Meox1在心肌梗死大鼠心肌中的表达情况
沈俊,袁平,唐俊明,杨建业,李兴元,张蕾,赵继先,张焕鑫,薛仕珍,冯怡,王家宁
· 论著 ·
Meox1在心肌梗死大鼠心肌中的表达情况
沈俊1,2,袁平2,唐俊明1,杨建业1,李兴元1,张蕾1,赵继先2,张焕鑫2,薛仕珍2,冯怡2,王家宁1,2
目的 分析同源盒基因Meox1在心肌梗死大鼠心肌中的表达情况。方法 清洁级雄性SD大鼠20只,随机分入心肌梗死组和假手术组,每组10只。心肌梗死组采用结扎左冠状动脉前降支构建心肌梗死模型,手术过程死亡2只。假手术组仅穿线不结扎。术后7 d处死大鼠,制作石蜡切片,HE染色以及Masson染色观察心肌组织变化,免疫组化法检测Meox1的表达水平。结果 HE染色结果:心肌梗死组可见梗死区心肌细胞显著减少,核碎裂,核消失,被大量排列紊乱的结缔组织代替,可见大量粒细胞、单核细胞浸润。梗死边缘区可见心肌细胞代偿性肥大,横纹模糊或消失,心肌间隙水肿增宽,部分心肌纤维溶解、断裂,可见炎性细胞浸润、成纤维细胞增生。梗死远离区及假手术组大鼠心肌形态正常,结构清晰,心肌纤维排列整齐。Masson染色结果,非梗死区染色呈红色为正常心肌组织,蓝色为胶原纤维。假手术组及梗死远离区心肌细胞排列整齐紧密,细胞间隙散在分布少量胶原纤维;心肌梗死组梗死区可见细胞间隙大量胶原纤维沉积,梗死边缘区的细胞间隙也有大量的胶原纤维沉积。免疫组化结果,梗死区及梗死边缘区的心肌细胞中可见棕黄色颗粒沉积,有Meox1表达;在梗死远离区的正常心肌细胞中未见Meox1的表达;假手术组心肌细胞中亦未见Meox1的表达。假手术组及梗死远离区毛细血管内皮细胞中未见Meox1的表达;心肌梗死组梗死区及梗死边缘区毛细血管内皮细胞中Mexo1表达不明显。结论 在心肌梗死大鼠心肌组织中Meox1表达增高,提示其可能参与心肌梗死后心肌重塑。
心肌梗死;同源盒基因Meox1;表达
在我国冠状动脉粥样硬化性心脏病(冠心病)是主要死亡原因之一,心肌梗死(MI)后1年内死亡率较高[1],梗死和非梗死区细胞外基质的异常集聚,导致组织结构改变、组织硬度增加,心肌广泛重构,最终引起心功能不全[2-8]。因此寻求有效治疗靶点非常必要。同源盒基因Meox1是控制发育的主要基因,对心血管系统发育及疾病的发生有调控作用[9]。本研究通过结扎SD大鼠冠状动脉前降支制备心肌梗死模型,采用HE染色、Masson染色及免疫组化的方法观察心肌梗死后心肌组织变化及Meox1的表达情况。
1 材料与方法
1.1 实验动物、主要材料与仪器 清洁级雄性SD大鼠20只由湖北医药学院实验动物中心提供,重量180~230 g。饲养条件:温度23℃,湿度95%,12 h明暗交替,大鼠专用饲料喂养。实验过程严格遵守伦理学准则。Meox1抗体(美国abcom公司)、DAB显色试剂盒(武汉博士德生物工程有限公司),RM2245型病理切片机、H11220型烘片机、H11210型摊片机(德国Leica公司)、BX-51型光学显微镜(日本Nikon公司)。手术器械包(手术剪、弯头和直头止血钳、弯形镊子、眼科剪、显微持针器、开胸器、手术线、干棉花、酒精棉、纱布、头皮针、注射器、三通管、棉线),6-0无菌医用非吸收性缝合线无损伤缝合针(杭州富阳医用缝合针线厂)。将20只大鼠随机分入心肌梗死组和假手术组,每组10只,制作心肌梗死模型死亡2只。
1.2 方法
