苍耳化学成分及生物活性研究
张文治 栗娜 白丽明 高鸿悦 张树军
摘要: 采用硅胶柱色谱、制备薄层色谱、正反高效液相色谱和重结晶等方法,对苍耳石油醚、乙酸乙酯萃取物进行分离,研究了中药苍耳(Xanthium sibiricum)全草的化学成分和抗菌活性。 结果表明:共得到14个化合物。根据理化性质和波谱数据分析,鉴定化合物结构分别为对甲氧基苯甲酸(1)、蒲公英赛醇(2)、羽扇豆醇(3)、熊果酸(4)、豆甾醇(5)、齐墩果酸(6)、lasidiol pmethoxybenzoate(7)、羽扇豆酮(8)、苍耳亭(9)、α菠甾醇(10)、槲皮素(11)、苍耳皂素(12)、芹菜素(13)、羽扇豆醇乙酸酯(14)。化合物2、7、8为首次从该植物中分离得到。以平板打孔法测试提取化合物对不同菌种的抑制作用,结果表明化合物2、8、9、12对蕃茄早疫、黄瓜枯萎、蕃茄灰霉和苹果腐烂病菌有较好的抑菌活性。
关键词: 苍耳, 化学成分, 分离, 抑菌活性
中图分类号: Q946.91, R284.1
文献標识码: A
文章编号: 10003142(2017)05062106
Abstract:
We studied the chemical constituents and bioactivity of Xanthium sibiricum, and fourteen compounds were isolated from the PE and EtOAc extract by the methods of column chromatography, thin layer chromatography, semiprepared HPLC, and recrystallization. The structures were elucidated by the analysis of spectral data and physicalchemical properties and identified as anisic acid(1), taraxerol(2), lupeol(3), ursolic acid(4), stimastero(5), oleanolic acid(6), lasidiol pmethoxybenzoate (7), lupenone(8), xanthatin(9), αspinasterol(10), quercetin (11), xanthinosin(12), apigenin(13) and oleanic acid(14); Compounds 2,7,8 were isolated from Xanthium sibiricum for the first time. The compounds were tested by plate diffusion method for the inhibitory effect of different strains. The results showed that the compounds taraxerol(2), lupenone(8), xanthatin(9) and xanthinosin(12) displayed antibacterial activity to Alternaria solani, Fusarium oxysporum. sp. susumebrium, Botrytis cinerea and Cytospora sp.
Key words: Xanthium sibiricum, chemical constituent, separation, antibacterial activity
苍耳(Xanthium sibiricum)为菊科苍耳属植物,广布我国各省区。其味辛苦,性温,在临床上被用来治疗风寒头痛、皮肤湿疹、鼻塞流涕等症状,具有抗炎、抗过敏、抗氧化、抗菌、抗肿瘤等药理作用(王蓓和赵卫星,2011)。本课题组前期对蒙古苍耳的茎叶及甲醇提取物进行了化学成分研究,分离得到25个化合物(张文治等,2009;张树军等,2015)。本文报道其全草95%乙醇提取物的化学成分,从中分离得到14个化合物,分别鉴定为对甲氧基苯甲酸(Anisic acid,1)、蒲公英赛醇(taraxerol,2)、羽扇豆醇(lupeol,3)、熊果酸(ursolic acid,4)、豆甾醇(stimastero,5)、齐墩果酸(oleanolic acid,6)、lasidiol pmethoxybenzoate(7)、羽扇豆酮(lupenone,8)、苍耳亭(xanthatin,9)、α菠甾醇(αspinasterol,10)、槲皮素(quercetin,11)、苍耳皂素(xanthinosin,12)、芹菜素(apigenin,13)、羽扇豆醇乙酸酯(oleanic acid,14)。化合物2、7、8为首次从该植物中分离得到。
