Fluorine-Substituted H4W10O32 as a Highly-Efficient Catalyst for Oxidative Degradation of Methyl Orange by Hydrogen Peroxide in Aqueous Solution Under Ultraviolet Light Irradiation
TANG Ze-yu, FU Zai-hui, TANG Sen-pei, ZHANG Shen-yi, ZHANG Chao, LIU Ya-chun, YIN Du-lin, LI Jian-wei
(National and Local United Engineering Laboratory for New Petrochemical Materials & Fine Utilization of Resources, Key Laboratory of Resource Fine-Processing and Advanced Materials of Hunan Province and Key Laboratory of Chemical Biology and Traditional Chinese Medicine Research (Ministry of Education of China), College of Chemistry and Chemical Engineering, Hunan Normal University, Changsha 410081, China)
Fluorine-Substituted H4W10O32as a Highly-Efficient Catalyst for Oxidative Degradation of Methyl Orange by Hydrogen Peroxide in Aqueous Solution Under Ultraviolet Light Irradiation
TANG Ze-yu, FU Zai-hui, TANG Sen-pei, ZHANG Shen-yi, ZHANG Chao, LIU Ya-chun, YIN Du-lin, LI Jian-wei
(National and Local United Engineering Laboratory for New Petrochemical Materials & Fine Utilization of Resources, Key Laboratory of Resource Fine-Processing and Advanced Materials of Hunan Province and Key Laboratory of Chemical Biology and Traditional Chinese Medicine Research (Ministry of Education of China), College of Chemistry and Chemical Engineering, Hunan Normal University, Changsha 410081, China)
AbstractA fluorine-substituted decatungstate acid (F-H4W10O32) was conveniently synthesized via a polymerization of sodium tungstate with hydrofluoric acid and characterized by FT-IR, UV-Vis and19F-NMR. And its photo-catalytic performance was evaluated using the oxidative degradation of methyl orange (MO) by hydrogen peroxide (H2O2) under ultraviolet light irradiation. Our results indicated that F atoms, as supported by FT-IR and19F-NMR, are likely incorporated into the framework of decatungstate via replacing side-bridged oxygen atoms of W-Oc-W bonds, which can significantly improve the stability of decatungstate and its activation capacity to H2O2in aqueous solution. As a result, it demonstrated an outstanding photo-catalytic activity for the oxidative degradation of MO by H2O2, achieving about 1.4~2.0 fold increase in degradation rate compared to the parent H4W10O32. Based on UV-Vis spectral characterizations, a reasonable photo-catalysis oxidation mechanism was proposed.
Keywordsfluorine-substituted decatungstate acid; hydrogen peroxide; methyl orange; photo-catalysis; oxidative degradation
During the past few decades, photo-catalytic oxidation technique has received extensive attention because of its potential applications in the synthesis of fine chemicals and especially environmental treatments[1-3]. Meanwhile, a large number of metal oxide and sulfide semi-conductors[4-6]and polyoxometalates[7-9]have been applied to effectively photo-catalyze the oxidative degradation of organic wastewater and the removal of waste gas. However, the photo-catalytic activity of most semiconductors is limited by their poor adsorption capacity for the organic dyes and the rapid recombination rate of their photo-generated electron-hole pairs. In general, the photo-catalytic performance of semiconductors can be markedly improved via the doping of various metal cations[10-11]and non-metal anions[12-23]. Among these doping agents, the doping of F-anion seems to be particularly effective and can exponentially increase the photo-catalytic activity of some semiconductors such as TiO2[20,24-28], SrTiO3[29], ZnWO4[19, 30],Bi2WO6[31],Bi2O3[32]and BiPO4[33]. The favorable effect of F-doping on the photo-catalytic activity of semiconductors may be originated from the following three reasons: i) the polarizability and dipole moment of semiconductor may be increased via F-anion replacing its lattice oxygen, which can lead to the improved adsorption capacity to organic contaminants in wastewater[33]. ii) F-doping can efficiently reduce the recombination of photo-generated electron-hole pairs on a semiconductor and thus improve the oxidative capacity of the photo-generated holes via lowering valence band edge[32]. And iii) F-doping may increase the surface hydroxyl density of semiconductor, which can efficiently induce the generation of hydroxyl radicals and improve the surface charge transfer efficiency[31, 34-35].
Polyoxometalates have been extensively studied over the past decades with some of them possessing interesting applications in catalysis[36]. Among them, decatungstate anion (W10O324-) is one of the most promising examples, due to its high photo-catalytic activity in the conversion of organic compounds to the corresponding oxidative products under ultraviolet or visible light[37-44]. But, it can only be used in small amounts as a photo-catalyst for the purification of wastewater[45-47]because of its instability in water. In order to solve this problem efficiently, we are inspired by the above success examples of F-doped semiconductors and for the first time we tried to prepare an F-substituted decatungstate acid (F-H4W10O32)viaa polymerization of sodium tungstate with hydrofluoric acid. Here, we report the initial results obtained from the synthesis of F-H4W10O32and the effect of F doping on the stability of decatungstate acid in water and its photo-catalytic performance for the oxidative degradation of methyl orange (MO) by hydrogen peroxide (H2O2) under ultraviolet light irradiation.
1 Experimental
1.1 Materials and apparatus
Materials and regents used in this study were sodium tungstate (Na2WO4·2H2O), 40% hydrofluoric acid (HF), 30% hydrogen peroxide (H2O2), 12-phosphotungstic acid (H3PW12O40),tetra-n-butylammonium bromide (TBABr), acetonitrile (CH3CN), concentrated hydrochloric acid (HCl), methyl orange (MO), all of which were of analytical grade without further purification. Distilled water was used throughout this experiment.
