Extranodal involvement in young patients with diffuse large B-cell lymphoma: distribution, prognostic value and treatment options
Shuna Yao, Junbo Li, Zhihua Yao, Yuanlin Xu, Junfeng Chu, Jiuyang Zhang, Shuiling Jin, Yangyang Huang, Jianbo Zhang, Jie Ma, Yan Zhao, Shujun Yang, Yanyan Liu
1Department of Internal Medicine, Henan Cancer Hospital & Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou 450008, China;2Department of Internal Medicine, the Second Affiliated Hospital of Zhengzhou University, Zhengzhou 450014, China;3Department of Pathology, Henan Cancer Hospital & Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou 450008, China
Extranodal involvement in young patients with diffuse large B-cell lymphoma: distribution, prognostic value and treatment options
Shuna Yao1*, Junbo Li2*, Zhihua Yao1*, Yuanlin Xu1, Junfeng Chu1, Jiuyang Zhang1, Shuiling Jin2, Yangyang Huang1, Jianbo Zhang3, Jie Ma3, Yan Zhao1, Shujun Yang1, Yanyan Liu1
1Department of Internal Medicine, Henan Cancer Hospital & Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou 450008, China;2Department of Internal Medicine, the Second Affiliated Hospital of Zhengzhou University, Zhengzhou 450014, China;3Department of Pathology, Henan Cancer Hospital & Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou 450008, China
Objective:Extranodal involvement represents a peculiar presentation of diffuse large B-cell lymphoma (DLBCL). Previous studies have suggested that older patients are more prone to extranodal involvement. This study retrospectively addressed the distribution, prognostic value and treatment options of extranodal involvement in young patients with DLBCL.
Diffuse large B-cell lymphoma; extranodal involvement; prognosis
View this article at: http://dx.doi.org/10.21147/j.issn.1000-9604.2017.01.07
Introduction
Diffuse large B-cell lymphoma (DLBCL) represents the most common type of lymphatic neoplasm and has obvious heterogeneity in its histology, immunology, genetics, molecular biology and clinical presentation (1). In fact, the significance of clinical presentation has been emphasized so emphatically that some subtypes of DLBCL have been separated based on presentation in the 2008 World Health Organization (WHO) lymphatic neoplasm classification (2), including primary mediastinal large B-cell lymphoma, DLBCL leg type, primary effusion lymphoma and primary central nervous system (CNS) lymphoma. The study retrospectively addressed extranodal involvement in young patients with DLBCL, which is a peculiar presentation compared to nodal disease.
The number of extranodal involvement sites has been defined as an independent prognostic factor in theinternational prognostic index (IPI) score model, aside from age, performance status, Ann Arbor stage and lactate dehydrogenase (LDH) level. However, the number of extranodal involvement sites is not included in the ageadjusted international prognostic index (aaIPI) model. Only performance status, Ann Arbor stage and LDH level are factors for young patients (3). The Ann Arbor staging is derived from Hodgkin’s lymphoma (HL) (4), and extranodal disease does not usually affect the prognosis of patients with nodal disease to the same anatomical extent because extranodal disease sites and contiguous nodal areas can be encompassed safely within the appropriate field for curative radiation therapy (5). As mentioned previously, extranodal involvement seems to have less significance in DLBCL based on traditional experience, especially in young patients. Nevertheless, recent studies have increasingly reported the importance of extranodal involvement in DLBCL. An analysis of the Surveillance, Epidemiology and End Results (SEER) database showed that approximately one-third of DLBCL patients presented with extranodal involvement that spread to 12 groups of anatomic sites (6). Extranodal sites have been reported to be associated with a distinct outcome in the analysis, and patients with extranodal sites tend to be older than patients with nodal DLBCL. Another study has even established an enhanced IPI (NCCN-IPI) formulation including involvement of the bone marrow, CNS, liver, gastrointestinal (GI) tract or lung as unfavorable factors. This formulation has proven to be a better prognostic tool than the traditional IPI for use in the rituximab era (7).