为了进一步研究其药理活性,开发利用植物资源,本研究对化合物2、8、9、12分别进行6种植物致病菌的抑菌活性筛选,其中4种化合物对蕃茄早疫、黄瓜枯萎、蕃茄灰霉和苹果腐烂病菌有较好的抑制作用。
1仪器与材料
瑞士BRUKER公司 AM600 型核磁共振仪,高效液相色谱仪 (Waters 2489),RE5200 旋转蒸发仪,Yanako熔点仪,硅胶(200 ~ 300目)为青岛海洋化工厂产品。实验用试剂如石油醚、乙酸乙酯、正丁醇、甲醇为天津凯通化学试剂公司所生产,HPLC使用色谱纯度的试剂。苹果腐烂病菌(Cytospora sp.)、蕃茄早疫病菌(Alternaria solani)、蕃茄灰霉病菌(Botrytis cinerea)、草莓灰霉病菌(Bofrytis cinerea)、黄瓜枯萎病菌(Fusarium oxysporum sp. susumebrium)、小麦赤霉病菌(Fusarium graminearum)6种菌种均从中国农业大学购买所得,葡萄糖为凯通化学试剂公司生产,琼脂为博兴生物技术公司生产。
所用材料于2013年8月采自黑龙江省兰西县。经齐齐哈尔大学生命学院杨晓杰教授鉴定为菊科植物苍耳(Xanthium sibiricum)。
2提取与分离
干燥苍耳全草 (30.0 kg) 粉碎,用90 L乙醇分四次浸泡7 d,浓缩至1 200 mL浸膏,加适量蒸馏水后,依次用三种不同极性的溶剂进行萃取,得到石油醚、乙酸乙酯和正丁醇萃取物,分别为198.8、160.2和55.5 g。
取石油醚萃取物 (198.8 g) 用石油醚和乙酸乙酯的混合溶剂进行硅胶柱色谱反复洗脱后,利用HPLC纯化得到化合物 1(20.9 mg)、化合物 2(14.6 mg)、 3(19.2 mg)和化合物 4(18.7 mg)。
取乙酸乙酯萃取物 (160.2 g) 用硅胶柱色谱进行反复多次洗脱后,利用HPLC纯化,得到化合物 5(187.2 mg)、6(30.2 mg)、7(11.2 mg)、8(53.7 mg)、9(84.3 mg)、10(22.9 mg)、11(12.7 mg)、12(15.5 mg)、13(17.6 mg)和 14(18.2 mg)。
3结构鉴定
化合物 1白色粉末(石油醚-乙酸乙酯),mp 181~186 ℃。易溶于甲醇。1HNMR(600 MHz,MeOD) δ: 7.98 (2H,dd,J=8.5,2.0 Hz,H2,6),6.99 (2H,d,J=8.5,2.0 Hz,H3,5),3.87 (3H,s,HOCH3);13CNMR(150MHz,MeOD) δ: 122.6 (C1),131.4 (C2),113.3 (C3),163.7 (C4),113.3 (C5),131.4 (C6),54.6 (C OCH3)。以上数据与华会明等(2005)对照,可鉴定化合物 1为对甲氧基苯甲酸。
化合物 2白色粉末(EtOAc),mp 271~272 ℃,易溶于氯仿。1HNMR (600 MHz,CDCl3) δ: 5.53 (1H,dd,J=10.5,4.0 Hz,H21),3.20 (1H,t,J=5.2,11.2 Hz,H3);1.63,2.05(6H,s),0.82,0.85,0.87,0.95,0.99,1.05(each 3H,s);13CNMR (150 MHz,CDCl3) δ:38.0 (C1),26.1 (C2),76.2 (C3),39.2 (C4),55.9 (C5),18.8 (C6),35.1 (C7),38.8 (C8),48.9 (C9),37.5 (C10),17.4 (C11),35.8 (C12),37.7 (C13),158.1 (C14),116.9 (C15),36.7 (C16),37.4 (C17),49.3 (C18),41.3 (C19),28.9 (C20),33.8 (C21),33.1 (C22),28.2 (C23),15.2 (C24),15.4 (C25),29.8 (C26),25.1 (C27),29.7 (C28),33.5 (C29),21.6 (C30)。以上结果与吴双庆等(2012)基本一致,可判断化合物 2 为蒲公英赛醇。