1.2 Characterization of samples
Liquid UV-Vis spectra of the samples in aqueous solution were recorded from 200 to 450 nm on UV-2450 spectrophotometer (Shimadzu, Japan) and their transmission FT-IR spectra were recorded from 400 to 4 000 cm-1on a Nicolet Nexus 510 P FT-IR spectroscopy using a KBr disk.19F-NMR spectrum of the sample F-H4W10O32was recorded on the Bruker Avance-500 superconductive Nuclear Magnetic Resonance, and its W and F contents measured on a sequential X-ray fluorescence spectrometer (XRF-1700, Shimadzu, Japan) with H3BO3as a binder.
1.3 Preparation of catalysts
A preparation procedure of the F-substituted decatungstate acid is described as follows: Na2WO4·2H2O (0.01 mol) was dissolved with 10 mL H2O to obtain a solution under magnetic stirring. After adding 12 mL of 40% HF, the solution was heated to 70 ℃ and continuously stirred at this constant temperature for a sufficiently long time until it was completely evaporated to dryness. The resulting white solid was dissolved with a small amount of water, then filtered to remove insoluble NaF. Finally the filtrate obtained was evaporated to dryness to yield the product, marked as F-H4W10O32. The W and F content of F-H4W10O32measured by XRF analysis was 92.75% and 2.16%, respectively, and the formula of as-prepared catalyst should be H4W10O30.75F2.5.
According to the literature[39,48], the preparation procedures for H4W10O32and TBA4W10O32are described as follows: 6.4 g (19.4 mmol) Na2WO4·2H2O was dissolved with 40 mL of water in a three-necked flask (200 mL), followed by the addition of 13.4 mL of 3 mol/L HCl solution. Then, the mixture was boiled for 5~10 min and then cooled to the room temperature to obtain aqueous H4W10O32. This solution was further treated with aqueous TBABr (1.3 mol/L, 6 mL) at 100 ℃ under continuous stirring to generate the white precipitate. And the precipitate obtained by filtering was repeatedly washed with water for three times and then dried at 60 ℃ under vacuum to yield the white solid TBA4W10O32.
1.4 Photo-catalytic oxidation degradation of methyl orange
The oxidative degradation of MO in water catalyzed by three decatungstates and a H3PW12O40under UV irradiation was carried out on a XPA photochemical reaction apparatus (Xujiang machine plant in Nanjing, China). In a typical experiment, A 10 mL of aqueous solution consisting of MO (100 mg/L, 9 mL) and catalyst ( 0.6 g/L,1 mL) and 30% H2O2(0.1 mL) was added to in a one-necked flask (50 mL) and its pH value was adjusted to 2 with HCl. Then, the resulting reaction mixture was irradiated continuously by a 300 W high-pressure mercury lamp from its side face (spacing, 10 cm) under magnetic stirring. After the desired irradiation time had elapsed, a small portion of the reaction mixture (0.5 mL) was sampled from the reactor, and its absorbance was determined on an UV-2450 spectrophotometer at 506 nm and the residual concentration of MO was calculated based on a curve obtained with its standard sample. So the degradation rate of MO solution was evaluated by the following formula:
Rt=(C0-Ct) /C0×100%,
Rt— degradation rate of MO solution at light irradiationttime;
C0—the initial concentration of MO solution;
Ct—the concentration of MO solution at light irradiationttime.
2 Results and Discussion
2.1 Characterizations

Fig.1 The FT-IR spectra of two solid samples F-H4W10O32 and TBA4W10O32 and two aqueous F-H4W10O32 and H4W10O32
Figure 1 is the FT-IR spectra of three decatungstates in 4 000~500 cm-1. A FT-IR spectrum of TBA4W10O32showed some characteristic peaks at 967, 891, 810, 585 and 435 cm-1in the range of 1 000~400 cm-1attributable to the isopolyacid anions, in agreement with previous reports[49-50]. Among these peaks, three characteristic peaks at 967, 891 and 810 cm-1can be attributed to stretching vibrations of W-Otconnecting with the terminal oxygen atoms, W-Ob-W, associated with corner-bridged oxygen atoms and W-Oc-W associated with side-bridged oxygen atoms in the decatungstate anion, respectively. H4W10O32also gave a very similar FT-IR spectrum to TBA4W10O32, but its three characteristic peaks attributable to decatungstate anion were significantly weaker than those of TBA4W10O32and two weak peaks in 600~400 cm-1were not found in its FT-IR spectrum. This may be because an aqueous H4W10O32is not instable and its partial degradation prior to measurement has likely occurred during drying its KBr tablet with an infrared lamp. Notably, FT-IR spectrum of solid F-H4W10O32also displayed some characteristic peaks at 967, 903, 779, 585 and 436 cm-1attributable to decatungstate anion. However, in comparison with TBA4W10O32, its characteristic peak attributable to the W-Ob-W band was shifted to a higher wavenumber (903 cm-1) and another peak attributable to the W-Oc-W bond became very weak and appeared at 779 cm-1. In addition, a new peak at 550 cm-1, which are unknown at present, could be also noticed in its FT-IR spectrum. These changes in the FT-IR spectrum of F-H4W10O32should be relative to the substitution of F atoms to the lattice oxygen atoms of decatungstate and such substitution likely occurs upon the side-bridged oxygen atoms of W-Oc-W bonds. It is worth mentioning that FT-IR spectrum of aqueous F-H4W10O32was very similar to that of its solid sample, implying that F-H4W10O32is stable in water.
