禾草内生真菌研究及应用进展
田沛,张光明,南志标
(1.草地农业生态系统国家重点实验室,兰州大学草地农业科技学院, 甘肃 兰州 730020;2.广东粤明电力工程有限公司,广东 珠海 519000 )
禾草内生真菌研究及应用进展
田沛1*,张光明2,南志标1
(1.草地农业生态系统国家重点实验室,兰州大学草地农业科技学院, 甘肃 兰州 730020;2.广东粤明电力工程有限公司,广东 珠海 519000 )
内生真菌感染大部分禾草形成互惠共生体,提高共生体对环境适应性,并进而对动物、微生物以及整个生态系统产生广泛的影响,使该领域成为近年来研究的热点问题。分子生物学技术、基因组学、蛋白组学和代谢组学以及相应的信息生物学技术的应用加快了禾草内生真菌的研究,尤其是内生真菌全基因组序列的测定,明确了次生代谢物多样性及其与基因的关系,鉴定相关的功能基因和蛋白,基因敲除技术阐释了维持内生真菌与寄主动态平衡的分子机制。利用基因组学等技术筛选对家畜无毒的内生真菌菌株, 通过接种技术建立新的既具有抗逆性,又对家畜无毒的禾草-内生真菌共生体,提高禾草的品质并确保对动物的安全性,在牧草及草坪草育种上获得了巨大成功。但是内生真菌的寄主特异性限制了内生真菌可利用的范围,因此后续应继续利用基因组学和代谢组学新技术深入研究内生真菌与寄主相互作用的机制,利用基因工程技术人工创造无毒菌株,克服共生体创制的瓶颈。本研究旨在对以上内容进行综述,以期为更广泛利用内生真菌进行牧草和草坪草育种奠定基础。
内生真菌;食物链;生态系统;微生物;基因组;牧草育种
禾草内生真菌指在禾草体内渡过全部或大部分生活周期,而禾草本身不显示任何外部症状的一大类真菌[1]。广泛研究的内生真菌主要是子囊菌门(Ascomycota)麦角科(Clavicipitaceae)的有性世代Epichloё和其所对应无性世代Neotyphodium属内生真菌。自1898年,从毒麦(Loliumtemulentum)种子内分离出第一株内生真菌至今,对内生真菌的研究经历了由浅入深的过程,尤其是在20世纪70年代以后,Bacon等[2]揭示了高羊茅(Festucaarundinacea)内生真菌与牛的狐茅中毒症(fescue toxicosis)的密切关系,随后Fletcher等[3]发现了多年生黑麦草(Loliumperenne)内生真菌与新西兰绵羊黑麦草蹒跚病(ryegrass staggers)的关系,禾草内生真菌的研究得到了突飞猛进的发展,对其分类、分布、生理、生态、代谢、遗传以及与寄主植物的相互作用等方面开展了大量的研究工作[4-6]。最近,Leuchtmann 等[7]根据内生真菌的基因序列和形态特征,依据 “一种真菌一个名称”的原则,将已发表鉴定的18种有性世代(Epichloё)和25种无性世代(Neotyphodium)内生真菌均划分为Epichloё 属内生真菌,原本Neotyphodium属认为暂时没有发现其有性世代,也被冠以Epichloё属。近年来,随着分子生物学、基因组学、代谢组学、生物信息学的快速发展以及在植物和真菌学领域内的广泛应用,内生真菌的研究方向主要集中在内生真菌与寄主相互作用的分子机制以及利用内生真菌进行牧草和草坪草育种等方面,本研究拟对近年来这方面的进展进行综述。
1 内生真菌对寄主的影响
大量以高羊茅-Epichloёcoenophiala和多年生黑麦草-Epichloёfestucaevar.lolii共生体为代表的研究形成了Epichloё属内生真菌与禾草形成互惠共生体,提高共生体对环境适应性的结论[8-9]。已证实内生真菌可以提高寄主的生长以及对食草动物[10-15]、昆虫[8,16]、线虫[16-17]、病原真菌、细菌[18-23]和竞争性植物[14,16,24]等生物胁迫的抗性和对干旱[20,24-25]、营养物质缺乏[25]和化学他感作用[26]等非生物胁迫的抗性。有关内生真菌对寄主的影响以及机理已有多篇文章和综述发表[1,4-5,27-30],笔者在这里不做赘述。随着对内生真菌资源调查的深入,发现大量新的禾草-内生真菌共生体[7,31-35],内生真菌与寄主植物共生关系表现出明显的不确定性,有证据表明Epichloё 属内生真菌并不能提高某些寄主尤其是野生禾草的适应性,比如内生真菌并没有提高寄主亚利桑那羊茅(Festucaarizonica)的生物量[15]、竞争力[36]和降低动物的采食[37]。这些不同的结果可能是由于高羊茅和多年生黑麦草等栽培牧草经长期驯化,遗传多样性远远低于野生禾草,内生真菌与寄主长期共生并协同进化,在演化过程中向更有利于寄主的方向进化。而新的内生真菌共生体,寄主植物遗传多样性高,其遗传背景足以影响植物的生长并超越内生真菌的作用,同时由于真菌繁殖模式、传播方式和环境的不同造成了不同的选择压力,使共生关系复杂化,因而对寄主造成了不同的影响[15,38-39],降低了在栽培牧草上得出结论的可靠性。内生真菌的传播方式也显著影响其对寄主的作用。水平传播的有性阶段内生真菌能在宿主生殖枝上产生子座,抑制宿主的开花和结实,对宿主的有益影响可能小于严格传播的无性阶段内生真菌[40]。有可能在自然群落中,内生真菌与寄主形成稳定的关系,但在温室或试验田中开展的试验改变了共生体的生存环境,因而进一步改变内生真菌与寄主的关系。这些都是在研究内生真菌与寄主相互作用时需要关注和思考的问题。
2 内生真菌对动物及食物链的影响
高羊茅内生真菌引起的狐茅中毒症和多年生黑麦草内生真菌引起的黑麦草蹒跚病给畜牧业造成了巨大的经济损失[41-42],内生真菌对动物尤其是家畜的影响已经开展了大量研究。已经明确内生真菌与禾草共生时,能产生多种次生代谢物,其中以吲哚双萜类(indolditerpene,以lolitrem B为代表),吡咯并吡嗪类(pyrrolopyrazine,以peramine为代表),麦角碱类(ergot,以ergovaline为代表)和饱和吡咯化合物(pyrrolizidine,以loline为代表)这四大类为代表[43]。除peramine以外的其余3类生物碱均对哺乳动物有毒,其中毒性最强的是lolitrem B和ergovaline,分别是引致狐茅中毒症和黑麦草蹒跚病的主要原因[10-12,44]。后又陆续发现禾草内生真菌共生体对多种家畜如家兔[45],鹿[46],骆驼[47],羊驼[48],山羊[49],马[41],驴[50]等的毒性。自黑麦草蹒跚症与内生真菌的关系确立不久,Prestidge等[51]发现内生真菌能提高多年生黑麦草对阿根廷茎象甲(Listronotusbonariensis)的抗性,这种保护作用是因为内生真菌产生对昆虫有强烈毒性的生物碱peramine和loline[52-53],这些生物碱可直接导致昆虫神经系统紊乱而死亡,或者在体内累积,影响对食物的消化利用,延缓生长发育,降低存活率。据不完全统计,内生真菌可对鞘翅目(Coleoptera),鳞翅目(Lepidoptera),异翅目(Heteroptera),同翅目(Homoptera),直翅目(Orthoptera),蜱螨目(Acarina)等目昆虫产生抗性[1,29,44,54]。近年来,逐渐发现一些新的生物碱,比如黑麦草内生真菌菌株AR37产生janthitrems[55],虽然尚未深入了解,但发现其可以提高寄主抗虫性[56],已经在育种中得到了重视。
共生体对动物的毒性是由内生真菌产生的次生代谢物引起的,这些次生代谢物可在采食者体内累积,帮助其防御捕食者,进而对上一级营养层甚至生态系统中多级营养层产生影响。比如内生真菌降低了寄主上蚜虫(Rhopalosiphumpadi)的密度,共生体产生的生物碱在蚜虫体内累积[57],经蚜虫食物链传递进而降低了蚜虫的寄生性天敌-寄生蜂和捕食性天敌-瓢虫的密度,并降低这些天敌的产卵量和生存率,延长发育期,其幼虫和成虫适应度显著降低,后代总数显著降低,这种级联作用能长期影响蚜虫天敌的种群动态[58]。同时也发现内生真菌对食物链的影响还包括蚜虫的采食促使内生真菌影响寄主分泌的挥发性有机物,这些有机物可降低其他昆虫的采食[59]。除了对蚜虫及食物链的应用,内生真菌也降低了阿根廷茎象甲和粘虫(Spodopterafrugiperda)的寄生蜂(Microctonushyperodae)的生长和存活率[60-61]。同时也发现采食被内生真菌感染(E+)植株的昆虫对线虫及其寄生细菌具有更强的抵抗力。比如多年生黑麦草和高羊茅E+植株上的小地虎(Agrostisipsilon)不易受到昆虫病原线虫(Steinernemacarpocapse)的感染[54,62]。这些例子说明禾草-内生真菌共生体与食草动物关系非常复杂,内生真菌的存在改变食草动物的取食行为,影响到植食性昆虫物种多样性和种群结构,对采食者的捕食性和寄生性天敌种类和数量产生影响,影响捕食者的捕食、结网(或寄生)和繁殖能力,从而影响食物链的能量流动和食物网结构,对生态系统产生不可低估的作用。在田间条件下,这种作用受到环境及共生体的基因型、生物量等各种因素影响,生物碱对食物链的影响可能会弱化。值得一提的是,虽有研究表明吲哚双萜类化合物lolitrem B和janthitrem在牛和羊的脂肪和牛奶中微量残留[63-64],但并不会对人类健康造成威胁[64-65]。