化合物 3白色无定形粉末(石油醚乙酸乙酯),易溶于氯仿,石油醚,乙酸乙酯,mp 216~218 ℃。1HNMR(600MHz,CDCl3) δ: 4.69(1H,d,J=2.5 Hz,H29b),4.57 (1H,d,J=2.5 Hz,H29a),3.18 (1H,dd,J=5.1,11.2 Hz,H3),1.69 (3H,s,H30),1.04 (3H,s,H26),0.97 (3H,s,H25),0.91 (3H,s,H23),0.83 (3H,s,H27),0.79 (3H,s,H28),0.76 (3H,s,H24);13CNMR (150MHz,CDCl3) δ: 38.7 (C1),27.5 (C2),78.8 (C3),38.9 (C4),55.3 (C5), 18.3 (C6), 34.2 (C7),40.8 (C8),
50.5 (C9),37.2 (C10),21.1 (C11),25.2 (C12),38.1 (C13),42.7 (C14),27.4 (C15),25.5 (C16),42.6 (C17),48.3 (C18),47.9 (C19),150.9 (C20),29.7 (C21),40.0 (C22),27.9 (C23),15.4 (C24),16.1 (C25),16.0 (C26),14.6(C27),18.1 (C28),109.3 (C29),19.4 (C30)。以上數据与张树军等(2015)对照基本一致,鉴定化合物 3 为羽扇豆醇。
化合物 4白色粉末(MeOH),mp 278~280 ℃,易溶于甲醇。1HNMR (600 MHz,DMSOd6) δ: 12.75 (1H,s,HOH),5.22 (1H,d,J=4.6 Hz,H12),3.81 (1H,m,H3),1.62 (3H,d,J=6.8 Hz,H29),1.71(3H,d,J=7.4 Hz,H30),1.48,1.52,1.56,1.68,1.70,1.72,1.85 (each 3H,s,H23~27); 13CNMR (150MHz,DMSOd6) δ: 38.6 (C1),24.1(C2),79.3 (C3),37.1 (C4),55.5 (C5),18.3(C6),32.6(C7),39.8(C8),47.7(C9),37.2 (C10),23.2(C11),125.9(C12),138.2(C13),41.3 (C14),27.7 (C15),23.4 (C16),47.6 (C17),53.3 (C18),39.8 (C19),38.6 (C20),30.8 (C21),34.4 (C22),28.2 (C23),16.6 (C24),15.4(C25),17.6 (C26),23.7(C27),182.2 (C28),17.1 (C29),19.6 (C30)。以上数据与张俊燕等(2014)比较分析,推断化合物 4为熊果酸。
化合物 5无色针晶(石油醚醋酸乙酯),mp 167~168 ℃,易溶于氯仿、丙酮。1HNMR (600 MHz,CDCl3) δ: 5.14 (1H,m,H6),5.02 (1H,dd,J=8.4,15.0 Hz,H22),5.01 (1H,dd,J=15.0,8.4 Hz,H23),3.57 (1H,m,H3),1.03 (3H,d,H21),1.01 (3H,s,H19),0.81 (3H,t,J=2.8 Hz,H29),0.79 (3H,s,H27),0.54 (3H,s,H18); 13CNMR (150MHz,CDCl3) δ: 37.1 (C1),31.8 (C2),71.8 (C3),43.2 (C4),121.7 (C5) 140.1 (C6),40.3 (C7),31.8 (C8), 50.2 (C9),36.5 (C10),21.2 (C11),39.8 (C12), 42.2 (C13),32.0(C14),25.4(C15),28.6(C16), 56.0(C17),12.2 (C18),19.2 (C19),40.1(C20),21.5(C21),138.2(C22),129.3(C23),51.3 (C24),31.8 (C25),21.7 (C26),19.1 (C27),25.3 (C28),12.3 (C29)。与吴希等(2008)的研究结果一致,可鉴定化合物 5 为豆甾醇。