In order to further confirm the substitution of F atoms to the lattice oxygen atoms of decatungstate, we measured the liquid19F NMR spectrum of F-H4W10O32. As shown in Fig.2, the F-containing H4W10O32clearly presented four19F resonance peaks respectively located at -75.9, -114.6, -131.1 and -150.7 ppm in its19F NMR spectrum, indicating that different coordination states of F-anions are in existence in the F-H4W10O32.Notably, these19F resonance peaks are not consistent with the two peaks at -129.7 and -160.0 ppm in HF and the one at -122.6 ppm in NaF reported by the literature[51]. Therefore, it is reasonable to suggest that F-anions should be doped into the crystal lattice of decatungstate via replacing the lattice O atoms rather than being absorbed on the surface of decatungstate in the form of HF or NaF, as supported by the above FT-IR spectral results.

Fig.2 The 19F NMR of aqueous F-H4W10O32
The liquid UV-Vis spectra of F-H4W10O32and H4W10O32in water were measured on UV-2450 spectrophotometer. As shown in Fig.3, the UV-Vis spectrum of H4W10O32showed two characteristic absorption bands in the 250~350 nm region, in agreement with previous reports[44-45]. The band atλmax=320 nm should be originated from oxygen-to-tungsten charge transition (LMCT) of four linear W-O-W bridge bonds in the decatungstate anion structure[46]and another weak band atλmax=262 nm can be assigned to oxygen-to-tungsten charge transition of the unstable structural subunit[W5O16]2-anions[50]. Notably, F-H4W10O32also exhibited such two LMCT bands in its spectrum, indicating that the substitution of F-anions does not lead to the destruction of decatungstate structure. And such substitution could result in a slight red-shift and an increase in the decatungstate’s LMCT band at 320 nm, along with a decrease in its another LMCT band at 262 nm. This likely implies that the substitution of F-anions can slightly reduce the band gap of decatungstate, and, on the other hand, it plays a positive role in preventing the conversion of decatungstate into the unstable {W5O18} structural subunit, which can be further supported by the following experiments of using an UV-Vis spectrophotometer to monitor the stability of both samples in water. As shown in Fig.4, the LMCT band of H4W10O32at 320 nm in water rapidly decayed with time prolonging and nearly disappeared after 1.5 h, along with an obvious increase in its another LMCT band at 262 nm. This indicates that H4W10O32is very unstable in water and can easily be converted to its unstable {W5O18} structural subunit even at room temperature. And this should be the real reason that solid H4W10O32has not been observed via the heating evaporation process. But this decaying phenomenon described-above was nearly neglected in an aqueous F-H4W10O32when the storage time was not more than 1 h and it became apparent after only 4 h. Still, this LMCT band at 320 nm was observed clearly in the UV spectrum of aqueous F-H4W10O32even up to 12 h. Undoubtedly, this should be due to the substitution of F atoms for their efficiently inhibiting the conversion of decatungstate to unstable {W5O18} structural subunit.

Fig.3 The UV-Vis spectra of aqueous H4W10O32 and F-H4W10O32

Fig.4 The influence of storage time on the stability of F-H4W10O32 and H4W10O32 in water
2.2 Photo-catalytic performance
The photo-catalytic performance of three decatungstates and a phosphotungstic acid (H3PW12O40) was evaluated by the oxidative degradation of aqueous MO by H2O2under UV light illumination, whose results are shown in Table 1. Entries 1-4 show that these catalysts were very active in this photo-oxidation and could achieve a complete degradation of MO solution from orange red to colorless in a shorter irradiation time. However, such oxidative degradation over F-H4W10O32was nearly neglected without UV light illumination (entry5), indicating that this oxidative degradation is indeed triggered by light. Also, the UV light-driven MO degradation could occur in the absence of H2O2or catalyst, but providing a very poor degradation rate (entries6and7). This suggests that the air in the reactor has a marginal contribution to this photo-catalysis degradation. On the other hand, the H2O2itself can be decomposed by UV light to generate small amount of hydroxyl radicals, which may directly oxidizes MO as well. Notably, the present photo-catalysis reaction could efficiently be triggered by visible light, but its degradation rate was much lower than that of UV light (entries8vs1). Among the catalysts examined, the F-H4W10O32showed the highest photo-catalytic efficiency for this degradation reaction. For example, when the concentration of MO was ca. 80 mg/L, the irradiation time to achieve a complete degradation of MO solution over F-H4W10O32only required 30 min, which was much shorter than that required by other catalysts (entries9vs10,11and12). The average degradation rate thus calculated over F-H4W10O32was ca.2.7 mg/L·min, which is 1.67-, 1.98- and 2.03-fold faster than that over H4W10O32, TBA4W10O32and H3PW12O40, respectively. And this predominance in catalysis rate was narrowed at a lower MO concentration (60 mg/L, entries1-4), but it became more noticeable at a higher MO concentration (100 mg/L, entries13-16).

Tab.1 The oxidative degradation of MO under various conditionsa
aCatalyst concentration, 0.6 g/L; H2O2dosage, 0.1 mL;bNo light irradiation;cNo H2O2.;dNo catalyst;eUsing 35 W of tungsten-bromine lamp as visible light source.
