R, Yuan Z G, Keller J. Partial nitrification to nitrite using low dissolved oxygen concentration as the main selection factor [J]. Biodegradation, 2008,19(2):303-312.
[19]李泽兵,刘常敬,赵白航,等.多基质时厌氧氨氧化菌、异养反硝化污泥活性及抑制特征 [J]. 中国环境科学, 2013,33(4):648-654.
[20]van de Graaf AA, Debruijn P, Robertson L A, et al. Autotrophic growth of anaerobic ammonium-oxidizing micro-organisms in a fluidized bed reactor [J]. Microbiology, 1996,142(8):2187-2196.
[21]曹相生,付昆明,钱 栋,等.甲醇为碳源时C/N对反硝化过程中亚硝酸盐积累的影响 [J]. 化工学报, 2010,(11):2938-2943.
[22]汪彩华,郑 平,唐崇俭,等.间歇性饥饿对厌氧氨氧化菌混培物保藏特性的影响 [J]. 环境科学学报, 2013,33(1):36-43.
[23]Tang C J, Zheng P, Mahmood Q, et al. Start-up and inhibition analysis of the Anammox process seeded with anaerobic granular sludge [J]. Journal of Industrial Microbiology and Biotechnology,2009,36(8):1093-1100.
[24]Blackburne R, Yuan Z Q, Keller J. Demonstration of nitrogen removal via nitrite in a sequencing batch reactor treating domestic wastewater [J]. Water Research, 2008,42(8/9):2166-2176.
[25]韩晓宇,张树军,甘一萍,等.以FA与FNA为控制因子的短程硝化启动与维持 [J]. 环境科学, 2009,30(3):809-814.
[26]Villaverde S, Fdz-Polanco F, Garcia P A. Nitrifying biofilm acclimation to free ammonia in submerged biofilters. Start-up influence [J]. Water Research, 2000,34(2):602-610.
[27]Fux C, Huang D, Monti A, et al. Difficulties in maintaining long-term partial nitritation of ammonium-rich sludge digester liquids in a moving-bed biofilm reactor (MBBR) [J]. Water Science and Technology, 2004,49(11/12):53-60.
[28]Wiesmann U. Biological nitrogen removal from wastewater [J]. Advances in Biochemical Engineering Biotechnology, 1994,51,113-154.
[29]Bae W, Baek S, Chung J, et al. Optimal operational factors for nitrite accumulation in batch reactors [J]. Biodegradation,2001,12(5):359-366.
[30]Tokutomi T. Operation of a nitrite-type airlift reactor at low DO concentration [J]. Water Science and Technology, 2004,49(5/6): 81-88.
[31]Philips S, Laanbroek H J, Verstraete W. Origin, causes and effects of increased nitrite concentrations in aquatic environments [J]. Environ. Sci. Biotechnol, 2002,1(2):115-141.
[32]Rathnayake R M L D, Song Y, Tumendelger A, et al. Source identification of nitrous oxide on autotrophic partial nitrification in a granular sludge reactor [J]. Water Research, 2013,47(19): 7078-7086.
[33]杨 洋,左剑恶,沈 平,等.温度、pH值和有机物对厌氧氨氧化污泥活性的影响 [J]. 环境科学, 2006,27(4):691-695.
[34]Molinuevo B, Garcia M C, Karakashev D, et al. Anammox for ammonia removal from pig manure effluents: Effect of organic matter content on process performance [J]. Bioresource Technology, 2009,100(7):2171-2175.
[35]van der Star W, Miclea A I, van Dongen U, et al. The membrane bioreactor: A novel tool to grow anammox bacteria as free cells [J]. Biotechnology and Bioengineering, 2008,101(2):286-294.
[36]Guo J, Peng Y Z, Wang S Y, et al. Long-term effect of dissolved oxygen on partial nitrification performance and microbial community structure [J]. Bioresource Technology, 2009,100(11): 2796-2802.
[37]An P, Xu X C, Yang F L, et al. Comparison of the characteristics of anammox granules of different sizes [J]. Biotechnology and Bioprocess Engineering, 2013,18(3):446-454.
[38]Zhong Y M, Jia X S. Simultaneous ANAMMOX and denitrification (SAD) process in batch tests [J]. World Journal of Microbiology and Biotechnology, 2013,29(1):51-61.
[39]Kartal B, Rattray J, van Niftrik L A, et al. Candidatus "Anammoxoglobus propionicus" a new propionate oxidizing species of anaerobic ammonium oxidizing bacteria [J]. Systematic and Applied Microbiology, 2007,30(1):39-49.
The study of SNAD process start-up on domestic wastewater.
ZHENG Zhao-ming, LI Ze-bing, LIU Chang-jing,CHEN Guang-hui, ZHENG Lin-xue, MA Jing, LI Jun*, ZHAO Bai-hang (Key Laboratory of Beijing for Water Quality Science and Water Environment Recovery Engineering, Beijing University of Technology, Beijing 100124, China). China Environmental Science, 2015,35(4):1072~1081
The SNAD (simultaneous partial nitrification, anaerobic ammonium oxidization and denitrification) process was successfully developed in a SBR to treat domestic wastewater. In the beginning, some anammox (anaerobic ammonium oxidation) granules were inoculated into the SBR to develop partial nitrification granules under high DO(500L/h). Afterwards, some anammox granules were added again to develop SNAD granules under low DO(40L/h). During the stable partial nitrification stage, the average ammonium removal rate was above 94% while the average nitrite accumulation rate was more than 95%. In the stable SNAD stage, 85% average total nitrogen removal rate was achieved. To the partial nitrification granules, batch tests indicated that the specific aerobic ammonium oxidation activity and nitrite oxidation activity were 0.234 and 0kgN/(kgVSS·d), respectively. To the SNAD granules, batch tests indicated that the specific anammox activity, denitrification activity over nitrite, aerobic ammonium oxidation activity and nitrite oxidation activity were 0.158、0.104、0.281、0kg/(kgVSS·d), respectively. The denitrification activity over nitrate was 0.061kg/(kgVSS·d) during 0~120min while the value was 0.104kg/(kgVSS·d) during 120~360min. The SEM observations indicated that the bacteria in the outer part of the SNAD granule were mainly short rod-shaped and spherical, which may be AOB and denitrification bacteria. In the inner part of the SNAD granule, the bacteria were mainly crater-shaped, which should be anammox bacteria.
anammox;partial nitrification;denitrification;domestic wastewater;granules
X703.5
A
1000-6923(2015)04-1072-10
郑照明(1989-),男,浙江嵊州市人,北京工业大学硕士研究生,主要研究厌氧氨氧化、亚硝化脱氮工艺.发表论文2篇.
2014-09-04
国家水体污染控制与治理科技重大专项(2014ZX 07201-011);北京市自然科学基金资助项目(8122005);国家自然科学基金青年基金(51308010);北京市教委面上项目(KM201210005028)
* 责任作者, 教授, jglijun@bjut.edu.cn