化合物 6白色粉末(EtOAc),mp 278~280 ℃,易溶于氯仿,丙酮。1HNMR (600 MHz,CDCl3) δ: 5.25(1H,s,H12),3.22 (1H,dd,J=4.9,5.4 Hz,H3),2.82 (1H,dd,J=13.7,4.3 Hz,H18),1.13 (3H,s,H27),0.99 (3H,s,H25),0.93 (3H,s,H30),0.91 (3H,s,H29),0.90 (3H,s,H24),0.77 (3H,s,H23),0.75 (3H,s,H26); 13CNMR (150 MHz,CDCl3) δ: 38.5 (C1),27.2 (C2),78.7 (C3),38.7 (C4),55.2 (C5),18.3 (C6),32.6 (C7),39.4 (C8),47.6 (C9),37.1 (C10),22.9 (C11),122.2 (C12),143.5 (C13),41.6(C14),27.7 (C15),23.4 (C16),46.6 (C17),41.3(C18),45.8 (C19),30.6 (C20),33.8 (C21),32.4 (C22),28.1 (C23),15.6 (C24),15.3(C25),16.8 (C26),25.9(C27),183.3(C28),33.1(C29),23.6(C30)。以上数据与陈勇等(2015)的报道一致,故确定化合物 6 为齐墩果酸。
化合物 7淡黄色蜡状物(EtOAc), mp 42.9~48.8 ℃。1HNMR (600 MHz,CDCl3) δ:8.01 (2H,d,J=9.0 Hz,H2′,6′),6.89 (2H,d,J=9.0 Hz,H3′,5′),5.49 (1H,br d,J=4.3 Hz,H2),5.32 (1H,d,J=4.3 Hz,Hl),3.86 (3H,s,OCH3),2.42 (1H,m,H4),2.32 (1H,m,H5),2.08 (1H,m,H10),2.07 (1H,d,H11),1.70 (3H,br s,H15),1.08 (3H,s,H14);13CNMR (150MHz,CDCl3) δ: 77.9 (C1),121.4 (C2),143.1 (C3),30.3 (C4),35.9 (C5),24.7 (C6),36.1 (C7),56.8 (C8),26.6 (C9),24.6(C10),21.5 (C11),25.2 (C12),24.6(C13),22.9 (C14), 25.8 (C15),165.5 (C1′),123.0(C2′),131.5(C3′),113.6 (C4′),163.3 (C5′),113.6 (C6′),131.5 (C7′),55.5 (C8′)。以上數据与刘旭刚等(2014)的报道一致,判定化合物 7 为 lasidiol pmethoxybenzoate。
化合物 8白色针晶(石油醚醋酸乙酯),mp 165~167 ℃。1HNMR(600MHz,CDCl3) δ: 4.57(1H, br s, J=2.4 Hz, H29b), 4.69 (1H, br s, J=2.4 Hz, H29a), 1.69 (3H, br s, H30), 1.07 (3H, s, H26), 1.05 (3H, s, H23), 1.03 (3H, s, H24), 0.96 (3H, s, H27), 0.93 (3H, s, H25), 0.80 (3H, s, H28); 13CNMR (150MHz, CDCl3) δ: 39.8 (C1), 34.2 (C2), 218.1 (C3), 47.5 (C4), 54.9 (C5), 19.6 (C6), 33.6 (C7), 40.7 (C8), 50.0 (C9), 36.9 (C10), 21.6 (C11), 25.2 (C12), 38.1 (C13), 42.9 (C14), 27.3 (C15), 35.5 (C16), 42.9 (C17), 48.3 (C18), 47.8 (C19), 150.9 (C20), 29.8 (C21), 39.9 (C22), 26.7 (C23), 21.0 (C24), 15.8 (C25), 15.9 (C26), 14.6(C27), 18.0 (C28), 109.3 (C29), 19.2 (C30)。以上数据与彭小冰等(2012)的结果对照,可确定化合物 8 为羽扇豆酮。