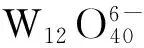
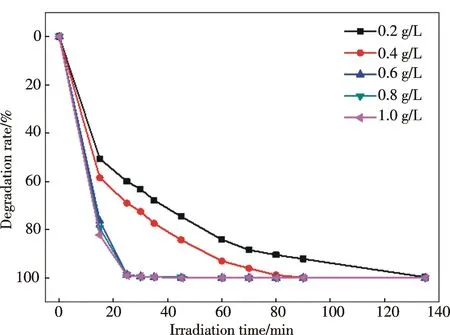
Fig.5 Effect of catalyst concentration on the photo-degradation rate of MO solution (100 mg/L) by H2O2 (0.1 mL) over F-H4W10O32

Fig.6 Effect of H2O2 dosage on the photo-degradation rate of MO solution (100 mg/L) over F-H4W10O32 (0.6 g/L)

Fig.7 Effect of pH on the photo-degradation rate of MO solution (100 mg/L) by H2O2 (0.1 mL) over F-H4W10O32 (0.6 g/L)
2.3MediatedmechanismofFdopingonthephoto-catalyticperformanceofdecatungstate
In order to unveil possible reasons that F-H4W10O32shows a much better catalysis reactivity than H4W10O32in the photo-catalytic degradation of MO by H2O2, the interaction of aqueous decatungstate with H2O2in the absence or presence of MO under UV light illumination was investigated using UV-Vis spectra. Fig.8 (See color pictures on the inside back cover) gives an UV spectral change of F-H4W10O32with the illumination time in the presence of only H2O2. In that, F-H4W10O32as described above in Fig.3 exhibited two characteristic LMCT bands in 250~375 nm in water (Curve A in Fig.8). When H2O2was introduced into the A solution, the band at 320 nm attributable to decatungstate’s structure hardly varied, but the trace in 250~280 nm was rapidly uplifted to the highest position (Curve B0), which may be indicative of an interaction of catalyst with H2O2. When the solution B0was illuminated by sustained UV light, the band at 320 nm continuously decayed with the illumination time, along with a gradual dropping of the trace in 250~280 nm (see curves B1~B5in Fig.8). In the co-existence case of MO and H2O2, the structure band of F-H4W10O32was continuously weakened with illumination time until 40 min, and, after that, it was gradually rebounded toward its starting position with further increasing time, along with a fast dropping of the trace in 250~280 nm in the whole illumination time (Fig.9 (See color pictures on the inside back cover)). Notably, the spectral changes described above could be noticed in a stepwise overlay of UV-Vis spectra for both the H4W10O32-H2O2and H4W10O32-H2O2-MO photoreaction systems (see the corresponding inserts in Figs. 8 and 9). These spectral changes may indicate that decatungstate rapidly reacts with H2O2to generate an intermediate, which can lead to a rapid increase in the absorbance in 250~280 nm. And such an intermediate is gradually decomposed to form an active radical and the reduced catalyst under UV light, thereby leading to the continuous decreases in the absorbance in 250~280 nm attributable to the intermediate and the structure band of original catalyst at 320 nm in the former stage of photoreaction. The resulting active radical may continuously oxidize MO, which can accelerate decomposition of the intermediates. And the reduced catalyst can be directly oxidized by H2O2to its starting oxidation state, and subsequently leads to a gradual recovery of the catalyst’s structure band in the latter stage of photoreaction. Based on the fact that the structure band of the catalyst cannot be recovered at all in the absence of MO, we believe that a re-oxidation of the reduced catalyst should be hampered seriously, which may be due to the rapid decomposition of H2O2initiated by such a radical. In order to further compare the change rate of catalyst and its reactive intermediate with H2O2in various photo-catalysis systems described-above, we assume that the two absorbencies at 320 and 255 nm, respectively, present the concentration (C) of catalyst and its intermediate, and such two absorbencies measured after adding H2O2and followed by various exposure time are defined as the initial and instantaneous concentrations (C0andCt), respectively, thus the change rate constants (k) of both the species can be obtained from the logarithm ofCt/C0vs the exposure time. The following results can be summarized from Fig.10 and Tab.2. i) F-H4W10O32under H2O2interaction and UV light illumination shows a slower decaying rate than that of H4W10O32whether the substrate MO is presence or not. ii) In the presence of MO, the decaying of both catalysts can be markedly accelerated in the former stage of light illumination. And the recovery rate of F-H4W10O32is slightly superior to that of H4W10O32in the later period of photo-irradiation. iii) F-H4W10O32gives a faster rate for decomposition of its intermediate under UV light illumination that of H4W10O32no matter if the substrate MO is presence or not. Notably, the substrate MO can accelerate decomposition of the intermediate under UV light irradiation and such superiority in acceleration became more noticeable upon F-H4W10O32. These findings evidently support that the substitution of F atoms can strengthen the decatungstate’s resistance against degradation by H2O2, thereby leading to significantly improved the catalyst stability under photo-reaction conditions. On the other hand, its substitution can bestow decatungstate with outstanding advantages in accelerating the photo-activated H2O2to oxidize MO and improving the efficiency of photo-catalysis recycling. Evidently, these outstanding advantages of F-H4W10O32should be responsible for its excellent photo-catalysis activity.