1.2.1 心肌梗死动物模型的构建 按照Kumar等[10]的改进方法,实验前大鼠禁食2 h以上,水合氯醛(300 mg/kg)进行腹腔注射麻醉,以有无翻正反射作为大鼠麻醉与否的评定标准。固定大鼠,手术区剃毛后,剪开皮肤,分离颈部肌肉,暴露气管,用气管穿刺针进行气管插管。插管成功后接小动物呼吸机辅助呼吸,潮气量30~50 ml/kg,呼吸频率55~65 次/min,呼吸比2:1。连接心电图机测肢体Ⅱ导联心电图。在三、四肋间心尖搏动最强点(大约为平左前肢与左胸骨2 mm处)切开皮肤,钝性分离第三、四肋骨的肋间肌,止血钳撑开肋间隙扩大手术视野。小心地撕开心包膜暴露心脏,在心脏收缩的一瞬间,用食指与拇指挤出心脏,快速从左心耳下方2.0~3.0 mm入针,进针深度为1.5~2.0 mm,然后快速还纳心脏,于左心耳和肺动脉圆锥与心尖连线中点处(约为左冠状动脉走行处)以6-0无创缝合线将留置软管一起进行缝扎,结扎时力度要适中,结扎缺血区心肌组织变白,同时心电图对应的导联抬高。假手术组仅开胸和分离冠状动脉,但是不结扎。观察心脏跳动情况,实验中若出现心律失常,则在腹腔注射少量的利多卡因。结扎后逐层缝合胸腔,并保持预留留置针通畅以防止气胸。待大鼠呼吸稳定后,放入恢复笼内饲养。腹腔注射3~5 ml生理盐水补液后,观察并及时清除气管痰液。待大鼠苏醒后肌肉注射青霉素钠以防感染。
1.2.2 组织的获得和处理 手术后7 d,麻醉并处死大鼠,开胸暴露心脏,将接有生理盐水的输液针头扎在右心耳,用眼科剪将心尖剪一个约2 mm小口,生理盐水灌洗心腔,冲洗干净后用4%多聚甲醛冲洗心腔2 h。取出心脏,去除心房。将心室置于4%多聚甲醛固定24 h,于结扎线处水平将心室切成1 mm薄片,置于包埋盒中流水冲洗过夜,常规脱水、透明、浸蜡、包埋等处理。使用切片机连续切片,厚度为5 μm,并按顺序编号。
1.2.3 HE染色 具体步骤如下:①切片常规二甲苯脱蜡,梯度酒精水化,用去离子水洗1~2 min;②苏木精染色8~10 min,自来水洗1~2 min;③体积分数为1%的盐酸-酒精分化1~2 s(显微镜下观察效果),自来水洗10~15 s;④体积分数为l%的氨水或肥皂水返蓝20~40 s,自来水洗1 min;⑤伊红染色4~5 min,自来水洗1 min;⑥切片依次入体积分数为95%乙醇5~10 s,无水乙醇3次,每次5~10 s,二甲苯3次,每次1~2 min;⑦中性树脂封片,镜下观察。
1.2.4 Masson染色 步骤:①切片常规二甲苯脱蜡,梯度酒精水化,去离子水洗1~2 min;②用配制的Weigert铁苏木素染色5~10 min;③体积分数为1%的盐酸-酒精分化1~2 s,自来水洗10~15 s;④Masson蓝化液返蓝20~40 s,自来水洗1 min,去离子水洗1 min;⑤丽春红染色5~10 min;⑥蒸馏水:弱酸溶液=2:1比例配置弱酸工作液,用弱酸工作液洗1 min;⑦磷钼酸溶液洗1~2 min,弱酸工作液洗1 min;⑧苯胺蓝染色1~2 min,弱酸工作液洗1 min;⑨切片依次入体积分数为95%乙醇5~10 s,无水乙醇3次,每次5~10 s,二甲苯3次,每次1~2 min;⑩中性树脂封片,镜下观察。