化合物 9块状透明晶体(EtOAc),mp 114.5~115 ℃。1HNMR (600 MHz, CDCl3) δ: 7.07 (1H, d, J=16.0 Hz, H2), 6.28 (1H, dd, J=9.1, 3.4 Hz, H5), 6.20 (1H, d, J=16.0 Hz, H3), 6.18 (1H, d, J=3.3 Hz, H13a), 5.48 (1H, d, J=3.3 Hz, H13b), 4.29 (1H, dt, J=12.2, 2.6 Hz, H8), 2.80 (1H, ddd, J=16.7, 9.1, 2.5 Hz, H6a), 2.56 (1H, dt, J=12.2, 2.6 Hz, H7), 2.38 (1H, ddd, J=12.8, 3.9, 2.6 Hz, H9b), 2.30 (3H, s, H15), 2.20 (1H, ddd, J=16.7, 12.2, 3.3 Hz, H6b), 1.83 (1H, dt, J=12.8, 3.9 Hz, H9a), 1.16 (3H, d, J=7.4 Hz, H14); 13CNMR (150 MHz, CDCl3) δ: 144.8 (C1), 148.5 (C2), 124.7 (C3), 198.5 (C4), 138.1 (C5), 27.2 (C6), 47.4 (C7), 81.5 (C8), 36.6 (C9), 29.1 (C10), 139.2 (C11), 169.7 (C12), 118.9 (C13), 18.8 (C14), 27.9 (C15)。结合张文治等(2009),可鉴定出化合物 9 为苍耳亭。
化合物 10无色针状结晶(石油醚乙酸乙酯), mp 169~171 ℃。1HNMR (600 MHz, CDCl3) δ: 5.36 (1H, dd, J=15.0, 8.5 Hz, H23), 5.18 (1H, dd, J=15.0, 8.5 Hz, H23), 4.97 (1H, m, H7), 3.53 (1H, m, H3); 13CNMR (150 MHz, CDCl3) δ: 37.4 (C1), 31.6 (C2), 71.2 (C3), 38.2 (C4), 40.4 (C5), 29.9 (C6), 177.3 (C7), 139.6 (C8), 49.7 (C9), 34.5 (C10), 21.7 (C11), 39.6 (C12), 43.4 (C13), 55.2 (C14), 23.3 (C15), 28.3 (C16), 56.2 (C17), 12.6 (C18), 13.3 (C19), 40.7 (C20), 21.1 (C21), 138.1 (C22), 129.7 (C23), 51.4 (C24), 31.8 (C25), 19.2 (C26), 21.6 (C27), 25.3 (C28), 12.1 (C29)。根據文献数据(赵晓亚等,2005),鉴定化合物 10 为α菠甾醇。
化合物 11黄色无定形粉末(甲醇), mp 311.9~314.9 ℃。1HNMR (600 MHz, DMSOd6) δ: 12.50 (1H, br s, 5OH), 10.80 (1H, br s, 7OH), 9.61 (1H, br s, 3′OH), 9.37 (1H, br s, 3OH), 9.32 (1H, s, 4′OH), 7.66 (1H, d, J=2.2 Hz, H2′), 7.54 (1H, dd, J=8.5, 2.2 Hz, H6′), 6.89 (1H, d, J=8.5Hz, H5′), 6.41 (1H, d, J=2.0 Hz, H8), 6.20 (1H, d, J=2.0 Hz, H6); 13CNMR (150 MHz, DMSOd6) δ: 156.1 (C2), 135.7 (C3), 176.1 (C4), 147.6 (C5), 98.1 (C6), 163.2 (C7), 93.3 (C8), 160.8 (C9), 102.9 (C10), 121.9 (C1′), 115.0 (C2′), 146.1 (C3′), 115.4 (C5′), 119.9 (C6′)。以上数据与杨宝等(2014)的研究结果一致,可确定化合物 11 为槲皮素。
化合物 12无色脂状物。1HNMR (600 MHz, CDCl3) δ: 6.16 (1H, d, J=3.4 Hz, H13b), 5.53 (1H, dd, J=8.9, 2.9 Hz, H5), 5.44 (1H, d, J=3.4 Hz, H13a), 4.24 (1H, dt, J=12.6, 2.9 Hz, H8), 2.52 (1H, m, H6a), 2.19 (1H, m, H9b), 2.12 (3H, s, H15), 2.04 (1H, m, H6b), 1.77 (1H, m, H9a), 1.15 (3H, d, J=7.3 Hz, H14); 13CNMR (150 MHz, CDCl3) δ: 147.1 (C1), 34.5 (C2), 42.8 (C3), 208.4 (C4), 122.1 (C5), 25.8 (C6), 48.1 (C7), 82.0 (C8), 37.0 (C9), 33.7 (C10), 138.9 (C11), 170.2 (C12), 118.5 (C13), 18.6 (C14), 30.2 (C15)。以上数据与张树军等(2015)的结果一致,因此推断化合物 12 为苍耳皂素。