Fig.8 The UV spectra (200~375 nm) of F-H4W10O32(8.49×10-5 mol/L) in water containing H2O2. (A) The system in a pure water (0.1 mL),(B0)Adding 0.2 mol/L H2O2(0.1 mL) to the system A, (B1→B5 ) After the system was irradiated using UV light for 10, 30, 40, 50 and 80 min. Inset is the UV spectra (200~375 nm) of H4W10O32 in water containing H2O2 under the same conditions

Fig.9 The UV spectra (200~375 nm) of F-H4W10O32(8.49×10-5 mol/L) in water containing H2O2 and MO. (A) The system in a pure water (0.2 mL),(B0)Adding 0.2 mol/L H2O2(0.1 mL) and 2.5 mg/L MO (0.1 mL) to the system A, (B1→B5 ) After the system was irradiated using UV light for 10, 20, 40, 50 and 80 min. Inset is the UV spectra (200~375 nm) of H4W10O32 in water containing H2O2 and MO under the same conditions
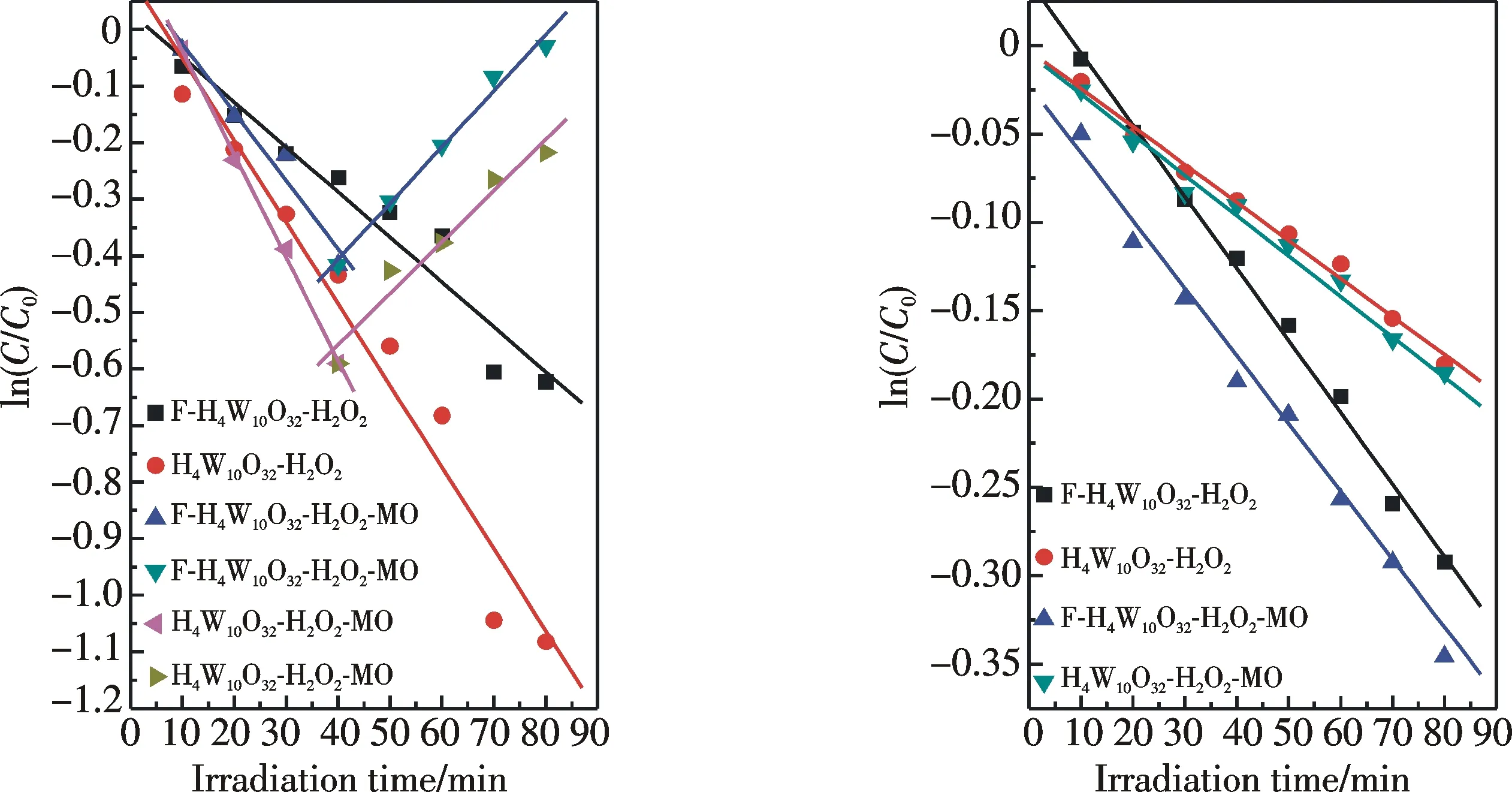
Fig.10 The change rate of catalyst and its reactive intermediate by H2O2 with irradiation time

SystemDecayingrate(k/min-1)at320nmRecoveringrate(k/min-1)at320nmDecomposedrate(k/min-1)at255nmH4W10O32-H2O20.01444—0.00215F-H4W10O32-H2O20.00749—0.00407H4W10O32-H2O2-MO0.012090.009930.00229F-H4W10O32-H2O2-MO0.018230.009080.04830
3 Photo-catalytic reaction mechanism
Based on the above results, as well as those from previous studies[52-54], a reasonable photo-catalysis mechanism is proposed as follows (see Scheme 1): Firstly, decatungstate W10O32-xF2x4-can rapidly capture H2O2to generate an adduct[W10O32-xF2x4--H2O2](Eq.1), and then this intermediate can be excited by UV light to generate a locally excited state[W10O32-xF2x4-*-H2O2]. The extremely reactive W10O32-xF2x4-*of the adduct can oxidize H2O2to generate a peroxy radical (·OOH) and the reduced catalyst HW10O32-xF2x4-(Eq.2) via a hydrogen atom abstraction or electron transfer mechanism[52-54]. The reduced catalyst is easily oxidized by H2O2to its starting oxidation state, along with the generation of hydroxyl radicals (·OH) (Eq.3). Finally, ·OOH and ·OH radicals as major active species are involved in the degradation of MO. In the absence of MO, the ·OOH radical can be further oxidized by W10O32-xF2x4-*(Eq.5) or react with ·OH radical (Eq.6) to achieve the decomposition of H2O2, which leads to a significantly hampered re-oxidation of the reduced catalyst under such conditions, as supported by the UV spectral characterization in Fig.8. Based on this mechanism, the efficiency of the present photo-catalysis reaction should be strongly dependent on the generated rate of ·OOH radicals and the regenerated rate of catalyst. We propose that the introduction of F-anion, with its strongest electro-negativity, can significantly accelerate a transfer of H atom or electron from H2O2to the excited catalyst to explain and rationalzie its outstanding capacities in attracting H2O2and trapping electron, which can lead to the improved generation rate of ·OOH radicals. On the other hand, the substitution of F atoms to the lattice O atoms can strengthen significantly decatungstate’s stability under reaction conditions, thus leading to a tremendous improvements in a photo-catalysis recycling, as supported by the above reaction and characterization results.