3 内生真菌与其他微生物的关系
在自然界中,植物同时与多种微生物类群共生,内生真菌对共生体-其他微生物的相互作用也产生显著影响。研究较多的是对共生体与地上部分病原真菌和细菌以及地下部分菌根真菌和土壤微生物群落的相互作用的影响。
通过离体培养平板对峙、离体叶片和活体植株接种以及田间发病率调查等方法已发现禾草内生真菌对多种病原真菌及其引起的植物病害产生影响[27,66]。内生真菌通过抵抗病原菌的侵入、抑制病原菌的生长、抑制孢子的萌发、抑制病斑的扩展和阻止传毒介体昆虫等机制提高寄主对22种牧草和草坪草病害的抗性[1,27]。但是由于植物抗病本身就是一个非常复杂的过程,受病原物种,寄主基因型,环境条件如植物营养供应(尤其是氮的供应)、温度、土壤湿度和水分条件和其他生物之间综合作用的影响[24,67],尤其田间条件下,内生真菌对寄主的抗病性影响出现不太一致,相对于内生真菌其他方面的研究,此方面研究涉及不多并较难得到一致的结论。
内生真菌能提高寄主对一些植物病毒,如黑麦草花叶病毒和大麦黄矮病毒的抗性[68-69]。但是对感染大多数真菌类群且不产生明显表现的真菌病毒关注较少。Marquez等[70]在Science杂志上报道了真菌Curvularia耐热病毒决定了内生真菌(Cryphonectriaprotuberata)与热带稗草(Dichantheliumlanuginosum)形成共生体的耐热性,只有被病毒感染的内生真菌才能提高寄主植物的耐热性,反之则不能。这就揭示了真菌病毒能够调整植物与内生真菌形成的共生体系,因而部分学者开始关注Epichloё属内生真菌与真菌病毒之间的关系。从草地羊茅(Festucarubra)分离的内生真菌E.festucae大部分被双链RNA病毒或者裸露RNA病毒感染,这些病毒对真菌的表现无明显影响,对共生体的影响也未知[71-72]。以后对内生真菌的研究应关注这方面的问题。
菌根真菌与植物根系建立互惠共生关系,增加宿主植物对土壤中营养元素特别是氮、磷的吸收,促进水分吸收和利用,碳水化合物代谢和提高光合速率等[73-74]。而Epichloё内生真菌与植物地上组织建立互惠共生关系。已开展了大量研究阐明单一共生真菌与寄主植物的相互关系,而菌根真菌和内生真菌双重感染时对寄主植物影响由于三者之间的互作受土壤营养水平、共生真菌的基因型以及不同的草地管理方式(单作或者混作,施肥,灌溉等)等多种因素的影响,尚不能得出统一的结论。有研究表明内生真菌与菌根真菌存在拮抗作用,内生真菌降低菌根真菌的侵染和扩展[18-19,75],但是在某些情况下促进菌根真菌的侵染[76]。比如内生真菌提高一种AM真菌对其寄主披碱草(Elymushystrix)的侵染,却抑制了另外一种AM真菌的侵染[77];同时也发现,AM真菌共寄生提高多年生黑麦草-E.festucaevar.lolii共生体的冠根比,却降低了多年生黑麦草-E.typhina共生体的冠根比[75],这些研究都强调了基因型的强烈影响。虽然二者单独寄生时均形成互惠共生体,有研究发现二者共同作用时,降低寄主生物碱含量和抗虫性[78-79],因此,如何同时利用这两种真菌以形成超级共生体对寄主提供全方面的保护,还需要更多深入的研究。
内生真菌除了影响与寄主共生的微生物,还能够改变土壤的营养元素水平,影响土壤微生物群落结构和功能[80]。初步证明了内生真菌直接影响根部生物量、形态和分泌物从而影响根部食物链和营养循环,增加输入到土壤中的有机营养,提高根际营养循环和土壤微生物活性,影响土壤微生物群落的代谢多样性[81-84];并可通过对共生体地上部分比如生物量,种群结构,周围微环境的综合作用和家畜采食间接影响地下部分。共生体产生生物碱或者其他化感作用物质可渗入到土壤中对土壤微生物和动物群落产生影响。但是也有不同的结果,有研究表明禾草内生真菌降低土壤微生物量和土壤呼吸作用,抑制土壤微生物活性,对土壤微生物群落的稳定性存在一定的副作用[85-86]。
4 内生真菌对生态系统的影响
上述内生真菌与寄主、草食动物和微生物的研究属于个体或种群水平的研究,随着内生真菌研究的深入,其已拓宽到内生真菌与寄主所在的整个生态系统的影响。通过上述提到的直接或间接影响,内生真菌在生物群落和整个生态系统中发挥重要作用[87]。比如内生真菌能提高寄主对环境的适应性,使具有较强抗逆性的带菌禾草能迅速占据生态位,提高其在群落中的竞争力,降低周围群落杂草的多样性,影响了植物群落和生态系统的多样性[88]。而生物碱等次生代谢物对草食动物及食物链的影响,改变自然群落中竞争和捕食关系,进而改变群落中动物的多样性[87],比如内生真菌导致高羊茅草地中小型哺乳动物、节肢动物多样性的降低[89-90],也有共生体为其他草类植物和节肢动物提供了庇护,从而保护生物多样性的报道[91]。内生真菌对寄主地下部分的影响包括对根际和土壤微生物两方面的作用,改变根系分泌物和根际代谢物[92],影响土壤真菌和细菌群落[93],进而影响地下部分生态系统营养循环;内生真菌对地上部分生物量及营养品质的影响,会进一步影响寄主枯叶的质量和降解速率,影响土壤碳释放和温室气体的排放,这些作用反过来影响地上和地下部分群落结构[94](图1)。由此可以看出内生真菌虽然作为微生物在寄主体内生存,但是对草原植物群落多样性、稳定性和草原生态系统均有非常重要的影响,微生物已经成为影响生态系统不可忽略的因素。虽然内生真菌对生态系统的研究相对较少,但却已取得了具有国际影响力的成果, Clay等[14]表明虽然内生真菌没有提高高羊茅地上部分的生物量,但却提高了寄主在群落中的优势地位,降低物种多样性,导致群落结构的改变。Omacini等[58]通过对内生真菌-蚜虫食物链的研究表明内生真菌干扰能量从植物向更高级营养水平的传递,从而影响生物链的能量传递,进而改变生物群落的多样性。这些成果分别在顶级学术期刊Science和Nature上发表,从而使内生真菌对生态系统的重要性在国际范围内引起关注。
5 禾草内生真菌的基因组学和代谢组学
随着分子生物学技术、基因组学、蛋白组学和代谢组学以及相应的生物信息学技术的迅速发展和日益成熟,内生真菌的研究也得到了空前的发展[4-6,96-97],相对于上述提到的几个方面,基因组学得到最多的关注和迅速的发展,开展了内生真菌及其寄主植物的全基因组序列测定,明确次生代谢物多样性及其与基因的关系,并鉴定相关的功能基因和蛋白。代表性工作有美国肯塔基大学Schardl实验室围绕生物碱合成的比较基因组学研究,现已完成26株麦角菌其中包括20个不同的Epichloё内生真菌的全基因组测序,其序列在http://www.endophyte.uky.edu/上公布,有助于全球科学家共同开展基因图谱构建等工作[6]。另外,澳大利亚维多利亚农业生物研究中心(AgriBio, Victoria)Spangenberg实验室也完成了16株羊茅属内生真菌和19株黑麦草属内生真菌的全基因组测序[98-99]。这些全基因组序列的获得为研究内生真菌的系统进化,深入分析生物碱合成相关基因,寻找真菌与寄主相互作用的基因奠定了重要的基础。比如以前广泛利用DNA分子标记技术如SSR等[100-101]和特定DNA片段如tubB、tefA 序列对内生真菌进行遗传多样性、系统发育、起源和进化研究[7,102]。但这些基因片段往往小于整个基因组的千分之一,数据不足以反映整个基因组的特点。而全基因组序列的获得,可以用更多的长片段基因比如线粒体基因进行真菌系统发育的研究,更清楚地阐明基因的结构变异、拷贝数目、碱基缺失等与真菌生态功能的关系,加速分子标记的开发,更准确地追溯真菌起源,对以前分类模糊的种进行精确的定性[34,98-99]。内生真菌产生的四大类生物碱的合成途径及调控基因已通过基因克隆和基因沉默等技术阐明[88,103-105],可根据生物碱合成途径基因出现与否推断内生真菌的产碱类型[97,106-108]。而全基因组序列的获得能克服基因克隆的片面性和AT富集区干扰片段的出现等问题,精确分析具有不同生活史和生态功能的生物碱合成相关基因的结构变化,转座子、微型反向重复转座元件、端粒以及基因内部碱基缺失对基因功能的影响,并发现了一些新的与生物碱合成相关的基因,阐明基因与内生真菌次生代谢物多样性的关系[5-6,109]。
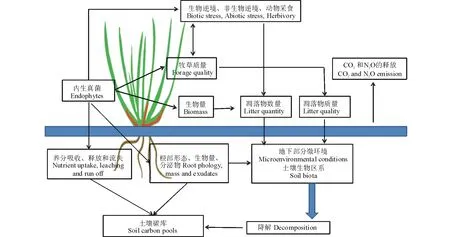
图1 内生真菌对地上和地下生态系统的影响[4,95]Fig.1 The influence of endophytes on ecosystem of both above and belowground[4,95]