化合物 13黄色结晶 (甲醇), mp 348~350 ℃。1HNMR (600 MHz, DMSOd6) δ: 12.92 (1H, s, 5OH), 10.81 (1H, s, 7OH), 10.37(1H, s, 4′OH), 7.92 (2H, d, J=8.6 Hz, H2′6′), 6.92 (2H, d, J=8.8 Hz, H3′, 5′),6.77 (1H, s, H3), 6.48 (1H, d, J=1.9 Hz, H8), 6.19 (1H, d, J=1.9 Hz, H6); 13CNMR (150 MHz, DMSOd6) δ: 164.1 (C2), 103.2 (C3), 182.3 (C4), 160.6 (C5), 99.1 (C6), 163.4 (C7), 94.5 (C8), 158.2 (C9), 104.9 (C10), 121.8 (C1′), 129.0 (C2′), 116.4 (C3′), 129.0 (C6′), 116.4 (C5′), 160.1 (C4′)。以上数据与卫强等(2015)对照, 可鉴定化合物 13 为芹菜素。
化合物 14无色针状结晶(石油醚乙酸乙酯), mp 213~215 ℃。1HNMR (600 MHz, CDCl3) δ: 4.67 (1H, s, H29), 4.62 (1H, s, H29), 4.51 (1H, dd, J=12.9, 6.4 Hz, H3), 2.18 (3H, s, COCH3), 1.67 (3H, s, H26), 1.05 (3H, s, H30), 0.99 (3H, s, H27), 0.96 (3H, s, H22), 0.88 (3H, s,H23), 0.87 (3H, s, H25), 0.86 (3H, s, H24), 0.83 (3H, s, H28);13CNMR (150 MHz, CDCl3) δ: 38.3 (C1), 23.3 (C2), 80.7 (C3), 37.7 (C4), 55.3 (C5), 18.2 (C6), 34.6 (C7), 39.3 (C8), 50.3 (C9), 36.9 (C10), 21.5 (C11), 25.4 (C12), 38.2 (C13), 42.9 (C14), 27.6 (C15), 36.5 (C16), 42.7 (C17), 48.7 (C18), 48.5 (C19), 150.6 (C20), 29.5 (C21), 40.7 (C22), 28.0 (C23), 16.2 (C24), 16.9 (C25), 16.4 (C26), 14.6 (C27), 18.0 (C28), 109.4 (C29), 19.1 (C30), 170.8 (CO), 21.3 (CH3COO)。以上结果与王延亮等(2014),徐菁等(2014)对照,鉴定化合物14为羽扇豆醇乙酸酯。
4生物活性
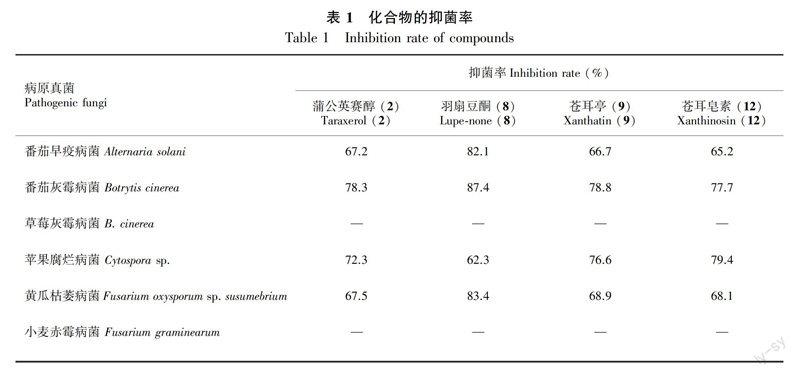
采用平板打孔法,测定化合物2、8、9、12对蕃茄早疫病菌、蕃茄灰霉病菌、草莓灰霉病菌、苹果腐烂病菌、黄瓜枯萎病菌、小麦赤霉病菌的抑菌作用。将实验所用菌种进行活化,并选用Φ = 9 cm的培养皿进行培养,实验样品用丙酮试剂溶解,对照样品为丙酮溶剂(马淑丽等,2015)。测量、计算、对比各菌落的半径大小可以体现出各单体化合物抑菌活性的强弱。
由表1可知,化合物2、8、9、12除對草莓灰霉病菌和小麦赤霉病菌外,对其它四种病原真菌体现出良好的的抑制作用。其中,化合物2、9、12对蕃茄早疫和黄瓜枯萎病菌的抑菌率在65%以上,对蕃茄灰霉病菌和苹果腐烂致病菌的抑菌率在75%以上;化合物8对蕃茄早疫和黄瓜枯萎病菌的抑制率均在80%以上,对蕃茄灰霉病菌的抑菌率为87.4%,且抑菌作用随着浓度的增加而增强。
参考文献:
CHEN Y, ZUO J, CHEN JW,et al, 2015. Chemical constituents from Claoxylon indicum stems [J]. J Chin Med Mat,38(4): 761-763. [陈勇,左坚,陈建伟,等,2015. 白桐树枝干化学成分研究 [J]. 中药材,38(4): 761-763.]