Young age is considered to be a favorable factor in patients with DLBCL (3), but refraction and relapse are still challenging. Although some studies have suggested that extranodal disease predominantly occurs in older patients, and certain sites are associated with a poorer prognosis (6,7), the conditions of extranodal involvement are unknown in young DLBCL patients. Rituximab, an anti-CD20 monoclonal antibody, has been used for the treatment of DLBCL due to its superior efficacy (8,9). Whether it can produce a positive effect on the outcome of patients with extranodal involvement has not been addressed. In addition, although radiotherapy has produced remarkable results for extranodal lesions in patients with HL (10), its significance is controversial in DLBCL with extranodal disease. This study was designed to retrospectively explore the distribution, prognostic value and treatment choices of extranodal involvement in young DLBCL patients. The effects of gender, aaIPI, the number and site of extranodal involvement, rituximab infusion and radiotherapy on patient outcomes were documented in the study.
Patients and methods
Patients and study design
This study retrospectively included patients who were hospitalized in the Department of Internal Medicine, Henan Cancer Hospital & Affiliated Cancer Hospital of Zhengzhou University from January 1, 2005 to July 1, 2012 and who were aged 16–59 years. These patients were newly diagnosed with DLBCL based on the 2008 WHO classification, and each was reviewed by at least two experienced pathologists. Enrolled patients received 4–6 cycles of systemic chemotherapy in the primary setting and salvage therapy at the time of relapse. Patients receiving autologous stem cell transplantation were excluded from the study. The patients’ clinical characteristics and treatment modalities were completely collected. The clinical characteristics included gender, performance status, Ann Arbor staging, LDH levels and aaIPI scores; and the treatment modalities consisted of rituximab infusion and radiotherapy. The study was approved by the Ethic Committee of Henan Cancer Hospital.
A total of 329 patients were enrolled in the study, including 195 males and 134 females, with a median age of 37.5 years. Among them, 294 had good performance status [Eastern Cooperative Oncology Group (ECOG) score 0–1], and 129 presented elevated LDH levels. Localized stage (I/II) was diagnosed in 213 cases, and advanced stage (III/IV) was diagnosed in 116 cases. Then, 254 patients were defined as having a good prognosis (aaIPI score 0–1), whereas 75 were defined as having a poor prognosis (aaIPI score 2–3) (Table 1). Rituximab was combined with chemotherapy in 59 cases, and 72 patients were given fieldinvolved radiotherapy.
The treatment response was assessed after every two cycles of chemotherapy using computed tomography (CT) or18F-fluorodeoxyglucose positron emission tomography (PET)/CT according to the Cheson criteria or the revised response criteria, which included PET (11,12). The followup time of these patients was from the time of diagnosis to July 1, 2014, with a median time of 68.5 (24–113) months. Progression-free survival (PFS) and overall survival (OS) were calculated. PFS was defined as the time from diagnosis to primary treatment failure, relapse or final follow-up. OS was defined as the time from diagnosis tothe final follow-up or death from any cause. Extranodal involvement in these patients was analyzed, including the distribution, prognostic value and effects of treatment modalities on survival.

Table 1 Comparison of clinical characteristics between patients with extranodal and nodal involvement
Statistical analysis
Categorical data were compared using χ2test. Means were compared usingt-test. Survival functions were estimated using the Kaplan-Meier method and compared using the log-rank test. The Cox proportional hazards regression model was used to assess the effect of multiple variables on survival, including the number and sites of extranodal involvement, gender, aaIPI, rituximab infusion and radiotherapy. Differences were considered statistically significant if the two-sided P<0.05. All statistical analyses were performed using SPSS software (Version 13.0; SPSS Inc., Chicago, IL, USA).