内生真菌与寄主相互作用非常复杂,其相互作用机制仍不清楚。研究者曾试图分析内生真菌及寄主的转录组以阐明内生真菌如何调控寄主。比如利用基因芯片技术分析了固体和液体培养基中黑麦草内生真菌[110]以及E+和E-高羊茅植株的差异表达基因[111];利用抑制消减杂交方法寻找寄主体内受内生真菌影响的基因[112]。但是这些技术需要已知基因序列,并且受探针数量的限制,不能覆盖全部基因组。而高通量转录组测序技术无需全基因组信息,直接对mRNA反转录生成的cDNA进行测序,根据转录组差异表达基因寻找内生真菌调控基因。尤其是内生真菌全基因谱图的构建,可快速阐明内生真菌转录组差异表达基因的功能,以期解释这些基因如何在寄主中发挥调控作用。比较E+和E-植株转录组的差异揭示了内生真菌通过改变寄主的代谢产物以调控内生真菌与寄主之间的信号和化学物质转导,提高寄主的生长和竞争能力[113-115];而通过比较某些基因缺失菌株和野生型菌株对寄主转录组的影响,发现突变菌株的水解酶类、转运蛋白和代谢物的基因表达显著上调,对寄主表现显著的致病作用,寄主植物抗病,转座子活性、激素合成及应答的基因表达上调,由互惠共生关系转变为致病关系[114]。同样的,分析不同逆境条件下共生体转录组表达差异,也发现了多种基因簇参与调控寄主体内代谢途径的改变,从而引起抗逆性的变化[115]。由于高通量测序信息量较大,受内生真菌调控影响的基因数目较多,经常有数百个在植物生理生化过程中起作用的功能蛋白及化合物的基因受到调控,通过基因功能分析可以推断内生真菌与寄主之间的物质转导,但是阐明这些基因间的关联以及如何受到信号调控还是现在的难点,随着生物信息的发展,网络谱图构建加快,相信很快会克服这些困难,阐明内生真菌与寄主相互作用的分子机制。
而以前基于基因敲除技术开展的单个目标基因的研究,也已获得了突破性的成果,阐释了维持内生真菌与寄主动态平衡的分子机制。首先发现内生真菌产生活性氧(reactive oxygen species,ROS)以阻止寄主的保护反应,NADPH氧化复合体调控胞间合成活性氧ROS,Nox复合体任何基因(NoxA,NoxR,RacA和BemA)缺失或突变都会导致寄主感病和矮化,菌丝无序生长,丧失互惠共生关系[116-119]。MAP激酶也调控Nox复合体的活性,编码该酶的SakA基因的缺失也会导致互惠共生关系破坏,寄主矮化和早熟[113]。另外也发现菌丝融合相关基因soft(so)和转录因子proA基因对维持内生真菌与寄主互惠共生关系的重要作用[102,120]。进行铁离子吸收和贮存的嗜铁蛋白-胞外嗜铁素A对维系内生真菌与多年生黑麦草互惠共生也是必需的。内生真菌利用铁通透酶介导的还原性铁吸收系统或直接通过嗜铁蛋白进行铁离子吸收[5,121]。非核糖体多肽合成酶是合成嗜铁蛋白的关键酶,其相关调控基因sidN的缺失,抑制内生真菌分泌铁载体,从而改变了共生铁离子的动态平衡,最终导致其互惠性向拮抗性转换[5]。这些机制的研究有助于阐明植物与真菌之间信号转导,物质交流过程,从而解决利用新内生真菌创制共生体时出现的不融合性,克服共生体创制的瓶颈。
随着蛋白组学和代谢组学的发展,尤其是色谱-质谱连用,核磁共振等技术的应用,可以对共生体次生代谢物进行更加细致深入的分析,对未知化合物定性也变得更加容易。结合蛋白组及代谢组的比较分析技术,同时分析共生体整体代谢物的变化,阐述多种代谢产物和代谢途径的动态变化过程,有助于阐明转录组分析识别的重要基因,阐明基因的功能以及基因组之间的相互作用。此类研究刚刚起步,但是已发现内生真菌感染能降低共生体中含氮化合物、氨基酸和镁含量,提高可溶性碳水化合物、脂类和一些有机酸的含量[122];提高寄主的葡萄糖、果糖、海藻糖、糖醇、脯氨酸和谷氨酸含量以及内生真菌次生代谢物甘露醇和生物碱loline含量以提高寄主抗旱性[123];提高与糖酵解、三羧酸循环和氨基酸合成途径的相关代谢物水平以提高寄主抗寒能力[124];全面影响寄主根系的分泌代谢物以改变植物地下部分以及土壤微生物群落[92]。结合上述提到的转录组学技术,同时分析转录组基因差异和代谢产物差异的一致性可能会更快的揭示内生真菌调控寄主的机制。
同时,由于技术的发展,对内生真菌产生的四大类生物碱合成途径中的化合物及前体物质的毒性及独特的药理作用展开了更深入的研究。以麦角碱为例,所有前体物质现都可进行定量检测以确定各代谢产物的关系以及对家畜的毒性[125]。其合成途径包含多种化合物,他们显著抑制了依赖于Na+/K+和Mg2+离子通道的两种ATP酶,或与D2多巴胺相结合而抑制AMP循环[126-128],从而使动物体温升高,采食量减少,体重、催乳激素及牛奶产量降低[9]。通过对前体物质进行分离纯化和毒性检测,发现ergoamide及衍生物主要对动物神经产生影响,ergopetines主要进行血管收缩和减少催乳激素分泌,其中ergovaline 和 ergotamine具有最强的血管收缩和与D2多巴胺结合的能力,而麦角酸收缩血管能力较弱。这些不同的毒性物质有不同的毒性动力学,与家畜体内的药物代谢酶作用能力也不尽相同[129]。这些研究证明了麦角碱类化合物功能非常复杂,其纯品化合物可用于不同的医疗用途,而共生体代谢途径的任何微小改变都可能引起共生体毒性及对家畜影响的变化。随着对化合物研究的深入,在以后利用内生真菌进行牧草育种时,不能只考虑终产物的影响,必须借助代谢组学筛选不含有任何有毒物质的菌株。
除了以生物碱为代表的活性物质,内生真菌还产生其他化学结构和功能多样的次生代谢物,De Battista等[130]很早就发现高羊茅内生真菌在离体培养条件下能产生植物生长素-吲哚乙酸。随后,自梯牧草内生真菌E.typhina中分离出数种抗真菌活性化合物后,陆续有吲哚类化合物[131],倍半萜烯等多种抗真菌化合物被分离鉴定[132-133]。Song 等[133]还在披碱草内生真菌中分离纯化出具有类似除草剂活性化合物,对多年生黑麦草和早熟禾幼苗生长具有明显的抑制作用。从这些研究可以看出,内生真菌是丰富的天然产物资源库,孕育着多种新型化合物,根据这些资源创制具有新分子骨架、新作用机制的新型杀虫剂、杀菌剂并在生产上进行开发利用还任重道远。
6 利用新内生真菌菌株进行新品种选育
由于内生真菌对家畜的毒性,曾尝试建立不带内生真菌的高羊茅(美国)和黑麦草(新西兰)草地以防治家畜中毒,但是不带内生真菌的牧草建植困难、生长缓慢、产量下降、易受虫害危害,难以管理[1,42],在某些逆境条件下甚至不能存活[25],证明内生真菌对寄主有极其重要的作用,建立不带内生真菌的草地并不可行。减少家畜病害的其他措施还包括E+牧草与三叶草(Trifoliumsp.)、苜蓿(Medicagosp.)等豆科牧草混播,与谷类混饲,制作青贮饲料等降低家畜采食的毒素[134];或者补饲药物或添加剂;加强放牧管理比如轮牧减少家畜对生物碱含量高的茎基部采食[10,135]。但是这些措施都不能从根本解决家畜中毒问题,而对家畜无毒的内生真菌菌株的发现为解决这一问题提出了新途径。从禾草内生真菌种质资源中筛选出不产生对家畜有毒生物碱的无毒菌株,通过接种技术建立新的既具有抗逆性又对家畜无毒的禾草-内生真菌共生体,提高禾草的品质并确保对动物的安全性,在育种上已获得了巨大成功[4-5,136]。表1列出了部分广泛用于多年生黑麦草和高羊茅育种的新内生真菌菌株。目前已有5种内生真菌用于黑麦草育种(Endosafe、AR1、NEA2、Endo5和AR37),尤其是AR1,投放市场3年内占据了新西兰黑麦草种子市场的60%[137]。还有澳大利亚维多利亚农业生物研究中心和新西兰Agriseeds联合发现了多株无毒菌株正拟投放市场[138]。高羊茅内生真菌E.coenophiala菌株AR542只产生对昆虫有毒的loline和peramine生物碱,将其接种到一些优质高羊茅中开发了MaxQ和 MaxP商用品种。这些共生体既不影响采食家畜的健康,又同时保持了植株的生长及抗逆优势,在畜牧业生产中取得了巨大的经济价值[136,139]。而进行草坪草育种时,不需要考虑内生真菌毒素引致的家畜中毒问题,其为寄主带来的抗逆、抗虫、耐践踏等特性正是草坪草业所需要的[1,67]。因此根据内生真菌的不同表现可以将其利用在不同方面,比如新西兰利用对家畜和昆虫有毒生物碱含量极高的内生真菌菌系AR95和AR601,分别接入到多年生黑麦草和高羊茅草坪草品种中,成功培育机场防鸟草Avanex品种[140]。