Scheme 1 A proposed photo-catalytic reaction mechanism
4 Conclusions
In summary, for the first time, we have designed and prepared an F-substituted decatungstate acid as a highly efficient catalyst for the photo-catalyzed oxidation degradation of MO by H2O2. The proposed catalyst has the following advantages: i) Such catalyst can be prepared conveniently under relatively mild conditions. ii) The introduction of F atoms can bestow the catalyst with an outstanding stability in aqueous solution and excellent capacity in accelerating the transfer of photo-generated electrons to H2O2, which can lead to a significantly improved photo-catalysis efficiency. With this well-behaved kind of catalysts synthesized, we are in a better position now to exploit its possible applications in other organic oxidations.
[1] ZHU L, JO S B, YE S,etal. Rhodamine B degradation and reactive oxygen species generation by a ZnSe-graphene/TiO2sonocatalyst[J]. Chin J Catal, 2014,35(11):1825-1832.
[2] LI X Y, CHEN G H, PO-LOCK Y,etal. Photocatalytic oxidation of cyclohexane over TiO2nanoparticles by molecular oxygen under mild conditions[J]. J Chem Technol Biotechnol, 2003,78(10):1246-1251.
[3] ALMEIDA A R, MOULIJN J A, MUL G. In situ ATR-FTIR study on the selective photo-oxidation of cyclohexane over anatase TiO2[J]. J Phy Chem C, 2008,112(10):1552-1561.
[4] HAMID S B A, TAN T L, LAI C W,etal. Multiwalled carbon nanotube/TiO2nanocomposite as a highly active photocatalyst for photodegradation of reactive blak 5 dye[J]. Chin J Catal, 2014,35(12):2014-2019.
[5] LETTMANN C, HILDENBRAND K, KISCH H,etal. Visible light photodegradation of 4-chlorophenol with a coke-containing titanium dioxide photocatalyst[J]. Appl Catal B, 2001,32(4):215-227.
[6] TARANTO J, FROCHOT D, PICHAT P. Combining cold plasma and TiO2photo-catalysis to purify gascous effluents: A preliminary study using methanol-contaminated air[J]. Ind Eng Chem Res, 2007,46(23):7611-7614.
[7] LIU M S, CAO X H, WENG L Y,etal. Characterization of phosphotungstovanado-heteropolyacid with dawson structure and its photocatalytic activity[J]. Environ Prot Chem Ind, 2012,32(2):177-180.
[8] WANG L. Technical study on removing inhaled PM2.5 by using thermophoresis in annular channel with turbulent flow[J]. Techniq Equip Environ Pollu Contr, 2006,7(3):129-134.
[9] EINAGA H, MISONO M. Photo-catalysis of H3P12W40for 4-cholorophenol decomposition in aqueous media[J]. Bull Chem Soc Jpn, 1996,69(3):3411-3435.
[10] NIU Y X, XING M Y, ZHANG J L,etal. Visible light activated sulfur and iron co-doped TiO2photocatalyst for the photocatalytic degradation of phenol[J]. Catal Today, 2013,201(3):159-166.
[11] CHEN X, MAO S S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and application[J]. Chem Rev, 2007,107(7):2891-2959.
[12] DONG F, WANG H, WU Z. One-step “Green” synthetic approach for mesoporous C-doped titanium dioxide with efficient visible light photocatalytic activity[J]. J Phy Chem C, 2009,113(38):16717-16723.
[13] DIWALD O, THOMPSON T L, ZUBKOV T,etal. Photochemical activity of nitrogen-doped rutile TiO2(110) in visible light[J]. J Phy Chem B, 2004,108(19):6004-6008.
[14] IZUMI Y, ITOI T, PENG S,etal. Site structure and photocatalytic role of sulfur or nitrogen-doped titanium oxide with uniform mesopores under visible light[J]. J Phy Chem C, 2009,113(16):6706-6718.
[15] GOPAL N O, LO H H, KE T F,etal. Visible light active phosphorus-doped TiO2nanoparticles: an EPR evidence for the enhanced charge separation[J]. J Phy Chem C, 2012,116(30):16191-16197.