1.2.5 免疫组化分析 具体步骤如下:①切片常规二甲苯脱蜡,梯度酒精水化,用去离子水洗1~2 min;②微波修复抗原15 min,3%H2O2孵育5~10 min,PBS洗3次,每次5 min;③10%马血清封闭,室温1 h;④加一抗:Meox1兔多克隆抗体(1:100),4℃湿盒过夜,PBS洗3次,每次5 min;⑤加二抗:鼠抗兔多克隆抗体(1:100),室温1 h,PBS洗3次,每次5 min;⑥DAB染色(现配现用),PBS洗3次,每次5 min;⑦苏木精染色8~10 min,自来水洗1~2 min;⑧体积分数为1%的盐酸-酒精分化1~2 s(显微镜下观察效果),自来水洗10~15 s;⑨体积分数为1%的氨水或肥皂水返蓝20~40 s,自来水洗1 min;⑩切片依次入体积分数为95%乙醇5~10 s,无水乙醇3次,每次5~10 s;二甲苯透明3次,每次1~2 min;⑪中性树脂封片。
2 结果
2.1 两组HE染色结果比较 HE染色结果:心肌梗死组可见梗死区心肌细胞显著减少,心肌纤维凝固性坏死,核碎裂,核消失,被大量排列紊乱的结缔组织代替,肌浆均质红染或呈不规则粗颗粒状,可见大量粒细胞、单核细胞浸润(图1)。梗死边缘区可见心肌细胞代偿性肥大,肌横纹模糊或消失,心肌间隙水肿增宽,部分心肌纤维溶解、断裂,可见炎性细胞浸润、成纤维细胞增生。梗死远离区及假手术组(图1)大鼠心肌形态正常,结构清晰,心肌纤维排列整齐有序,肌纤维间无成纤维细胞聚集、增生现象。

图1 大鼠心肌组织HE染色结果(A:假手术组;B:心肌梗死组梗死区;C:心肌梗死组梗死边缘区;D:心肌梗死组梗死远离区,×200)
2.2 两组Masson染色结果比较 Masson染色结果,非梗死区染色呈红色为正常心肌组织,蓝色为胶原纤维。假手术组及梗死远离区可见红色的心肌细胞排列整齐紧密,细胞间隙内有散在分布少量蓝色胶原纤维;心肌梗死组可见梗死区细胞间隙大量胶原纤维沉积,梗死边缘区红色的细胞间隙有大量的胶原纤维沉积(图2)。
2.3 Meox1在心肌细胞中的表达情况 在梗死区及梗死边缘区的心肌细胞中可见棕黄色颗粒沉积,提示Meox1在心肌细胞中表达;在梗死远离区的正常心肌细胞中未见Meox1的表达;假手术组心肌细胞中亦未见Meox1的表达(图3)。
2.4 Meox1在毛细血管内皮细胞中的表达情况 假手术组及梗死远离区毛细血管内皮细胞中未见Meox1表达;心肌梗死组梗死区及梗死边缘区毛细血管内皮细胞中Mexo1表达不明显,其动态变化可能在心肌梗死后基质重塑中起作用(图4)。
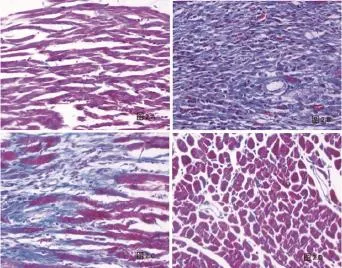
图2 大鼠心肌组织Masson染色结果(A:假手术组;B:心肌梗死组梗死区;C:心肌梗死组梗死边缘区;D:心肌梗死组梗死远离区,×200)
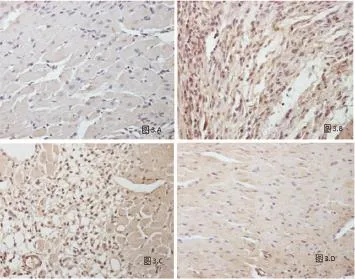
图3 Meox1在心肌细胞中的表达情况(A:假手术组;B:心肌梗死组梗死区;C:心肌梗死组梗死边缘区;D:心肌梗死组梗死远离区,×200)
3 讨论
心肌梗死后伴心室重构,梗死和非梗死区细胞外基质的异常集聚,导致组织结构破坏、组织硬度增加,引起左心腔扩大及心功能不全[11-16]。心肌梗死后的心室重构是一个慢性过程,研究表明[17-24],细胞外基质的合成及降解、肾素-血管紧张素-醛固酮系统、转化生长因子β、金属蛋白酶、Micro RNA及各种转录生长因子等都参与了心肌纤维化的过程。