由于内生真菌的寄主特异性,利用人工接种建立新的禾草-内生真菌共生体时,会发生一些不融合反应(incompatability),这就限制了内生真菌可利用的范围。其次寄主和内生真菌的相互关系受植物和内生真菌基因型的强烈影响[13,15]。同一内生真菌接种到不同寄主中后其生物碱种类和含量会发生改变。有时候新的共生体产生的生物碱与期待并不一致,一个很明显的例子就是Endosafe,Endosafe是只产生吡咯并吡嗪类化合物(peramine)却不产生吲哚双萜类化合物(lolitrem B)的E.festucaevar.lolii(N.lolii)菌株,当它接种到黑麦草中却产生了麦角碱(ergovaline)[149],因此该菌株后来被AR1取代,并提醒育种者后续利用内生真菌育种时必须开展长期严格评估才能投放市场[5]。研究也发现不产生动物毒素的内生真菌对寄主的保护作用有所降低,使其在实际生产中的应用有所下降[150]。比如接种了新内生真菌的黑麦草,产生的吡咯并吡嗪类化合物(peramine)含量降低,因而更容易受阿根廷茎象甲的危害[147,150],这些都是利用内生真菌育种时需要解决的问题。基于这些问题,一方面正在利用基因组学和代谢组学新技术深入研究内生真菌与寄主相互作用的机制,从而解决利用内生真菌创制共生体时出现的不融合性,克服共生体创制的瓶颈。另一方面利用基因工程技术人工创造无毒菌株,而生物碱合成途径及相关调控基因的阐明也为采用生物工程方法创制对家畜无毒菌株奠定了基础[6,102,113]。比如澳大利亚Spangenberg实验室将菌株进行基因突变,定向筛选缺少lolitrem B合成基因的菌株,获得了不产生lolitrems的内生真菌菌株;或者利用CRISPR(Clustered Regularly Interspaced Short Palindromic Repeats)基因编辑技术,切除有毒生物碱的基因以获得无毒菌株;利用根癌农杆菌诱导转化将抗虫peramine基因导入到只产生有益生物碱janthitrems的菌株中,获得了可以同时产生这两种抗虫生物碱的菌株(Spangenberg G,个人通讯)。通过生物技术创制的菌株接回原始分离寄主则可以减少内生真菌与寄主不融合性的出现。
表1 用于多年生黑麦草和高羊茅育种的新内生真菌菌株
Table 1 Novel endophytes strains and their outcomes

内生真菌Endophyticfungi菌株Strain产碱特性Alkaliproperty生产中表现Performanceinproduction参考文献Reference多年生黑麦草Perennialryegrass,内生真菌E.festucaevar.loliiAR1吡咯并吡嗪类化合物Peramine无黑麦草蹒跚症,提高家畜体重。Noryegrassstaggers,improvelivestockbodyweight.[141]提高种子产量和萌发率。Improveseedsproductionandgermination.[142]无黑麦草蹒跚症,并提高牧草产量和家畜奶产量。Noryegrassstaggers,improveforageandlivestockmilkproduction.[143]AR37Janthitrems无黑麦草蹒跚症,提高寄主对害虫Wiseanacervinata的抗性。Noryegrassstaggers,improvehostresistancetopest-Wiseanacervinata.[144]提高牧草产量和草地持久性。Improveforageproductionandgrasslandpersistence.[145]NEA2较少的吲哚双萜类,麦角碱类和吡咯并吡嗪类化合物lowlevelsofLolitremB+Ergot+Peramine提高寄主对阿根廷茎象甲的抗性。ImprovehostresistancetoListronotusbonariensis.[146]接种到不同黑麦草品种中,生物碱含量有变化。Alkaloidscontentsvariedinoculatedintodifferenthostcultivar.[100]Endosafe麦角碱类和吡咯并吡嗪类化合物Ergot+Peramine无黑麦草蹒跚症,提高对阿根廷茎象甲的抗性。Noryegrassstaggers,im-provehostresistancetoListronotusbonariensis.[141]Endo5麦角碱类和吡咯并吡嗪类化合物Ergot+Peramine提高寄主抗虫性。Improvehostresistancetopest.[137]AR6麦角碱类和吡咯并吡嗪类化合物Ergot+Peramine无黑麦草蹒跚症,提高对阿根廷茎象甲的抗性。Noryegrassstaggers,im-provehostresistancetoListronotusbonariensis.[147]AR95吲哚双萜类,麦角碱类和吡咯并吡嗪类化合物LolitremB+Ergot+Peramine在机场草坪中,减少昆虫和鸟的采食。Birdandwildlifedeterrentinryegrasssportfields.[140]高羊茅Tallfescue,内生真菌E.coenophi-alaAR542,AR548吡咯并吡嗪类和饱和吡咯化合物Peramine+Lolines无狐茅中毒症,提高牧草产量,草地持久性,提高家畜体重。Nofescuetoxico-sis,improveforageproduction,grasslandpersistenceandlivestockbodyweight.[139]提高寄主对蚜虫的抗性。ImprovehostresistancetoRhopalosiphumpadi.[136]ArkPlus吡咯并吡嗪类和饱和吡咯化合物Peramine+Lolines无狐茅中毒症,提高家畜体重。Nofescuetoxicosis,improvelivestockbodyweight.[148]AR601麦角碱类和饱和吡咯化合物Ergot+Lolines在机场草坪中,减少昆虫和鸟的采食。Birdandwildlifedeterrentinryegrasssportfields.[140]
7 展望
经过多年的努力,内生真菌的研究已经获得了显著的成果,发现了越来越多的Epichloё属内生真菌,这将有助于整体认识和理解内生真菌的系统发育过程,进行优良禾草的选育和改良,加强对内生真菌资源的保护。但是除了高羊茅和黑麦草内生真菌,其余菌株还需深入研究其进化起源以及与寄主植物的关系。有关内生真菌在生态系统水平对植物群落结构、生产力和多样性影响的研究还较少,为更好利用内生真菌保护草原生态系统的稳定性,需要加强了解内生真菌在植物和真菌进化过程中的作用和在天然草地群落中的作用。
由于禾本科植物内生真菌对寄主植物品质及抗逆性的影响,内生真菌已经广泛应用到牧草和草坪草育种中,而小麦(Triticumaestivum)、水稻(Oryzasativa)等粮食作物也属于禾本科植物,新西兰研究者尝试通过人工接种,将小麦族植物中分离的内生真菌转接到小麦、大麦(Hordeumvulgare)等麦类作物中,但是由于寄主特异性和不融合性,尚未获得成功,不能直接用于粮食作物中[151]。因此,应进一步扩大内生真菌资源调查的范围,尤其是从冷季型草坪草扩宽到暖季型植物,以期发现更多可以在粮食生产中应用的内生真菌。同时,应尽快解决内生真菌与寄主不融合性的问题,借助基因组学和代谢组学阐明真菌与植物之间信号转导和物质交流过程,从而利用基因组编辑技术创制兼容性无毒菌株,为内生真菌应用到粮食作物育种,保障粮食安全奠定基础。
[1] Nan Z B, Li C J. Roles of the grass-Neotyphodiumassociation in pastoral agriculture systrems. Acta Ecologica Sinica, 2004, 24(3): 605-616.
[2] Bacon C W, Porter J K, Robbins J D,etal.Epichloё typhina from toxic tall fescue grasses. Applied and Environmental Microbiology, 1977, 34(5): 576-581.
[3] Fletcher L R, Harvey I C. An association of aLoliumendophyte with ryegrass staggers. New Zealand Veterinary Journal, 1981, 29(10): 185-186.
[4] Young C A, Hume D E, Mcculley R L. Forages and pastures symposium: Fungal endophytes of tall fescue and perennial ryegrass: Pasture friend or foe. Journal of Animal Science, 2013, 91(5): 2379-2394.