Results
Widespread distribution of extranodal involvement in young patients with DLBCL
Of the 329 patients enrolled in the study, 193 patients (59%) had extranodal involvement, and 136 patients (41%) had nodal disease alone. The groups presented similar clinical characteristics with the exception of performance status (Table 1). Extranodal lesions were associated with poorer performance status at diagnosis than in patients with nodal disease. Thirty patients had more than one extranodal lesion, and poor clinical features were observed in them. All of these patients had advanced disease (30/30), 67% (20/30) presented poor performance status at diagnosis, 60% (18/30) had high LDH levels, and 83% (25/30) were assigned to the poor prognosis group.
Sixteen sites of extranodal involvement were classified in the anatomy. The GI tract was the most common site, accounting for 33% (64/193) of the anatomic sites. Waldeyer’s ring ranked second, accounting for 22% (43/193) of the sites. The patients with other extranodal lesions included 14 in the bone tissue, 13 in the thorax, 12 in the pancreas, 11 in the thyroid, 11 in the breast, 10 in the liver, 9 in the adrenal gland, 9 in bone marrow, 7 in the paranasal sinuses, 5 in the brain, 5 in female genitals, 4 in salivary glands, 2 in the skin and 2 in the testicles. Special extranodal sites showed a high incidence of more than one extranodal lesion, including the adrenal gland (7/9, 78%), the pancreas (9/12, 75%) and the liver (7/10, 70%). The frequency of other sites was 54% (7/13) in the thorax, 40% (2/5) in the brain, 33% (3/9) in the bone marrow, 23% (15/64) in the GI, 21% (3/14) in the bone, 18% (2/11) in the breast, 18% (2/11) in the thyroid and 14% (1/7) in the paranasal sinuses.
Patients with more than one instance of extranodal involvement exhibited the poorest survival, which was attributed to a high aaIPI score
Based on the number of extranodal involvements, 136 of the patients were classified as having only nodal disease, 163 were classified as having extranodal disease with onesite involved, and 30 patients were classified as having extranodal disease with more than one site involved. The highest percentage of poor prognosis was found in patients with more extranodal lesions (83%vs. 21%vs. 14%, P<0.001), while the percentage of men (61%vs. 56%vs. 70%, P=0.300), patients receiving rituximab infusion (16%vs. 20%vs. 19%, P=0.778), and patients receiving radiotherapy (20%vs. 26%vs. 7%, P=0.053) were similar among the three groups. The survival outcomes were compared among these groups using the Kaplan-Meier method. Patients with more than one extranodal lesion displayed the poorest survival, with a 3-year PFS of 23.3%±7.7% and a 3-year OS of 30.0%±8.4%, which was significantly worse compared to the survival of patients with nodal disease, who had a 3-year PFS of 58.5%±4.3% (Log rank=17.417, P<0.001) and a 3-year OS of 69.2%±4.0% (Log rank=24.250, P<0.001), and patients with extranodal disease with one site involved, who had a 3-year PFS of 67.8%±3.7% (Log rank=26.984, P<0.001) and a 3-year OS of 74.3%±3.5% (Log rank=28.228, P<0.001). However, there was no significant difference between patients with only nodal disease and patients with one extranodal lesion (PFS: Log rank=3.704, P=0.054; OS: Log rank=1.985, P=0.159) (Figure 1).
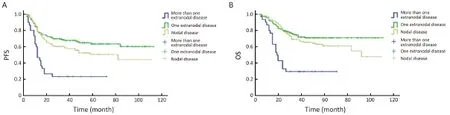
Figure 1 Survival curves of patients with different numbers of extranodal involvement sites. (A) Patients with more than one extranodal involvement siteshowed poorer progression-free survival (PFS) than patients with one extranodal lesion (Log rank=26.984, P<0.001) and patients with nodal disease (Log rank=17.417, P<0.001). However, there was no significant difference in the PFS between patients with nodal disease and patients with one extranodal lesion (Log rank=3.704, P=0.054); (B) Patients with more than one site of extranodal involvement showed poorer overall survival (OS) than patients with one extranodal lesion (Log rank=28.228, P<0.001) and patients with nodal disease (Log rank=24.250, P<0.001). However, there was no significant difference between patients with nodal disease and patients with one extranodal lesion in OS (Log rank=1.985, P=0.159).