同源盒蛋白是广泛存在于真核生物的一类转录调控因子[25-27]。同源盒结构域高度保守,通过螺旋-转折-螺旋结构模式与启动子或增强子的序列结合,激活或抑制靶基因的转录[28-30]。同源盒基因Meox所编码的蛋白质也属于转录因子[31,32]。近年关于同源盒基因的报道较多,而关于Meox1在心血管系统的作用却较少。王书美等[32]通过建立心脏特异表达Meox1的转基因小鼠,发现Meox1在心脏过表达引起扩张型心肌病。

图4 Meox1在毛细血管内皮细胞中的表达情况(A:假手术组;B:心肌梗死组梗死区;C:心肌梗死组梗死边缘区;D:心肌梗死组梗死远离区,×200)
通过免疫组化染色发现大鼠心肌梗死后心肌组织中表达Meox1,在心肌梗死区及梗死边缘区毛细血管内皮细胞中表达,而远离区及正常内皮细胞中无表达。还发现心肌梗死区及梗死边缘区心肌细胞亦表达Meox1,而远离区及正常心肌细胞则无Meox1表达。推测:梗死区及梗死边缘区心肌细胞及血管内皮细胞为缺血缺氧细胞,缺血缺氧诱导Meox1表达,而心肌梗死后Meox1表达短暂升高或与心肌细胞的存活相关,其机制不清。本研究表明,Meox1在心肌梗死大鼠的心脏血管内皮细胞及心肌细胞中表达,提示其参与心肌梗死后的心肌重构,具体机制需更深入的研究。
[1] Sutton MJ,Douglas L,Rouleau JL,et al. Left ventricular remodeling and ventricular arrhythmias after myocardial infarction[J]. Circulation, 2003,107(20):2577-82.
[2] Weber KT. Fibrosis and hypertensive heart disease[J]. Curr Opin Cardiol,2000,15(4):264-72.
[3] Ruwhof C,van Wamel AE,Egas JM,et al. Cyclic stretch induces the release of growth promoting factors from cultured neonatal cardiomyocytes and cardiac fibroblasts[J]. Mol Cell Biochem,2000, 208(1-2):89-98.
[4] Weber KT. Cardiac interstitimu in heart and disease,the fibrillar collagen netvork[J]. J Am Coll Cardiol,1989,13(7):1637-52.
[5] Porter KE,Turner NA. Cardiac fibroblasts:at the heart of myocardial remodeling[J]. Pharmacol Ther,2009,123(2):255-78.
[6] Manso AM,Kang SM,Boss RS. Integrins,focal adhesions, and cardiac fihroblasts[J]. J Investig Med,2009,57(8):856-60.
[7] Sun Y,Zhang JQ,Zhang J,et al. Cardiac remodeling by fibrous tissue after infarction in rats[J]. J Lab Clin Med,2000,135(4):316-23.