HUA HM,LI X,XING SE,et al,2005. Study on the chemical constituents of Linaria vulgaris [J]. Chin Pharm J,40(9): 653-656. [华会明,李铣,邢素娥,等,2005. 柳穿鱼化学成分的研究 [J]. 中国药学杂志,40(9): 653-656.]
ISAEV IM, MAMEDOVA RP, AGZAMOVA MA, et al,2007. Triterpene glycosides from Astragalus and their genins. L X X V. Sterols and triterpenoids from Astragalus orbiculatus [J]. Chem Nat Comp,43(3): 358-359.
LIU XY,XIE YF,ZHANG H,et al,2014. Chemical constituents from Pouzolzia zeylanica (L.) Benn. var. microphylla (Wedd.) W. T. Wang. [J]. Chin J Exp Trad Med Form,20(6): 43-47. [刘旭阳,谢郁峰,张慧,等,2014. 多枝雾水葛化学成分(I) [J]. 中国实验方剂学杂志,20(6): 43-47. ]
MA SL, LI HH, LI N, et al, 2015. Chemical constituents and bioactivity of Crepis tectrum linn. [J]. J Qiqihar Univ, 31(2): 45-47. [马淑丽, 李慧慧, 栗娜, 等, 2015. 还阳参化学成分及生物活性研究[J]. 齐齐哈尔大学学报, 31(2): 45-47. ]
PENG XB, GAO WL, HU DQ, et al, 2012. Chemical constituents from the aerial part of Stauntonia obovatifoliola Hayata subso. urophylla [J]. J Chin Med Mat, 36(11): 1795-1798. [彭小冰, 高伟略, 胡冬群, 等, 2012. 尾叶那藤地上部分化学成分研究 [J]. 中药材, 36(11): 1795-1798.]
WANG B, ZHAO WX, 2011. The application and chemical constituents of Cocklebur [J]. Chem Ind Times, 25(7): 47-49. [王蓓, 赵卫星, 2011. 苍耳化学成分研究及应用 [J]. 化工时刊, 25(7): 47-49.]
WANG YL, DUAN SL, ZHANG QY, et al, 2014. Chemical constituents from stems of Ficus tsiangii [J]. Chin Trad Herb Drugs, 45(3): 333-336. [王延亮, 段松冷, 张庆英, 等, 2014. 岩木瓜茎干的化学成分研究 [J]. 中草药, 45(3): 333-336.]
WEI Q, JI XY, LONG XS, et al, 2015. Chemical constituents from leavea of “Chuju” Chrysanthemum morifolium and their antioxidant activities in vitro [J]. J Chin Med Mat, 38(2): 305-310. [卫强, 纪小影, 龙先顺, 等, 2015. 滁菊叶化学成分及其体外抗氧化活性研究 [J]. 中药材, 38(2): 305-310.]
WU SQ, SUN Q, CHU CJ, et al, 2012. Chemical constituents of Eupatorium lindleyanum [J]. Chin J Chin Mat Med, 37(7): 937-940. [吴双庆, 孙群, 褚纯隽, 等, 2012. 野马追化学成分的研究 [J]. 中国中药杂志, 37(7): 937-940.]
WU X, XIA HL, HUANG LH, et al, 2008. Chemical constituents from rhizomes of Cyperus rotundus [J]. J Chin Med Mat, 31 (7): 990-992. [吴希, 夏厚林, 黄立华, 等, 2008. 香附化学成分的研究 [J]. 中药材, 31 (7): 990-992.]