[16] SHEN G Z, CHO J H, YOO J K,etal. Synthesis and optical properties of S-doped ZnO nanostructures: nanonails and nanowires[J]. J Phy Chem B, 2005,109(12):5491-5496.
[17] LIU G, NIU P, SUN C,etal. Unique electronic strucre induced high photoreactivity of sulfur-doped graphitic C3N4[J]. J Am Chem Soc, 2010,132(33):11642-11648.
[18] HUANG G, SHI R, ZHU Y. Photocatalytic activity and photoelectric performance enhancement for ZnWO4by fluorine substitution[J]. J Mole Catal A-Chem, 2011,348(1-2):100-105.
[19] HUANG G, ZHU Y. Enhanced photocatalytic activity of ZnWO4catalyst via fluorne doping[J]. J Phy Chem C, 2007,111(32):11952-11958.
[20] LI D, HANEDA H, HISHITA S,etal. Fluorine-doped TiO2powders prepared by spray pyrolysis and their improved photocatalytic activity for decomposition of gasphase acetaldehyde[J]. J Fluorine Chem, 2005,126(1):69-77.
[21] SHI R, HUANG G, LIN J,etal. Photocatalytic activity enhancement for Bi2WO6by fluorine substitution[J]. J Phy Chem C, 2009,113(45):19633-19638.
[22] YU J G, WANG W G, CHENG B,etal. Enhancement of photocatalytic activity of mesporous TiO2powders by hydrothermal surface fluorination treatment[J]. J Phy Chem C, 2009,113(16):6743-6750.
[23] HONG X T, WANG Z P, CAI W M,etal. Visible-light-activated nanoparticle photocatalyst of lodine-doped titanium dioxide[J]. Chem Mater, 2005,17(6):1548-1552.
[24] PARK H, CHOI W. Effects of TiO2surface fluorination on photocatalytic reactions and photoelectrochemical behaviors[J]. J Phy Chem B, 2004,108(13):4086-4093.
[25] YU J C, YU J, HO W,etal. Effects of F-doping on the photocatalytic activity and microstructures of nanocrystalline TiO2powders[J]. Chem Mater, 2002,14(9):3808-3816.
[26] XIANG Q, LV K, YU J. Pivotal role of fluorine in enhanced photocatalytic activity of anatase TiO2nanosheets with dominant (001) facets for the photocatalytic degradation of acetone in air[J]. Appl Catal B: Environ, 2010,96(3-4):557-564.
[27] LV K, CHENG B, YU J,etal. Fluorine ions-mediated morphology control of anatase TiO2with enhanced photocatalytic activity[J]. Phy Chem Chem Phy, 2012,14(16):5349-5362.
[28] YU J, LI Q, LIU S,etal. Ionic-liquid-assisted synthesis of uniform fluorinated B/C-codoped TiO2nanocrystals and their enhanced visible-light photocatalytic activity[J]. Chem-A Eur J, 2013,19(7):2433-2441.
[29] WANG J, YIN S, ZHANG Q,etal. Synthesis and photocatalytic activity of fluorine doped SrTiO3[J]. J Mater Sci, 2004,39(2):715-717.
[30] HUANG G L, SHI R, ZHU Y F. Photocatalytic activity and photoelectric performance enhancement for ZnWO4by fluorine substitution[J]. J Mol Catal A: Chem, 2011,348(1):100-105.
[31] SHI R, HUANG G L, LIN J,etal. Photocatalytic activity enhancement for Bi2WO6by fluorine substitution[J]. J Phy Chem C, 2009,113(45):19633-19638.
[32] JIANG H Y, LIU J J, CHENG K,etal. Enhanced visible light photocatalysis of Bi2O3upon fluorination[J]. J Phy Chem C, 2013,117(39):20029-20036.
[33] LIU Y F, LV Y H, ZHU Y Y,etal. Fluorine mediated photocatalytic activity of BiPO4[J]. Appl Catal B: Environ, 2014,147(5):851-857.
[34] MINERO C, MARIELLA G, MAURINO V,etal. Photocatalytic transformation of organic compounds in the presence of inorganic anions. 1. hydroxyl-mediated and direct electron-transfer reactions of phenol on a titanium dioxide-fluoride system[J]. Langmuir, 2000,16(6):2632-2641.
[35] MINERO C, MARIELLA G, MAURINO V,etal. Photocatalytic transformation of organic compounds in the presence of inorganic ions.2 competitive reactions of phenol and alcohols on a titanium dioxide-fluoride system[J]. Langmuir, 2000,16(23):8964-8972.
[36] HILL C L. Progress and challenges in polyoxometalate-based catalysis and catalytic materiials chemistry[J]. J Mol Catal A: Chem, 2007,262(1-2):2-6.
[37] TZIRAKIS M D, LYKAKIS I N, ORFANOPOULOS M. Decatungstate as an efficient photocatalyst in organic chemistry[J]. Chem Soc Rev, 2009,38(3):2609-2621.
[38] LYKAKIS I N, EVGENIDOU E, ORFANOPOULOS M. Photo-catalysis and polyoxo-anion decatungstate in organic chemistry: a manifold concept for green chemistry[J]. Curr Org Chem, 2012,16(20):2400-2414.
[39] FILOWITZ M, HO R K C, KLEMPERER W G,etal. Oxygen-17 nuclear magnetic resonance spectroscopy of polyoxometalates. 1. Sensitivity and resolution[J]. Inorg Chem, 1979,18(1):93-103.