[5] Johnson L J, Bonth A C M, Briggs L R,etal. The exploitation ofepichloaeendophytes for agricultural benefit. Fungal Diversity, 2013, 60(1): 171-188.
[6] Schardl C L, Young C A, Hesse U,etal. Plant-symbiotic fungi as chemical engineers: multi-genome analysis of the clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genet, 2013, 9(2): e1003323.
[7] Leuchtmann A, Bacon C W, Schardl C L,etal. Nomenclatural realignment ofNeotyphodiumspecies with genusEpichloё. Mycologia, 2014, 106(2): 202-215.
[8] Siegel M R, Bush L P. Toxin Production in Grass/Endophyte Associations[M]//Carroll G C, Tudzynski P. The Mycota V. Plant Relationships, Part B. Berlin: Heidelberg Springer, 1997: 185-208.
[9] Bush L P, Wilkinson H H, Schardl C L. Bioprotective alkaloids of grass-fungal endophyte symbioses. Plant Physiology, 1997, 114(1): 1-7.
[10] Prestidge R A. Causes and control of perennial ryegrass staggers in New Zealand. Agriculture, Ecosystems & Environment, 1993, 44(1): 283-300.
[11] Schmidt S P, Osborn T G. Effects of endophyte-infected tall fescue on animal performance. Agriculture, Ecosystems & Environment, 1993, 44(1): 233-262.
[12] Paterson J, Forcherio C, Larson B,etal. The effects of fescue toxicosis on beef cattle productivity. Journal of Animal Science, 1995, 73(3): 889-898.
[13] Faeth S H, Bultman T L. Endophytic Fungi and Interactions Among Host Plants, Herbivores and Natural Enemies[M]//Tscharntke T, Hawkins B A. Multitrophic level interactions. Cambridge: Cambridge University Press, 2002: 89-123.
[14] Clay K, Holah J. Fungal endophyte symbiosis and plant diversity in successional fields. Science, 1999, 285: 1742-1744.
[15] Faeth S H, Sullivan T J. Mutualistic asexual endophytes in a native grass are usually parasitic. The American Naturalist, 2003, 161(2): 310-325.
[16] Latch G C M. Physiological interactions of endophytic fungi and their hosts. Biotic stress tolerance imparted to grasses by endophytes. Agriculture, Ecosystems & Environment, 1993, 44(1): 143-156.
[17] Eerens J P J, Visker M H P W, Lucas R J,etal. Influence of the ryegrass endophyte (Neotyphodiumlolii) in a cool moist environment. IV. Plant parasitic nematodes. New Zealand Journal of Agricultural Research, 1998, 41(2): 209-217.
[18] Chu-Chou M, Guo B, An Z Q,etal. Suppression of mycorrhizal fungi in fescue by theAcremoniumcoenophialumendophtyes. Soil Biology and Biochemistry, 1992, 24(7): 633-637.
[19] Guo B Z, Hendrix J, An Z Q,etal. Role ofAcremoniumendophyte of fescue on inhibition of colonisation and reproduction of mycorrhizal fungi. Mycologia, 1992, 84(6): 882-885.
[20] West C P, Gwinn K D. Role ofAcremoniumin Drought, Pest, and Disease Tolerance of Grasses[C]//Hume D E. Proceeding of the 2nd International Symposium onAcremonium/Grass Interactions. Palmerstown North: New Zealand Grassland Association, 1993: 3-5.
[21] Nan Z B, Li C J.Neotyphodiumin Native Grasses in China and Observations on Endophyte/Host Interactions[C]//Paul V H, Dapprich P D. Proceedings of 4th InternationalNeotyphodium/Grass Interactions Symposium. Soest, 2000: 41-55.
[22] Tian P, Nan Z B, Li C J,etal. Effect of the endophyteNeotyphodiumloliion susceptibility and host physiological response of perennial ryegrass to fungal pathogens. European Journal of Plant Pathology, 2008, 122(4): 593-602.
[23] Ma M Z, Nan Z B. Effect of fungal endophytes against rust disease of perennial ryegrass (Loliumperenne) on growth and physiological indices. Acta Prataculturae Sinica, 2011, 20(6): 150-156.
[24] Bacon C W. Abiotic stress tolerances (moisture, nutrients) and photosynthesis in endophyte-infected tall fescue. Agriculture, Ecosystems & Environment, 1993, 44(1): 123-141.
[25] Malinowski D P, Belesky D P. Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Science, 2000, 40(4): 923-940.
[26] Matthews J W, Clay K. Influence of fungal endophyte infection on plant-soil feedback and community interactions. Ecology, 2001, 82(2): 500-509.
[27] Li X Z, Fang A G, Li C J,etal. Advances in the researches on the effects of grass endophytes on other microbes. Acta Ecologica Sinica, 2015, 35(6): 1660-1671.
[28] Wang Z W, Chen Y G, Wang Q C,etal. Progresses and perspectives of studies on plant endophytic microbes in China. Microbiology China, 2014, 41(3): 482-496.
[29] Wang Z W, Ji Y L, Chen Y G. Grass endophytes and their potential applications in agriculture. Journal of Nanjing Agricultural University, 2011, 34(5): 144-154.
[30] Zhang X X, Nan Z B, Li C J. Research progress of improved resistance of the grass to the heavy metal stress by endophyte. Pratacultural Science, 2014, 31(8): 1466-1474.
[31] Kaiser W J, Bruehl G W, Davitt C M,etal.Acremoniumisolates fromStiparobusta. Mycologia, 1996, 88(4): 539-547.
[32] Li C J, Nan Z B, Volker H P,etal. A newNeotyphodiumspecies symbiotic with drunken horse grass (Achnatheruminebrians) in China. Mycotaxon, 2004, 90(1): 141-147.
[33] Ji Y L, Zhan L H, Kang Y,etal. A new stromata-producingNeotyphodiumspecies symbiotic with clonal grassCalamagrostisepigeios(L.) Roth. grown in China. Mycologia, 2009, 101(2): 200-205.
[34] Chen L, Li X Z, Li C J,etal. Two distinctEpichloё species symbiotic withAchnatheruminebrians, drunken horse grass. Mycologia, 2015, 107(4): 15-19.
[35] Jin W J, Li C J, Wang Z F. Research advances on diversity of grassEpichloё endophytes. Acta Prataculturae Sinica, 2015, 24(1): 168-175.
[36] Faeth S H, Helander M L, Saikkonen K T. AsexualNeotyphodiumendophytes in a native grass reduce competitive abilities. Ecology Letters, 2004, 7(4): 304-313.
[37] Tibbets T M, Faeth S H.Neotyphodiumendophytes in grasses: deterrents or promoters of herbivory by leaf-cutting ants. Oecologia, 1999, 118(3): 297-305.
[38] Cheplick G P, Perera A, Koulouris K. Effect of drought on the growth ofLoliumperennegenotypes with and without fungal endophytes. Functional Ecology, 2000, 14(6): 657-667.
[39] Cheplick G P. Recovery from drought stress inLoliumperenne(Poaceae): are fungal endophytes detrimental. American Journal of Botany, 2004, 91(12): 1960-1968.
[40] Jia T, Ren A Z, Wei M Y,etal. Effects of endophyte transmission on ecophysiological characteristics ofAchnatherumsibiricum. Chinese Journal of Plant Ecology, 2015, 39(1): 72-80.
[41] Hoveland C S. Importance and economic significance of theAcremoniumendophytes to performance of animals and grass plant. Agriculture, Ecosystems & Environment, 1993, 44(1): 3-12.
[42] Joost R E.Acremoniumin fescue and ryegrass: boon or bane? A review. Journal of Animal Science, 1995, 73(3): 881-888.
[43] Schardl C L, Leuchtmann A, Spiering M J. Symbioses of grasses with seedborne fungal endophytes. Annual Review of Plant Biology, 2004, 55: 315-340.
[44] Siegel M R, Latch G C, Johnson M C. Fungal endophytes of grasses. Annual Review of Phytopathology, 1987, 25(1): 293-315.
[45] Li C J, Nan Z B, Zhang C J,etal. Effects of drunken horse grass infected with endophyte on Chinese rabbit. Journal of Agricultural Science and Technology, 2009, 11(2): 90.
[46] Mackintosh C G, Orr M B, Gallagher R T,etal. Ryegrass staggers in Canadian Wapiti deer. New Zealand Veterinary Journal, 1982, 30(7): 106-107.
[47] Alabdouli K, Blythe L, Duringer J,etal. Physiological effects of endophyte-infected perennial ryegrass straw on female camels in the Middle East. Emirates Journal of Food and Agriculture, 2014, 26(1): 82-92.
[48] Reed K, Vaughan J, Cummins L,etal. Impact of mycotoxins and of a mycotoxin deactivator on alpacas grazing perennial ryegrass infected with wild endophyte (Neotyphodiumspp.). Animal Production Science, 2010, 50(9): 902-908.
[49] Browning R. Effect of the wild-type tall fescue endophyte on growth rate and feed consumption in nulliparous meat goat does. Small Ruminant Research, 2012, 105(1): 29-32.
[50] Di Menna M, Finch S, Popay A,etal. A review of theNeotyphodiumlolii/Loliumperennesymbiosis and its associated effects on animal and plant health, with particular emphasis on ryegrass staggers. New Zealand Veterinary Journal, 2012, 60(6): 315-328.