XU J, GAO HY, MA SL, et al, 2014. Chemical constituents and bioactivity of Kalimeris indica [J]. Chin Trad Herb Drugs, 45(22): 3246-3250. [徐菁, 高鸿悦, 马淑丽, 等, 2014.马兰化学成分及生物活性研究 [J]. 中草药, 45(22): 3246-3250.]
YANG B, FAN Z, ZHU JP, et al, 2014. Chemical constituents from Phymatopteris hastate [J]. Chin Trad Herb Drugs, 45(21): 3053-3056. [杨宝, 范真, 朱锦萍, 等, 2014. 金鸡脚化学成分研究 [J]. 中草药, 45(21): 3053-3056.]
ZHANG JY, WANG Y, CHEN WH, et al, 2014. Chemical constituents from the stem of Saprosma hainanense [J]. Nat Prod Res Dev, 26(4): 1944-1947. [张俊燕, 王燕, 陈文豪, 等, 2014. 海南染木树茎的化学成分研究(I) [J]. 天然产物研究与开发, 26(4): 1944-1947.]
ZHANG SJ, LIU H, LI J, et al, 2015. Study on the chemical constituents from whole herbs of Xanthium mongolicum [J]. Chin Trad Herb Drugs, 46(3): 329-333. [张树军, 刘焕, 李军, 等, 2015. 蒙古苍耳全草化学成分研究 [J]. 中草药, 46(3): 329-333.]
ZHANG WZ, HAN W, LI Y, et al, 2009. Chemical constituents from Xanthium mongolicum [J]. Chin J Chin Mat Med, 34(13): 1687-1689. [张文治, 韩巍, 李盈, 等, 2009. 蒙古苍耳化学成分研究 [J]. 中国中药杂志, 34(13): 1687-1689.]
ZHAO XY, SUN HD, WU JZ, et al, 2005. Studies on chemical constituents from rhizome of Impatiens pritzellii var. hupehensis [J]. Chin J Chin Mat Med, 30(8): 584-586. [赵晓亚, 孙汉董, 吴继洲, 等, 2005. 冷水七根茎的化学成分研究 [J]. 中国中药杂志, 30(8): 584-586.]
XU M, WANG D, ZHANG YJ, et al, 2007. Dammarane triterpenoids from the roots of Gentiana rigescens [J]. J Nat Prod, 70(5): 880-883.
XU M, YANG CR, ZHANG YJ, 2009. Minor antifungal aromatic glycosides from the roots of Gentiana rigescens (Gentianaceae) [J]. Chin Chem Lett, 20(10): 1215-1217.
YANG HX, MA F, DU YZ, et al, 2014. Study on the Tibetan medicine Swertia mussotii Franch and its extracts by Fourier transform infrared spectroscopy [J]. Spectrosc Spectr Anal, 34(11): 2973-2977. [楊红霞, 马芳, 杜玉枝, 等, 2014. 藏药川西獐牙菜及其不同提取物的红外光谱分析 [J]. 光谱学与光谱分析, 34(11): 2973-2977.]
YU H, MACGREGOR JF, 2004. Post processing methods (PLSCCA): simple alternatives to preprocessing methods (OSCPLS) [J]. Chemometr Intell Lab Syst, 73(2): 199-205.
YU XZ, LI QH, SUN DJ, et al, 2015. Determination of the peroxide value of edible oils by FTIR spectroscopy using polyethylene films [J]. Anal Method, 7(5): 1727-1731.
ZHANG J, WIDER B, SHANG H, et al, 2012. Quality of herbal medicines: challenges and solutions [J]. Compl Ther Med, 20(1): 100-106.
ZHAO YL, ZHANG J, JIN H, et al, 2015. Discrimination of Gentiana rigescens from different origins by Fourier transform infrared spectroscopy combined with chemometric methods [J]. J AOAC Int, 98(1): 22-26.
ZHAO YL, ZHANG J, YUAN TJ, et al, 2014. Discrimination of wild Paris based on near infrared spectroscopy and high performance liquid chromatography combined with multivariate analysis [J]. PLoS ONE, 9(2): e89100.