The influence of clinical features (gender, aaIPI, and the number of extranodal sites involved) and treatment options (rituximab infusion and radiotherapy) on survival were analyzed in this study. A univariate survival analysis showed that aaIPI was negatively associated with PFS [hazard ratio (HR), 2.343; 95% confidence interval (95% CI), 1.657–3.314; P<0.001] and OS (HR, 2.343; 95% CI, 1.657–3.314; P<0.001); rituximab infusion positively affected PFS (HR, 0.574; 95% CI, 0.346–0.953; P=0.032) and OS (HR, 0.541; 95% CI, 0.304–0.964; P=0.037); and the number of extranodal sites involved was not related to PFS, but was associated with poor OS (HR, 1.396; 95% CI, 1.034–1.885; P=0.029). Gender and radiotherapy were not shown to affect survival. Multivariate analyses revealed that both aaIPI and rituximab infusion, but not the number of extranodal involvement sites, played independent prognostic roles in both PFS (aaIPI: HR, 2.423; 95% CI, 1.711–3.430; P<0.001; rituximab: HR, 0.536; 95% CI, 0.323–0.892; P=0.016) and OS (aaIPI: HR, 2.598; 95% CI, 1.779–3.796; P<0.001; rituximab: HR, 0.516; 95% CI, 0.289–0.919; P=0.025).
Extranodal involvement sites displayed important prognostic value in patients with one extranodal lesion
In this study, 163 patients had extranodal disease with one affected site and this group had similar survival rates to patients with nodal disease. This study evaluated the effect of distinct extranodal sites on survival in these patients. To reduce the error from the sampling limitation, we excluded patients with lesions in the adrenal gland, skin and testicles due to the very small sample size (n=2, respectively). Eventually, 157 patients were included. A significant difference was demonstrated in PFS (Log rank=23.470, P=0.024) and OS (Log rank=26.922, P=0.008) among distinct sites. The survival rate for each extranodal site is listed inTable 2. The best survival rate occurred in patientswith lesions in Waldeyer’s ring, and the worst occurred in patients with lesions in the brain. Regarding the effects of multiple factors on survival, including clinical features (gender, aaIPI and extranodal involved site) and treatment options (rituximab infusion and radiotherapy), multivariate analyses revealed that distinct extranodal sites (PFS: HR, 0.942; 95% CI, 0.897–0.988; P=0.014; OS: HR, 0.922; 95% CI, 0.872–0.975; P=0.005) and radiotherapy (PFS: HR, 1.804; 95% CI, 1.018–3.197; P=0.043; OS: HR, 1.962; 95% CI, 1.041–3.968; P=0.037) were independently related to the outcome. Disappointingly, radiotherapy was proven to have an adverse effect on the survival of these patients.

Table 2 The PFS and OS of patients with one extranodal involvement site (N=157)
Patients with Waldeyer’s ring and GI disease did not show poorer outcomes than those with nodal disease alone
Waldeyer’s ring (n=43) and the GI tract (n=49) were the most common sites in patients with one extranodal involvement site. The survival of patients with these lesions was compared to the survival of patients with only nodal disease. The groups with Waldeyer’s ring and GI lesions showed similar clinical features and treatment choices, including the percentage of males (Waldeyer’s ring: 72%vs. 61%, P=0.189; GI: 59%vs. 61%, P=0.821), good prognosis (Waldeyer’s ring: 91%vs. 79%, P=0.092; GI: 90%vs. 79%, P=0.104), rituximab infusion (Waldeyer’s ring: 28%vs. 16%, P=0.087; GI: 18%vs. 16%, P=0.725) and radiotherapy (Waldeyer’s ring: 30%vs. 20%, P=0.154; GI: 16%vs. 20%, P=0.589). Patients with Waldeyer’s ring lesions exhibited better survival than patients with nodal disease (PFS: Log rank=7.022, P=0.008; OS: Log rank=7.097, P=0.008) (Figure 2), whereas patients with GI involvement showed similar survival to patients with nodal disease (PFS: Log rank=3.691, P=0.055; OS: Log rank=1.478, P=0.224) (Figure 3).