[51] Prestidge R A, Pottinger R P, Barker G M. An Association ofLoliumEndophyte with Ryegrass Resistance to Argentine Stem Weevil[C]//New Zealand Weed and Pest Control Society. Proceedings of the 35th New Zealand Weed and Pest Control Conference. Hamilton, New Zealand, 1982: 199-222.
[52] Ball O J P, Christensen M J, Prestidge R A. Effect of Selected Isolates ofAcremoniumEndophytes on Adult Black Beetle (Heteronychusarator) Feeding[C]//New Zealand Plant Pretection Society. Proceedings of the New Zealand Plant Protection Conference. New Zealand: Waitangi, 1994: 227.
[53] Porter J K. Analysis of endophyte toxins: fescue and other grasses toxic to livestock. Journal of Animal Science, 1995, 73(3): 871-880.
[54] Richmond D S, Kunkel B A, Somasekhar N,etal. Top-down and bottom-up regulation of herbivores:Spodopterafrugiperdaturns tables on endophyte-mediated plant defence and virulence of an entomopathogenic nematode. Ecological Entomology, 2004, 29(3): 353-360.
[55] Tapper B A, Lane G A. Janthitrems Found in aNeotyphodiumEndophyte of Perennial Ryegrass[C]//Kallenbach R, Rosenkrans C F, Lock T R. 5th International Symposium onNeotyphodium/Grass Interactions. Arkansas: Fayetteville, 2004: 301.
[56] Popay A J, Gerard P J. Cultivar and Endophyte effects on a root aphid,Aploneuralentisci, in perennial ryegrass. New Zealand Plant Protection, 2007, 60: 223-227.
[57] Fuchs B, Krischke M, Mueller M J,etal. Peramine and lolitrem b from endophyte-grass associations cascade up the food chain. Journal of Chemical Ecology, 2013, 39(11/12): 1385-1389.
[58] Omacini M, Chaneton E J, Ghersa C M,etal. Symbiotic fungal endophytes control insect host-parasite interaction webs. Nature, 2001, 409: 78-81.
[59] Li T, Blande J D, Gundel P E,etal.Epichloё endophytes alter inducible indirect defences in host grasses. Plos One, 2014, 9(6): e101331.
[60] Bultman T L, Rodstrom J L, Radabaugh K R,etal. Influence of genetic variation in the fungal endophyte of a grass on an herbivore and its parasitoid. Entomologia Experimentalis et Applicata, 2009, 130(2): 173-180.
[61] Bultman T L, Mcneill M R, Goldson S L. Isolate-dependent impacts of fungal endophytes in a multitrophic interaction. Oikos, 2003, 102(3): 491-496.
[62] Richmond D S, Bigelow C A. Variation in endophyte-plant associations influence black cutworm (lepidoptera: noctuidae) performance and susceptibility to the parasitic nematodeSteinernemacarpocapsae. Environmental Entomology, 2009, 38(4): 996-1004.
[63] Miyazaki S, Ikeda T, Hanazumi M,etal. Toxicological Evaluation of Endophyte-infected Perennial Ryegrass Straw to Japanese Black Steers[C]//Proceedings of the 6th International Symposium on Fungal Endophytes of Grasses. Christchurch, New Zealand: New Zealand Grassland Association, 2007: 415-418.
[64] Finch S, Thom E, Babu J,etal. The evaluation of fungal endophyte toxin residues in milk. New Zealand Veterinary Journal, 2013, 61(1): 11-17.
[65] Finch S, Fletcher L, Babu J. The evaluation of endophyte toxin residues in sheep fat. New Zealand Veterinary Journal, 2012, 60(1): 56-60.
[66] Tian P. Interactions of Ryegrass,Neotyphodiumloliiand Several Plant Pathogenic Fungi[D]. Lanzhou: Lanzhou University, 2009.
[67] Bacon C W, Richardson M D, White J F. Modification and uses of endophyte-enhanced turfgrasses: a role for molecular technology. Crop Science, 1997, 37(37): 1415-1425.
[68] Lewis G C. Effect of cutting height on perennial ryegrass with and without infection with endophyte and ryegrass mosaic virus. Iobc Wprs Bulletin, 1996, 19: 55-58.
[69] Guy P L, Davis L T. Variation in the incidence of Barley yellow dwarf virus and in the ability ofNeotyphodiumendophytes to deter feeding by aphids (Rhopalosiphumpadi) on Australasian tall fescue. Australasian Plant Pathology, 2002, 31(3): 307-308.
[70] Márquez L M, Redman R S, Rodriguez R J,etal. A virus in a fungus in a plant: three-way symbiosis required for thermal tolerance. Science, 2007, 315: 513-515.
[71] Herrero N, Márquez S S, Zabalgogeazcoa I. Mycoviruses are common among different species of endophytic fungi of grasses. Archives of Virology, 2009, 154(2): 327-330.
[72] Romo M, Leuchtmann A, García B,etal. A totivirus infecting the mutualistic fungal endophyteEpichloёfestucae. Virus Research, 2007, 124(1): 38-43.
[73] Marulanda A, Azcon R, Ruiz-Lozano J M. Contribution of six arbuscular mycorrhizal fungal isolates to water uptake byLactucasativaplants under drought stress. Physiologia Plantarum, 2003, 119(4): 526-533.
[74] Yang G W, Liu N, Yang X,etal. Relationship between arbuscular mycorrhizal fungi and individual plant and their effects on plant productivity and species diversity of plant community. Acta Prataculturae Sinica, 2015, 24(6): 188-203.
[75] Müller J. Artificial infection by endophytes affects growth and mycorrhizal colonisation ofLoliumperenne. Functional Plant Biology, 2003, 30(4): 419-424.
[76] Novas M V, Iannone L J, Godeas A M,etal. Evidence for leaf endophyte regulation of root symbionts: effect ofNeotyphodiumendophytes on the pre-infective state of mycorrhizal fungi. Symbiosis, 2011, 55(1): 19-28.
[77] Larimer A L, Bever J D, Clay K. The interactive effects of plant microbial symbionts: a review and meta-analysis. Symbiosis, 2010, 51(2): 139-148.
[78] Liu Q H, Parsons A J, Xue H,etal. Competition between foliarNeotyphodiumloliiendophytes and mycorrhizalGlomusspp. fungi inLoliumperennedepends on resource supply and host carbohydrate content. Functional Ecology, 2011, 25(4): 910-920.
[79] Vicari M, Hatcher P E, Ayres P G. Combined effect of foliar and mycorrhizal endophytes on an insect herbivore. Ecology, 2002, 83(9): 2452-2464.
[80] Mcnear D H Jr, Mcculley R L. Influence of theNeotyphodium——Tall fescue symbiosis on belowground processes. Plant and Soil Sciences Faculty Publications, 2012, http: //uknowledge.uky.edu/pss_facpub/44.
[81] Casas C, Omacini M, Montecchia M S,etal. Soil microbial community responses to the fungal endophyteNeotyphodiumin Italian ryegrass. Plant and Soil, 2011, 340(1/2): 347-355.
[82] Iqbal J, Siegrist J A, Nelson J A,etal. Fungal endophyte infection increases carbon sequestration potential of southeastern USA tall fescue stands. Soil Biology and Biochemistry, 2012, 44(1): 81-92.
[83] Roberts E L, Ferraro A. Rhizosphere microbiome selection byEpichloё endophytes ofFestucaarundinacea. Plant and Soil, 2015, 396(1/2): 229-239.
[84] Nourbakhsh F, Abbasi S, Mirlohi A,etal. Endophyte (Epichloёcoenophiala) symbiosis increased enzyme activities in the rhizosphere of tall fescue under greenhouse condition. Advances in Environmental Biology, 2014, 8(22): 380-384.
[85] Van Hecke M M, Treonis A M, Kaufman J R. How does the fungal endophyteNeotyphodiumcoenophialumaffect tall fescue (Festucaarundinacea) rhizodeposition and soil microorganisms. Plant and Soil, 2005, 275(1/2): 101-109.
[86] Franzluebbers A, Stuedemann J. Soil carbon and nitrogen pools in response to tall fescue endophyte infection, fertilization, and cultivar. Soil Science Society of America Journal, 2005, 69(2): 396-403.
[87] Rudgers J A, Clay K. Endophyte symbiosis with tall fescue: how strong are the impacts on communities and ecosystems. Fungal Biology Reviews, 2007, 21(2): 107-124.
[88] Saikkonen K, Gundel P, Helander M. Chemical ecology mediated by fungal endophytes in grasses. Journal of Chemical Ecology, 2013, 39(7): 962-968.
[89] Coley A B, Fribourg H A, Pelton M R,etal. Effects of tall fescue endophyte infestation on relative abundance of small mammals. Journal of Environmental Quality, 1995, 24(3): 472-475.
[90] Rudgers J A, Clay K. An invasive plant-fungal mutualism reduces arthropod diversity. Ecology Letters, 2008, 11(8): 831-840.
[91] Yao X, Christensen M J, Bao G,etal. A toxic endophyte-infected grass helps reverse degradation and loss of biodiversity of over-grazed grasslands in northwest China. Scientific Reports, 2015, 5: 18527.