[40] RENNEKE R F, PASQUALI M, HILL C L. Polyoxometalate systems for the catalytic catalytic selective production of nonthermodynamic alkenes form alkanes. Nature of excited-state deactivation processes and control of subsequent thermal processes in polyoxometalate photoredox chemistry[J]. J Am Chem Soc, 1990,112(18):6585-6594.
[41] CHAMBERS R C, HILL C L. Excited states of polyoxometalates as oxidatively resistant initiators of hydrocarbon autoxidation. Selective production of hydroperoxides[J]. Inorg Chem, 1989,28(13):2509-2511.
[42] BIGI F, CORRADINI A, QUARANTELLI C,etal. Silica-bound decatungstates as heterogeneous catalysts for H2O2activation in selective sulfide oxidation[J]. J Catal, 2007,250(2):222-230.
[43] TERMES S C, POPE M T. Reduction of the decatungstate anion in nonaqueous solution and its confirmation as “polytungstate-Y”[J]. Inorg Chem, 1978,17(2):500-501.
[44] WU W F, FU Z H, TANG S B,etal. (nBu4N)4W10O32-catalyzed selective oxygenation of cyclohexane by molecular oxygen under visible light irradiation[J]. Appl Catal B: Environ, 2015,164(3):113-119.
[45] RAFQAH S, CHUNG P W W, FORANO C,etal. Photocatalytic degradation of metsulfuron methyl in aqueous solution by decatungstate anions[J]. J Photochem Photobiol A, 2008,1999(2):297-302.
[46] ALLAOUI A, MALOUKI M A, CHUNG P W W. Efficient degradation of methabenzthiazuron photoinduced by decatungstate anion in water: kinetics and mechanistic studies[J]. Chemosphere, 2011,85(4):558-564.
[47] COLLINS I R, HEDGES B, HARRIS L M,etal. The development of a novel environmentally friendly dual function corrosion and scale inhibitor[J]. SPE Inter Sym Oil Chem, 2001(4):241-249.
[48] RENNEKE R F, PASQUALI M, HILL C L. Polyoxometalate systems for the catalytic selective production of nonthermodynamic alkenes form alkanes. Nature of excited-state deactivation processes and control of subsequent thermal processes in polyoxometalate photoredox chemistry[J]. J Am Chem Soc, 1990,112(18):6585-6594.
[49] ZHU W S, ZHU G P, Li H,etal. Oxidative desulfurization of fuel catalyzed by metal-based surfactant-type ionic liquids[J]. J Mol Catal A: Chem, 2011,347(1):8-14.
[50] FUCHS V J, HARTL H, SCHILLER W,etal. Die kristallstryktur des tributylammoniumdekawolframats[(C4H9)2NH]4W10O32[J].Acta Cryst B, 1976,32:740-749.
[51] QIU Z W, LIU Y L, YE N H,etal. A confirmation of the structure of a new sildenafil analogue[J]. Chin J Anal Sci, 2013,32(4):483-488.
[52] DUNCAN D C, NETZEL T L, HILL C . Early-time dynamics and reactivity of polyxometalate excited states. identification of a short-lived imct excited state and a reactive long-lived charge-transfer intermediate following picosecond flash excitation of[W10O32]4-in acetonitrile[J]. Inorg Chem, 1995,34(18):4640-4646.
[53] TANIELIAN C, DUFFY K, JONES A. Kinetic and mechanistic aspects of photocatalysis by polyoxotungstates: a laser flash photolysis, pulse radiolysis, and continuous photolysis study[J]. J Phy Chem B, 1997,101(21):4276-4282.
[54] HILL C L. Introduction: Polyoxometalates-multicomponent molecular vehicles to probe fundamental issues and practical problems[J]. Chem Rev, 1998,98(1):1-2.
(编辑 WJ)
O643.32
A
1000-2537(2017)05-0059-12
紫外光下氟取代H4W10O32作为一种高效催化剂催化双氧水氧化降解甲基橙
唐泽玉,伏再辉*,汤森培,张慎益,张 超,刘亚纯,尹笃林,李建伟
(国家和地方新型石化材料联合工程实验室,湖南省优质资源优良加工和先进材料利用以及化学生物学与中医药研究重点实验室(中国教育部),湖南师范大学化学化工学院, 中国 长沙 410081)
以钨酸钠与氢氟酸为原料聚合得到氟取代的十聚钨酸(F-H4W10O32),并采用FT-IR,UV-vis和19F-NMR进行表征.由FT-IR和19F-NMR的表征结果得出,F原子可能通过取代W-Oc-W键的侧桥氧原子而被结合到十聚钨酸盐的框架中,显著改善了十聚钨酸盐的稳定性及其对水溶液中H2O2的活化能力.在紫外光照射下催化过氧化氢(H2O2)氧化降解的甲基橙(MO)实验显示出氟取代的十聚钨酸出色的光催化活性,其与亲本H4W10O32相比,降解速率增加到1.4~2.0倍.基于UV-Vis光谱表征,提出了一种合理的光催化氧化机理.
氟取代十聚钨酸;过氧化氢;甲基橙;光催化;氧化性降解
2017-01-04
国家自然科学基金资助项目(21546010,20873040);高等教育博士点专业研究基金资助项目(20124306110005);湖南省自然科学基金资助项目(10JJ2007,14JJ2148)
*通讯作者,E-mail:fzhhnnu@126.com
10.7612/j.issn.1000-2537.2017.05.009