Figure 2 Survival curves of patients with Waldeyer’s ring lesions and nodal disease. (A) Patients with Waldeyer’s ring lesions displayed better progression-free survival (PFS) than patients with nodal disease (Log rank=7.022, P=0.008); (B) Patients with Waldeyer’s ring lesions showed better overall survival (OS) than patients with nodal disease (Log rank=7.097, P=0.008).
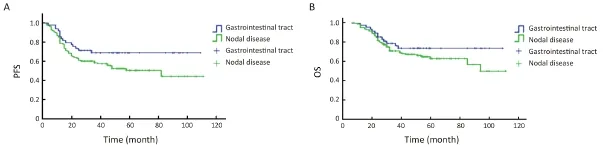
Figure 3 Survival curves of patients with gastrointestinal (GI) involvement and nodal disease. (A) Patients with GI involvement showed a similar progression-free survival (PFS) to patients with nodal disease (Log rank=3.691, P=0.055); (B) Patients with GI involvement had similar overall survival (OS) to patients with nodal disease (Log rank=1.478, P=0.224).
Discussion
This study revealed that extranodal involvement was common and ubiquitous at anatomic sites in young patients with DLBCL, which was consistent with data from the SEER database in a whole population with DLBCL (6). In addition, the most common extranodal sites in this study were Waldeyer’s ring and the GI tract, while the most common sites in the analysis of the SEER database were the GI tract and the head/neck. In fact, these two sites have a higher chance of contacting external antigens and stimulation, which increases the risk of malignant transformation. The present study also suggested that extranodal involvement resulted in a poorer performance status compared with nodal disease, but did not affect other important clinical features, such as the staging, LDH levels and aaIPI scores. However, very poor clinical characteristics were observed in patients with more than one extranodal lesion, including poor performance statuses, advanced stages, high LDH levels, and consequently, high aaIPI scores. Poor clinical characteristics were presumed to be associated with more aggressive tumor potential and to mediate a poor prognosis (3). Intriguingly, some extranodal sites were found to have high incidence of more than one extranodal lesion, including the adrenal gland, pancreas and liver, and these lesions may produce more aggressive biological behavior. In summary, extranodal lesions should receive more attention at the time of diagnosis in young patients with DLBCL due to their widespread distribution. Extranodal disease involving more than one site was associated with a poorer outcome than nodal disease alone or extranodal disease with one site. However, the prognostic value of the incidence of multiple extranodal sites was not demonstrated in a multivariate analysis that included gender, aaIPI, rituximab infusion and radiotherapy. Instead, the aaIPI score and rituximab infusion were shown to independently influence the outcome. Therefore, we concluded that the aaIPI exhibited powerful prognostic significance in the young patients, and poor survival with multiple extranodal lesions was attributed to a high aaIPI score. However, significant differences in the survival rates of patients with distinct sites of extranodal involvement were shown in patients with one extranodal site, who had a similar survival rate to patients with nodal disease in the study. Moreover, the prognostic value of the site of extranodal involvement was so strong that by comparison, the aaIPI and rituximab infusion had no significant prognostic value in these patients. The discriminative prognosis provided by distinct extranodal sites has been validated by other studies (13,14). Several reasons may be responsible for the high prognostic value. First, extranodal tissues and organs usually perform unique physiological functions. If major organs are involved, the performance status will be more severely affected. Poorer performance statuses have been observed in patients with extranodal involvement, which will reduce the tolerance of patients to standard treatments (3). Second, tumor cells from distinct extranodal sites may have different degrees of aggressive potential. More aggressive characteristics usually result in poorer responses to treatment or rapid disease progression. For example, diseases of the adrenal gland, pancreas and liver have been found to develop more extranodal lesions. More interestingly, distinct extranodal sites can provide a specific tumor microenvironment (15), which has been demonstrated to have a critical effect on the biological behavior of tumors (16). Some extranodal involvement sites have been shown to have an increased risk of a CNS event in DLBCL, including the paranasal sinus, testicles, epidermis, bone marrow, renal and adrenal glands, and the breast (17-20). It is well-known that metastasis to the CNS is a major cause of treatment failure. Therefore, it is worthclarifying the mechanism of the prognostic differences among distinct extranodal diseases to improve outcomes for these patients.