[8] Swynghedauw B. Molecular mechanisms of myocardial remodeling[J]. Physiol Rev,1999, 79(1):215-62.
[9] Mcginnis W,Krumlauf R. Homeobox genes and axial patterning[J]. Cell,1992,68(2):283-302.
[10] Kumar M,Kasala ER,Bodduluru LN,et al. Animal models of myocardial infarction: Mainstay in clinical translation[J]. Regul Toxicol Pharmacol,2016,76:221-30.
[11] Agnoletti G,Cargnoni A,Agnoletti L,et al. Experimental ischemic cardiomyopathy: insights into remodeling, physiological adaptation, and humoral response[J]. Ann Clin Lab Sci,2006,36(3):333-40.
[12] Jalil JE,Doering CW,Janicki JS,et al. Fibillar collagenand myocardial stiffness in the intact hypertrophied rat left ventricle[J]. Circ Res,1989,64(4):1041-50.
[13] Cohn JN,Ferrari R,Sharpe N. Cardiac remodeling concepts and clinical implications:a consensus paper from an international forum on cardiac remodeling[J]. J Am Coll Cardiol,2000, 35(3):569-82.
[14] Weber KT. Fibrosis in hypertensive heart disease:focus on cardiac libroblasts[J]. J Hypertens,2004,22(1):47-50.
[15] Camelliti P,Borg TK,Kohl P. Structural and functional characterization of cardiac libroblasts[J]. Cardiovasc Res,2005,65(1):40-51.
[16] Brown RD,Ambler SK,Mitchell MD,et al. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure[J]. Annu Rev Pharmacol Toxicol,2005,45:657-87.
[17] Gabbiani G. The myofibroblast diseasesin wound healing and fibrocontractive[J]. J Pathol, 2003,200(4):500-3.
[18] Desmouliere A,Redard M,Darby I,et al. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar[J]. Am J Pathol,1995,146(1):56-66.
[19] Sun Y,Weber KT. Infarct scar: a dynamic tissue[J]. Cardiovasc Res,2000,46(2):250-6.
[20] Frangogiannis NG. Targeting the inflammatory response in healing myocardial infarcts[J]. Curr Med Chem,2006,13(16):1877-93.
[21] Porter KE,Turner NA,O'Regan DJ,et al. Simvastatin reduces human atrial myofibroblast proliferation independently of cholesterol lowering via inhibition of RhoA[J]. Cardiovasc Res,2004,61(4):745-55.
[22] Li PF,Dietz R,von Harsdorf R. Superoxide induces apoptosis in cardiomyocytes,but proliferation and expression of transforming growth factor-betal in cardiac fibroblasts[J]. FEBS Lett,1999,448 (2-3):206-10.
[23] Ono K,Han J. The p38 signal transduction pathway: activation and function[J]. Cell Signal, 2000,12(1):1-13.
[24] Bolognese L,Carrabba N,Parodi G,et al. Impact of microvascular dysfunction on left ventricular remodeling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction[J]. Circulation,2004,109(9):1121-6.
[25] Sham MH,Hunt P,Nonchev S,et al. Analysis of the murine of the murine HOX-2.7 gene:conserved alternative transcripts with differential distributions in the nervous system and the potential for shared regulatory regions[J]. EMBO J,1992,11(5):1825-36.
[26] Candia AF,Hu J,Crosby J,et al. Mox-1 and Mox-2 define a novel homeobox gene subfamily and are differentially expressed during early mesodermal patterning in mouse embryos[J]. Development,116 (4):1123-36.
[27] Candia AF,Wright CV. Differential localization of Mox-1 and Mox-2 proteins indicates distinct roles during development[J]. Int J Dev Biol,1996,40(6):1179-84.
[28] Gehring WJ,Affolter M,Bürglin T. Homeodomain proteins[J]. Annu Rev Biochem,1994,63:487-526.