[92] Guo J, Mcculley R L, Mcnear Jr D H. Tall fescue cultivar and fungal endophyte combinations influence plant growth and root exudate composition. Frontiers in Plant Science, 2015, 6: 183.
[93] Wakelin S, Harrison S, Mander C,etal. Impacts of endophyte infection of ryegrass on rhizosphere metabolome and microbial community. Crop and Pasture Science, 2015, 66(10): 1049-1057.
[94] Iqbal J, Nelson J A, Mcculley R L. Fungal endophyte presence and genotype affect plant diversity and soil-to-atmosphere trace gas fluxes. Plant and Soil, 2013, 364(1/2): 15-27.
[95] Mcculley R L. Does Aboveground Fungal Endophyte Genotype Similarly Affect Belowground Arbuscular Mycorrhizal Fungal Colonization of Tall Fescue Roots and Associated Plant and Soil Parameters[C]//9th International Symposium on Fungal Endophytes of Grasses and 1st International Symposium on Plant Microbiomes. Australian: Melbourne, 2015: 52.
[96] Rasmussen S, Parsons A, Newman J A. Metabolomics analysis of theLoliumperenne—Neotyphodiumloliisymbiosis: more than just alkaloids. Phytochemistry Reviews, 2009, 8(3): 535-550.
[97] Schardl C L, Young C A, Faulkner J R,etal. Chemotypic diversity of epichloae, fungal symbionts of grasses. Fungal Ecology, 2012, 5(3): 331-344.
[98] Ekanayake P, Rabinovich M, Guthridge K,etal. Phylogenomics of fescue grass-derived fungal endophytes based on selected nuclear genes and the mitochondrial gene complement. BMC Evolutionary Biology, 2013, 13: 270.
[99] Hettiarachchige I K, Ekanayake P N, Mann R C,etal. Phylogenomics of asexualEpichloё fungal endophytes forming associations with perennial ryegrass. BMC Evolutionary Biology, 2015, 15: 72.
[100] Van Zijll De Jong E, Dobrowolski M P, Sandford A,etal. Detection and characterisation of novel fungal endophyte genotypic variation in cultivars of perennial ryegrass (LoliumperenneL.). Australian Journal of Agricultural Research, 2008, 59(3): 214-221.
[101] Van Zijll De Jong E, Dobrowolski M P, Bannan N,etal. Global genetic diversity of the perennial ryegrass fungal endophyteNeotyphodiumlolii. Crop Science, 2008, 48(4): 1487-1501.
[102] Charlton N D, Shoji J Y, Ghimire S R,etal. Deletion of the fungal gene soft disrupts mutualistic symbiosis between the grass endophyte epichloё festucae and the host plant. Eukaryotic Cell, 2012, 11(12): 1463-1471.
[103] Panaccione D G, Johnson R D, Wang J,etal. Elimination of ergovaline from a grass-Neotyphodiumendophyte symbiosis by genetic modification of the endophyte. Proceedings of the National Academy of Sciences of the United States of America, 2001, 98(22): 12820-12825.
[104] Young C A, Felitti S, Shields K,etal. A complex gene cluster for indole-diterpene biosynthesis in the grass endophyteNeotyphodiumlolii. Fungal Genet Biology, 2006, 43(10): 679-693.
[105] Fleetwood D J, Scott B, Lane G A,etal. A complex ergovaline gene cluster inEpichloё endophytes of grasses. Applied and Enviromental Microbiology, 2007, 73(8): 2571-2579.
[106] Young C A, Tapper B A, May K,etal. Indole-diterpene biosynthetic capability ofEpichloё endophytes as predicted byltmgene analysis. Applied and Environmental Microbiology, 2009, 75(7): 2200-2211.
[107] Takach J E, Mittal S, Swoboda G A,etal. Genotypic and chemotypic diversity ofNeotyphodiumendophytes in tall fescue from greece. Applied and Environmental Microbiology, 2012, 78(16): 5501-5510.
[108] Charlton N D, Craven K D, Afkhami M E,etal. Interspecific hybridization and bioactive alkaloid variation increases diversity in endophyticEpichloё species ofBromuslaevipes. FEMS Microbiology Ecology, 2014, 90(1): 276-289.
[109] Pan J, Bhardwaj M, Faulkner J R,etal. Ether bridge formation in loline alkaloid biosynthesis. Phytochemistry, 2014, 98: 60-68.
[110] Felitti S, Shields K, Ramsperger M,etal. Transcriptome analysis ofNeotyphodiumandEpichloё grass endophytes. Fungal Genetics and Biology, 2006, 43(7): 465-475.
[111] Dinkins R D, Barnes A, Waters W. Microarray analysis of endophyte-infected and endophyte-free tall fescue. Journal of Plant Physiology, 2010, 167(14): 1197-1203.
[112] Johnson L J, Johnson R D, Schardl C L,etal. Identification of differentially expressed genes in the mutualistic association of tall fescue withNeotyphodiumcoenophialum. Physiological and Molecular Plant Pathology, 2003, 63(6): 305-317.
[113] Eaton C J, Cox M P, Ambrose B,etal. Disruption of signaling in a fungal-grass symbiosis leads to pathogenesis. Plant Physiology, 2010, 153(4): 1780-1794.
[114] Eaton C J, Dupont P Y, Solomon P,etal. A core gene set describes the molecular basis of mutualism and antagonism inEpichloё spp. Molecular Plant-Microbe Interactions, 2015, 28(3): 218-231.
[115] Dupont P Y, Eaton C J, Wargent J J,etal. Fungal endophyte infection of ryegrass reprograms host metabolism and alters development. New Phytologist, 2015, 208(4): 1227-1240.
[116] Tanaka A, Christensen M J, Takemoto D,etal. Reactive oxygen species play a role in regulating a fungus-perennial ryegrass mutualistic interaction. Plant Cell, 2006, 18(4): 1052-1066.
[117] Tanaka A, Takemoto D, Hyon G S,etal. NoxA activation by the small GTPase RacA is required to maintain a mutualistic symbiotic association betweenEpichloёfestucaeand perennial ryegrass. Molecular Microbiology, 2008, 68(5): 1165-1178.
[118] Takemoto D, Tanaka A, Kayano Y,etal. Reactive Oxygen as a Signal in Grass-Epichloё Symbiosis[C]//Young C A, Aiken G E, McCulley R L,etal. Epichloae, Endophytes of Cool Season Grasses: Implications, Utilization and Biology. Proceedings of the 7th International Symposium on Fungal Endophytes of Grasses. USA: Lexington, Kentucky, 2012: 109-112.
[119] Scott B, Eaton C J. Role of reactive oxygen species in fungal cellular differentiations. Current Opinion in Microbiology, 2008, 11(6): 488-493.
[120] Tanaka A, Cartwright G M, Saikia S,etal. ProA, a transcriptional regulator of fungal fruiting body development, regulates leaf hyphal network development in theEpichloёfestucae-Loliumperennesymbiosis. Molecular Microbiology, 2013, 90(3): 551-568.
[121] Koulman A, Lee T V, Fraser K,etal. Identification of extracellular siderophores and a related peptide from the endophytic fungusEpichloёfestucaein culture and endophyte-infectedLoliumperenne. Phytochemistry, 2012, 75: 128-139.
[122] Rasmussen S, Parsons A J, Fraser K,etal. Metabolic profiles ofLoliumperenneare differentially affected by nitrogen supply, carbohydrate content, and fungal endophyte infection. Plant Physiology, 2008, 146(3): 1440-1453.
[123] Nagabhyru P, Dinkins R D, Wood C L,etal. Tall fescue endophyte effects on tolerance to water-deficit stress. BMC Plant Biology, 2013, 13: 127.
[124] Zhou L Y. Physio-Biochemical Mechanism ofFestucasinensis—Epichloё Symbiossis Exposure to Cold Stress[D]. Lanzhou: Lanzhou University, 2015.
[125] Lehner A F, Craig M, Fannin N,etal. Electrospray[+] tandem quadrupole mass spectrometry in the elucidation of ergot alkaloids chromatographed by HPLC: screening of grass or forage samples for novel toxic compounds. Journal of Mass Spectrometry, 2005, 40(11): 1484-1502.
[126] Browning R, Leite-Browning M L. Effect of ergotamine and ergonovine on thermal regulation and cardiovascular function in cattle. Journal of Animal Science, 1997, 75(1): 176-181.
[127] Larson B T, Harmon D L, Piper E L,etal. Alkaloid binding and activation of D2dopamine receptors in cell culture. Journal of Animal Science, 1999, 77(4): 942-947.
[128] Moubarak A S, Johnson Z B, Rosenkrans Jr C F. Antagonistic effects of simultaneous exposure of ergot alkaloids on kidney adenosine triphosphatase system.InVitroCellular & Developmental Biology-Animal, 2003, 39(8/9): 395-398.
[129] Guerre P. Ergot alkaloids produced by endophytic fungi of the genusEpichloё. Toxins, 2015, 7(3): 773-790.
[130] De Battista J P, Bouton J H, Bacon C W,etal. Rhizome and herbage production of endophyte-removed tall fescue clones and populations. Agronomy Journal, 1990, 82(4): 651-654.