Controversies remain regarding the prognostic value of some distinct extranodal sites. The International Extranodal Lymphoma Study Group (IELSG) reported that the survival of patients with DLBCL of the head and neck was inferior to that of patients with nodal DLBCL (21); in that report, DLBCL of the head and neck included lesions in Waldeyer’s ring, the parotid and salivary glands, the thyroid gland, the nasal cavity and paranasal sinus, the palate and the oral cavity. This was contrary to the results from the SEER database, which showed that the disease of the head and neck was associated with better survival (6). In the present study, patients with Waldeyer’s ring lesions displayed better survival than those with nodal disease. Although lesions in other sites of the head and neck, such as the salivary and thyroid glands and the paranasal sinus, were not compared with nodal disease because of the sample limitation in this study, better survival tended to occur in patients with lesions in the salivary and thyroid glands, whereas poor survival tended to occur in patients with lesions in the paranasal sinus. Waldeyer’s ring is actually not defined as an extranodal site but as a lymphatic tissue in the Ann Arbor staging (5). Takahashiet al. (14) also reported better survival of patients with Waldeyer’s ring lesions. The GI tract is another site that is commonly involved and has been associated with a poor prognosis in some studies (7,22). However, the present study did not confirm the unfavorable effect of DLBCL in the GI tract on survival compared with nodal disease. The divergence may be generated from a sample choice (young patients), but it remains unknown whether this divergence was caused instead by regional disparity. In our center, DLBCL of the GI tract was often found in combination withHelicobacter pyloriinfection, which may have given rise to distinct entity with lower aggressiveness and higher chemosensitivity (23). To answer this question, the epidemiology ofHelicobacter pyloriin different regions should be compared. An additional argument exists regarding the prognosis of patients with female genital tract involvement. Ahmadet al. (24) reported a more favorable prognosis in these patients, while a study from China showed that these patients had a high risk of CNS relapse and therefore had poorer prognosis (25). These issues definitely require comprehensive cooperation to achieve accurate insight into these rare extranodal diseases.
In the study, rituximab infusion was shown to have a positive effect on the outcome of young patients but did not affect the outcome of patients with one extranodal lesion. Radiotherapy was not shown to influence survival in young patients, and more disappointingly, it led to an adverse effect on survival in patients with one extranodal lesion. Radiotherapy efficaciously exerts local control of disease in patients with DLBCL (26). The addition of radiotherapy after systemic therapy is expected to improve the survival of DLBCL patients with extranodal disease in clinical practice. Unfortunately, this study did not demonstrate that these patients benefited from the use of radiotherapy. Likewise, whole brain radiotherapy has not been proven to significantly increase survival compared with chemotherapy alone in primary CNS lymphoma (27). In the future, novel treatments are expected to improve the poor outcomes of distinct extranodal involvement sites. For example, lenalidomide, an oral non-chemotherapy immunomodulator with effects on tumor cells and the microenvironment, has been demonstrated to be safe in combination with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) and shows promising efficacy in the treatment of DLBCL (28). Programmed cell death ligand 1 (PD-L1) has also been found to be expressed on select DLBCL tumor cells and on tumor-infiltrating nonmalignant cells (29). Immunotherapy targeting the PD-1/PD-L1 pathway should be considered in this distinct DLBCL subgroup. A recent study also supported the development of ibrutinib, which targets B-cell receptor signaling and acts as an inhibitor of Bruton’s tyrosine kinase, for use in DLBCL treatment (30).