[29] Gianakopoulos PJ,Skerjanc IS. Hedgehog signaling induces cardiomyogenesis in P19 cells[J]. J Biol Chem,2005,280(22):21022-8.
[30] Dou ville JM,Wigle JT. Regulation and function of homeodomain proteins in the embryonic and adult vascular systems[J]. Can J Physiol Pharmacol,2007,85(1):55-65.
[31] Friedman LS,Ostermeyer EA,Lynch ED,et al. 22 genes from chromosome 17q21: cloning, sequencing, and characterization of mutations in breast cancer families and tumors[J]. Genomics, 1995,25(1):256-63.
[32] 王书美,吕丹,陈炜,等. Meox1在心脏过表达引起转基因小鼠扩张性心肌病[J]. 中国比较医学杂志,2010,04(20):9-18.
本文编辑:姚璐,田国祥
Changes in the expression of Meox1 after acute myocardial infarction in rats
SHEN Jun*, YUAN Ping, Tang Jun-ming, YANG Jian-ye, LI Xing-yuan, Zhang Lei, ZHAO Ji-xian, ZHANG Huan-xin, XUE Shi-zhen, FENG Yi, WANG Jia-ning.*Institute of Clinical Medicine and Department of Cardiology, Renmin Hospital, Hubei University of Medicine, Shiyan, Hubei 442000, China.
Objective To analyze the expression of Meox1 in myocardium in rats with acute myocardial infarction (AMI). Methods Male SD rats (n=20) were randomly divided into AMI group and sham-operation group (each n=10). The model of AMI was established by ligation of left anterior descending branch of coronary artery in AMI group (2 rats died during the procedure), and sham-operation group was given the same procedure except of ligation. The rats were killed 7 d after the procedure for preparing paraffin sections, and changes of myocardial tissue were observed after HE staining and Masson staining and expression of Meox1 was detected by using immunohistochemistry technique. Results The results of HE staining showed that cardiomyocyte decreased significantly at infarction zone, and there were karyorrhexis, nuclear disappearing, disorganized connective tissue in quantity, and severe infiltration of granulocytes and monocytes in AMI group. The compensatory hypertrophy and cross striatation blurring or disappearing of cardiomyocytes, widened myocardial gap edema, fibrinolysis and fragmentation of partial myocardial fibers, infiltration of inflammatory cells and proliferation of fibroblast were observed at infarction marginal zone. The normal myocardial histology, clear structure and aligned myocardial fibers were observed at infarction remote zone and in sham-operation group. The results of Masson staining showed that red was normal myocardial tissue and blue was collagen fibers at non-infarction zone. The cardiomyocytes were aligned and tight, and there were less collagen fibers in intercellular space in sham-operation group and at infarction remote zone. There was deposition of collagen fibers in quantity in intercellular space and at infarction zone and infarction marginal zone in AMI group. The results of immunohistochemistry technique showed that there was deposition of brown granules and Meox1 expression in cardiomyocytes at infarction zone and infarction marginal zone, and there was no Meox1 expression in normal cardiomyocytes at infarction remote zone and in sham-operation group. There was no Meox1 expression observed in capillary endothelial cells in sham-operation group and at infarction remote zone, and which was not significant at infarction zone and infarction marginal zone in AMI group. Conclusion The expression of Meox1 increases in myocardial tissue in AMI rats, which indicates that Meox1 expression may take part in the myocardial remodeling after AMI.
Myocardial infarction; Homeobox gene Meox1; Expression
R541.4
A
1674-4055(2016)11-1329-04
国家自然科学基金(81270221)
1442000 十堰,湖北医药学院附属人民医院临床医学研究所;2442000 十堰,湖北医药学院附属人民医院心脏病中心1病区
王家宁,E-mail:rywjn@vip.163.com
10.3969/j.issn.1674-4055.2016.11.13