[131] Koshino H, Yoshihara T, Ichihara A,etal. Two sphingoid derivatives from stromata ofEpichloetyphinA onPhleumpratense. Phytochemistry, 1992, 31(11): 3757-3759.
[132] Niones J T, Takemoto D. An isolate ofEpichloёfestucae, an endophytic fungus of temperate grasses, has growth inhibitory activity against selected grass pathogens. Journal of General Plant Pathology, 2014, 80(4): 337-347.
[133] Song Q Y, Nan Z B, Gao K,etal. Antifungal, phytotoxic, and cytotoxic activities of metabolites fromEpichloёbromicola, a fungus obtained fromElymustangutorumgrass. Journal of Agricultural and Food Chemistry, 2015, 63(40): 8787-8792.
[134] Fletcher L R. Managin Ryegrass-Endophyte Toxicoses[M]//Roberts C A, West C P, Spiers D E.Neotyphodiumin Cool-Season Grasses. Blackwell, 2005: 229-241.
[135] Reed K F M, Nie Z N, Walker L V,etal. Fluctuations in the concentration of ergovaline and lolitrem B produced by the wild-type endophyte (Neotyphodiumlolii) in perennial ryegrass (Loliumperenne) pasture. Animal Production Science, 2011, 51(12): 1098-1108.
[136] Hunt M G, Newman J A. Reduced herbivore resistance from a novel grass-endophyte association. Journal of Applied Ecology, 2005, 42(4): 762-769.
[137] Milne G. Technology Transfer of Novel Ryegrass Endophytes in New Zealand[C]//Proceedings 6th International Symposium on Fungal Endophytes of Grasses. New Zealand, Christchurch: Grassland Research and Practice Series, 2007: 237-239.
[138] Kaur J, Ekanayake P, Tian P,etal. Discovery and characterisation of novel asexualEpichloё endophytes from perennial ryegrass (LoliumperenneL.). Crop and Pasture Science, 2015, 66(10): 1058-1070.
[139] Gunter S A, Beck P A. Novel endophyte-infected tall fescue for growing beef cattle. Journal of Animal Science, 2004, 82(13_suppl): 75-82.
[140] Pennell C G L, Rolston M P. Novel Uses of Grass Endophyte Technology[C]//The 8thInternational Symposium on Fungal Endophyte of Grasses. Lanzhou, 2012: 211-214.
[141] Fletcher L. “Non-toxic” endophytes in ryegrass and their effect on livestock health and production. Ryegrass endophyte: an essential New Zealand symbiosis. Grassland Research and Practice Series, 1999, 7: 133-139.
[142] Popay A, Marshall S, Baltus J. Endophyte infection influences disappearance of perennial ryegrass seed. New Zealand Plant Protection, 2000, 23: 398-405.
[143] Bluett S J, Thom E R, Clark D A,etal. Effects of perennial ryegrass infected with either AR1 or wild endophyte on dairy production in the Waikato. New Zealand Journal of Agricultural Research, 2005, 48(2): 197-212.
[144] Jensen J G, Popay A J. Perennial ryegrass infected with AR37 endophyte reduces survival of porina larvae. New Zealand Plant Protection, 2004, 57: 323.
[145] Hume D E, Popay A J, Cooper B M,etal. Effect of a Novel Endophyte on the Productivity of Perennial Ryegrass (Loliumperenne) in New Zealand[C]//5th International Symposium onNeotyphodium/Grass Interactions. USA: Fayetteville, Arkansas, Poster, 2004.
[146] Edwards G R, Bryant R H. What Perennial Ryegrass Should You Sow[C]//South Island Dairy Event (SIDE) Conference. Canterbury: Lincoln University, 2011: 27-29.
[147] Popay A J, Hume D E, Mainland R A,etal. Field resistance to Argentine stem weevil (Listronotusbonariensis) in different ryegrass cultivars infected with an endophyte deficient in lolitrem B. New Zealand Journal of Agricultural Research, 1995, 38(4): 519-528.
[148] Nihsen M E, Piper E L, West C P,etal. Growth rate and physiology of steers grazing tall fescue inoculated with novel endophytes. Journal of Animal Science, 2004, 82(3): 878-883.
[149] Tapper B A, Latch G C M. Selection Against Toxin Production in Endophyte-infected Perennial Ryegrass[C]//Woodfield D R, Mattews C. Ryegrass Endophyte: An Essential New Zealand Symbiosis. Napier, New Zealand: New Zealand Grassland Association, 1999: 107-111.
[150] Popay A J, Hume D E, Davis K L,etal. Interactions between endophyte (Neotyphodiumspp.) and ploidy in hybrid and perennial ryegrass cultivars and their effects on Argentine stem weevil (Listronotusbonariensis). New Zealand Journal of Agricultural Research, 2003, 46(4): 311-319.
[151] Simpson W R, Faville M J, Moraga R A,etal.Epichloё fungal endophytes and the formation of synthetic symbioses in Hordeeae (=Triticeae) grasses. Journal of Systematics and Evolution, 2014, 52: 794-806.
[1] 南志标, 李春杰. 禾草内生真菌共生体在草地农业系统中的作用. 生态学报, 2004, 24(3): 605-616.
[23] 马敏芝, 南志标. 内生真菌对感染锈病黑麦草生长和生理的影响. 草业学报, 2011, 20(6): 150-156.
[27] 李秀璋, 方爱国, 李春杰, 等. 禾草内生真菌对其它微生物的影响研究进展. 生态学报, 2015, 35(6): 1660-1671.
[28] 王志伟, 陈永敢, 王庆璨, 等. 中国植物内生微生物研究的发展和展望. 微生物学通报, 2014, 41(3): 482-496.
[29] 王志伟, 纪燕玲, 陈永敢. 禾本科植物内生真菌及其在农业上的应用潜力. 南京农业大学学报, 2011, 34(5): 144-154.
[30] 张兴旭, 南志标, 李春杰. 内生真菌提高禾草耐重金属胁迫的研究进展. 草业科学, 2014, 31(8): 1466-1474.
[35] 金文进, 李春杰, 王正凤. 禾草内生真菌的多样性及意义. 草业学报, 2015, 24(1): 168-175.
[40] 贾彤, 任安芝, 魏茂英, 等. 不同传播方式的内生真菌感染对羽茅的生理生态影响. 植物生态学报, 2015, 39(1): 72-80.
[45] 李春杰, 南志标, 张昌吉, 等. 醉马草内生真菌对家兔的影响. 中国农业科技导报, 2009, 11(2): 90.
[66] 田沛. 多年生黑麦草, 内生真菌与数种植物病原真菌的互作[D]. 兰州: 兰州大学, 2009.
[74] 杨高文, 刘楠, 杨鑫, 等. 丛枝菌根真菌与个体植物的关系及其对群落生产力和物种多样性的影响. 草业学报, 2015, 24(6): 188-203.
[124] 周连玉. 基于代谢组学的中华羊茅-内生真菌共生体响应低温胁迫的生化机制[D]. 兰州: 兰州大学, 2015.
Advances in research on grass endophytes in agricultural systems and applications in forage breeding
TIAN Pei1*, ZHANG Guang-Ming2, NAN Zhi-Biao1
1.StateKeyLaboratoryofGrasslandAgro-ecosystems,CollegeofPastoralAgricultureScienceandTechnology,LanzhouUniversity,Lanzhou730020,China; 2.GuangdongYuemingElectricPowerEngineeringCo.Ltd,Zhuhai519000,China
TheEpichloё endophyte forms mutually beneficial associations with its hosts, which enhance their survival under abiotic and biotic stresses. Modern techniques in molecular biology, genomics, proteomics, metabolomics, and bioinformatics have accelerated research on endophytes. The diversity of secondary metabolites and the genes related to their biosynthesis have been identified and the molecular mechanisms of mutualism between theEpichloё endophyte and its hosts have been clarified. These endophytes are widely utilized in agriculture as they are animal-safe grass endophytes that improve the growth and stress resistance of their hosts, leading to increased pasture persistence, sustainability, and production. Thus, severalEpichloё endophytes have been developed and commercialized. In this paper, we review recent research on the interactions between grasses and endophytes, and the effects of endophytes on livestock and on microbial and pasture ecosystems. We also discuss the selection of endophyte strains and forage breeding based on multidisciplinary research.
endophyte; trophic cascade; ecosystem; microbe; genomics; forage breeding
10.11686/cyxb2016049
http://cyxb.lzu.edu.cn
2016-01-25;改回日期:2016-03-08
国家基础研究发展规划“973”(2014CB138702),国家自然科学基金项目(31502001)和企事业单位委托科技项目[(15)0065]资助。
田沛(1979-),女,河南新郑人,副教授,博士。 E-mail: tianp@lzu.edu.cn*通信作者Corresponding author.
田沛, 张光明, 南志标. 禾草内生真菌研究及应用进展. 草业学报, 2016, 25(12): 206-220.
TIAN Pei, ZHANG Guang-Ming, NAN Zhi-Biao. Advances in research on grass endophytes in agricultural systems and applications in forage breeding. Acta Prataculturae Sinica, 2016, 25(12): 206-220.