Conclusions
Extranodal involvement is common and associated with many anatomical sites in young DLBCL patients. Patients with more than one extranodal involvement site present a poorer prognosis than those with one extranodal lesion or with only nodal disease, which is attributed to a high aaIPI score. Among patients with extranodal disease, the anatomical sites involved can be used instead of the traditional aaIPI score to predict the outcome. Not all extranodal involvement sites indicate a poorer prognosis than that observed in nodal disease. Waldeyer’s ring lesions may exhibit better survival; GI involvement may show similar survival; and CNS involvement may manifest poor survival compared with nodal disease. Radiotherapy for extranodal lesions does not improve patient outcomes, andnovel therapy may be promising for young patients with extranodal disease.
Acknowledgements
Funding: This work was supported by the National Nature Science Foundation of China (No. 81071938, 81470365).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
1.Pon JR, Marra MA. Clinical impact of molecular features in diffuse large B-cell lymphoma and follicular lymphoma. Blood 2016;127:181-6.
2.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th edition. Lyon: IARC Press, 2008.
3.A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med 1993;329:987-94.
4.Rosenberg SA, Boiron M, DeVita VT Jr, et al. Report of the Committee on Hodgkin’s Disease Staging Procedures. Cancer Res 1971;31:1862-3.
5.Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol 1989;7:1630-6.
6.Castillo JJ, Winer ES, Olszewski AJ. Sites of extranodal involvement are prognostic in patients with diffuse large B-cell lymphoma in the rituximab era: an analysis of the Surveillance, Epidemiology and End Results database. Am J Hematol 2014;89:310-4.
7.Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCNIPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2014;123:837-42.
8.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002;346:235-42.
9.Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol 2005;23:5027-33.
10.Bonadonna G, Bonfante V, Viviani S, et al. ABVD plus subtotal nodal versus involved-field radiotherapy in early-stage Hodgkin’s disease: long-term results. J Clin Oncol 2004;22:2835-41.
11.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999;17:1244.
12.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579-86.
13.Lu CS, Chen JH, Huang TC, et al. Diffuse large B-cell lymphoma: sites of extranodal involvement are a stronger prognostic indicator than number of extranodal sites in the rituximab era. Leuk Lymphoma 2015;56:2047-55.
14.Takahashi H, Tomita N, Yokoyama M, et al. Prognostic impact of extranodal involvement in diffuse large B-cell lymphoma in the rituximab era. Cancer 2012;118:4166-72.
15.Middle S, Coupland SE, Taktak A, et al. Immunohistochemical analysis indicates that the anatomical location of B-cell non-Hodgkin’s lymphoma is determined by differentially expressed chemokine receptors, sphingosine-1-phosphate receptors and integrins. Exp Hematol Oncol 2015; 4:10.
16.Lenz G, Wright G, Dave SS, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med 2008;359:2313-23.
17.Tilly H, Vitolo U, Walewski J, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii78-82.
18.Shimazu Y, Notohara K, Ueda Y. Diffuse large B-cell lymphoma with central nervous system relapse: prognosis and risk factors according to retrospective analysis from a single-center experience. Int J Hematol 2009;89:577-83.
19.Zucca E, Conconi A, Mughal TI, et al. Patterns of outcome and prognostic factors in primary large-celllymphoma of the testis in a survey by the International Extranodal Lymphoma Study Group. J Clin Oncol 2003;21:20-7.
20.Fletcher CD, Kahl BS. Central nervous system involvement in diffuse large B-cell lymphoma: an analysis of risks and prevention strategies in the postrituximab era. Leuk Lymphoma 2014;55:2228-40.
21.Mian M, Capello D, Ventre MB, et al. Early-stage diffuse large B cell lymphoma of the head and neck: clinico-biological characterization and 18 year followup of 488 patients (IELSG 23 study). Ann Hematol 2014;93:221-31.
22.Ghimire P, Wu GY, Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol 2011;17:697-707.
23.Kuo SH, Yeh KH, Chen LT, et al. Helicobacter pylori-related diffuse large B-cell lymphoma of the stomach: a distinct entity with lower aggressiveness and higher chemosensitivity. Blood Cancer J 2014; 4:e220.
24.Ahmad AK, Hui P, Litkouhi B, et al. Institutional review of primary non-hodgkin lymphoma of the female genital tract: a 33-year experience. Int J of Gynecol Cancer 2014;24:1250-5.
25.Cao XX, Li J, Zhang W, et al. Patients with primary diffuse large B-cell lymphoma of female genital tract have high risk of central nervous system relapse. Ann Hematol 2014;93:1001-5.
26.Ghielmini M, Vitolo U, Kimby E, et al. ESMO Guidelines consensus conference on malignant lymphoma 2011 part 1: diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL) and chronic lymphocytic leukemia (CLL). Ann Oncol 2013; 24:561-76.
27.Korfel A, Thiel E, Martus P, et al. Randomized phase study of whole-brain radiotherapy for primary CNS lymphoma. Neurology 2015;84:1242-8.
28.Nowakowski GS, LaPlant B, Macon WR, et al. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: a phase study. J Clin Oncol 2015;33:251-7.
29.Kiyasu J, Miyoshi H, Hirata A, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood 2015;126:2193-201.
30.Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med 2015;21:922-6.
Cite this article as: Yao S, Li J, Yao Z, Xu Y, Chu J, Zhang J, Jin S, Huang Y, Zhang J, Ma J, Zhao Y, Yang S, Liu Y. Extranodal involvement in young patients with diffuse large B-cell lymphoma: distribution, prognostic value and treatment options. Chin J Cancer Res 2017;29(1):57-65. doi: 10.21147/j.issn.1000-9604.2017.01.07
10.21147/j.issn.1000-9604.2017.01.07
*These authors contributed equally to this work.
Yanyan Liu. Department of Internal Medicine, Henan Cancer Hospital & Affiliated Cancer Hospital of Zhengzhou University, No. 127 Dong Ming Road, Zhengzhou 450008, China. Email: yyliu@zzu.edu.cn.
Methods:A total of 329 patients were enrolled according to the inclusion requirements. The effects of gender, extranodal involvement, age-adjusted international prognostic index (aaIPI), rituximab infusion and radiotherapy on patient outcomes were evaluated.
Results:Among these patients, 59% presented extranodal involvement in 16 anatomic sites. More than one instance was linked to many poorer clinical characteristics and poorer survival compared with either nodal disease or one instance. In patients with one extranodal lesion, multivariate analysis revealed that the site of extranodal involvement, but not the aaIPI or rituximab infusion, was independently related to the outcome, and radiotherapy had a negative influence on survival.
Conclusions:Extranodal involvement is common in younger patients and exhibits a ubiquitous distribution. The site of extranodal involvement is of strong prognostic significance. Radiotherapy for extranodal lesions does not improve patient outcomes.
Submitted Aug 31, 2016. Accepted for publication Jan 09, 2017.
 Chinese Journal of Cancer Research2017年1期
Chinese Journal of Cancer Research2017年1期
- Chinese Journal of Cancer Research的其它文章
- Cancer incidence and mortality in China in 2013: an analysis based on urbanization level
- Influencing factors of inpatient expenditure pattern for cancer in China, 2015
- Clinical characteristics and response to tyrosine kinase inhibitors of patients with non-small cell lung cancer harboring uncommon epidermal growth factor receptor mutations
- Prognostic factors for transarterial chemoembolization combined with sustained oxaliplatin-based hepatic arterial infusion chemotherapy of colorectal cancer liver metastasis
- Upregulation of kazrin F by miR-186 suppresses apoptosis but promotes epithelial-mesenchymal transition to contribute to malignancy in human cervical cancer cells
- PEG-asparaginase in BFM-90 regimen improves outcomes in adults with newly diagnosed lymphoblastic lymphoma
