Synthesis,adsorption and selectivity of inverse emulsion Cd(II)imprinted polymers☆
Luwei Li,Fang Zhu*,Yanhong Lu,Jian Guan
College of Environmental Science and Engineering,Taiyuan University of Technology,Taiyuan 030024,China
1.Introduction
With the development of industry,heavy metal pollution caused serious environmental problem and ecological damage.Compared with organic pollutants,heavy metals could not be degraded by microbial and existed for a long time in the environment.Among all heavy metals,cadmium is a kind of toxic heavy metal and can pose a threat to plants,animals and human safety when the concentration of Cd(II)is high in the environment[1].The methods for treating wastewater containing cadmium include the precipitation method[2],adsorption[3],the ionexchange method[4]and membrane separation[5].Among these methods,adsorption has long been concerned because of its low cost and good removal effect.However,commonly used adsorbent has poor selectively for cadmium ions.Currently,ion imprinted polymer with high selectively and good adsorption capacity has greatly drawn people's attention[6–9].
Ion imprinted technology is developed based on molecular imprinted technology.Ion imprinted polymer can recognize target ion and retain the advantages of molecular imprinted polymer[6].Ion imprinted polymer has served as selective adsorbent for removing target ions[7].A variety of methods have been applied to synthesize Cd(II)imprinted polymer for removing Cd(II)in aqueous sample[8,9].Xi et al.[10]utilized precipitation polymerization method to synthesize cadmium imprinted polymers for removal of Cd(II)from aqueous solutions and environmental samples.Adsorption experiments suggested that the maximum adsorption capacity that could be achieved is46.5 mg·g−1.Li et al.[11]prepared a surface-grafted Cd(II)imprinted polymer using surface imprinting technology.The Cd(II)imprinted polymer had good thermal stability and selectivity and the maximum adsorption amount was 38.3 mg·g−1.Alizadeh[12]applied the copolymerization method to synthesize Cd(II)imprinted polymer and the maximum adsorption capacity for Cd(II)in water samples was 45 mg·g−1.Li et al.[13]employed a one-step hydrothermal process to prepare Cd(II)imprinted particles.The particles had good recognition performance and the adsorption capacity could reach 40 mg·g−1.
Among all of the synthesis methods of molecular or ion imprinted polymer,the emulsion polymerization method including emulsion,inverse emulsion and inverse microemulsion polymerization could be used to prepare molecularly or ion imprinted polymer[14,15].The inverse emulsion polymerization method is able to speed up the polymerization rate and the product has good performance[16].Luo et al.[17]used crown ether as functional monomer to synthesis novel Pb(II)ion imprinted polymer(IIP)by inverse emulsion polymerization.Compared with the non-imprinted polymer,IIP had high adsorption capacity(27.95 mg·g−1)and selectivity coefficients.Younis et al.[18]prepared ion imprinted polymer for removing Cu(II)by O/W/O emulsion polymerization.The superior adsorption capacity could reach 84 mg·g−1.The selectivity for Cu(II)is four times than that of other bivalent ions.
In this work,inverse emulsion polymerization wasemployed to prepare inverse emulsion Cd(II)imprinted polymers(IEIIP),with Cd(II)as template,acrylamide and β-cyclodextrin as functional monomers,epichlorohydrin as cross linking agent,ammonium persulfate as initiator and liquid paraf fin as oil phase.SEM,FTIR and TG were used for characterization of IEIIP.The adsorption properties of IEIIP were investigated under different adsorption conditions.The selectivity of polymers was studied in binary solutions.The adsorption property of IEIIP was evaluated by kinetics and thermodynamics.
2.Experimental
2.1.Materials and instruments
Acrylamide(AM), β-cyclodextrin(β-CD),Span 80,Tween 20,CdCl2·2.5H2O,epichlorohydrin(ECH),liquid paraf fin,ammonium persulfate,CuCl2·2H2O,Zn(NO3)2·6H2O,acetic acid,methyl alcohol and ethyl alcohol were supplied by Sinopharm Chemical Reagent Co.,Ltd.,China.Lead nitrate was purchased from Tianjin Damao Chemical Reagent Industry,China.NaOH and HNO3for pH adjustment were supplied by Sinopharm Chemical Reagent Co.and Tian Jin Chemical Co.,Ltd.,China,respectively.
The static adsorption experiments were conducted in a SHAC constant-temperature shaker,which was purchased from Changzhou Guohua Instrument Co.,Ltd.,China.ATAS-990 flame atomic adsorption spectrometer(FAAS,Beijing Purkinje General Instrument Co.,Ltd.,China)was used to measure the concentrations of Cd(II),Zn(II),Cu(II)and Pb(II).A JSM-6700F Field Emission Scanning electron microscope(SEM)(Japan Electron Optics Laboratory Co.,Ltd.,Japan)was used to analyze the surface morphology of IEIIP.The Fourier transmission infrared spectra(FTIR,450 cm−1–4500 cm−1)with KBr pellets by a Bruker Tensor 27 FTIR spectrometer(Bruker,Germany)were used to analyze various functional groups of IEIIP.The TG 209 F3 thermogravimetric analyzer was applied to analyze the thermal stability of IEIIP as the temperature changes from 30 °C to 850 °C with the heating rate of 10 °C·min−1under argon atmosphere.
2.2.Synthesis of IEIIP
IEIIP was synthesized by inverse emulsion polymerization.4 g β-cyclodextrin was dissolved in sodium hydroxide aqueous solution and 0.75 g CdCl2·2.5H2O was dissolved in distilled water.Two kinds of solution were mixed and stirred slowly for 30 min at 303 K.Subsequently,3.11 g AM was added into the solution and continue to stir for 30 min to mix uniformity.Then,a mixed emulsifier(Span 80 and Tween 20)solved in the liquid paraf fin was mixed with the above mixture and rapidly stirred for 30 min to form an inverse emulsion.After rising to 60°C,3.4 mlECH and 0.006 g ammonium persulfate were added to the inverse elution and stirred for 3 h.After the end of the reaction,the product was demulsified by ethanol.Cd(II)imprinted polymer was eluted with methanol and acetic acid(Vol:Vol=9:1)and distilled water to get IEIIP.Finally,IEIIP was dried in vacuum at 70°C for 24 h.
2.3.Adsorption experiments
The adsorption ability of IEIIP was studied by static adsorption experiments.0.01 g IEIIP was put into 50 ml Cd(II)solutions.The adsorption time was 0–80 min.The concentration ofCd(II)was 0–150 mg·L−1.The pH value of Cd(II)aqueous solution was adjusted to 5,6 and 7 by NaOH and HCl dilute solution.The reaction temperature was 293 K,303 K and 313 K.The adsorption experiments were conducted in the constant temperature oscillator under different conditions.IEIIP was filtered and the residual concentration was determined by FAAS.The adsorption capacity(mg·g−1)was calculated by the following equation:

where,Co,Ctand Ce(mg·L−1)are the initial concentration of Cd(II),concentration of Cd(II)at t min and the equilibrium Cd(II)concentration,respectively.
The selective property of IEIIP was verified by competition adsorption experiments.Zn(II),Cu(II)and Pb(II)that had similar ionic radius and same charge with Cd(II)were used as competing ions.The study of selectivity was carried out in 50 ml binary solutions of Cd2+/Pb2+,Cd2+/Cu2+and Cd2+/Zn2+under 303 K for 80 min.The concentrations of Cd(II)and other metal ions were measured using FAAS after adsorption reached equilibrium.
3.Results and Discussion
3.1.Synthesis and characterization

Fig.1.The schematics of reaction process.

Fig.2.SEM images of Cd(II)imprinted polymers.
Fig.1 presents the schematic of reaction for crosslinked polymer formation.Oil phase and water phase formed an inverse emulsion through an emulsifier.Then,β-cyclodextrin and AM as functional monomer reacted with Cd(II)to form an imprinted polymer under crosslinking ECH and initiator ammonium per sulfates.The chemical structure of the resulted polymer was showed in Fig.1.
The morphology of IEIIP before and after elution was analyzed using a scanning electron microscope(SEM),as shown in Fig.2.The approximate thickness of IEIIP is about 1 μm.The surface of IEIIP before elution is smooth.While,the surface of IEIIP after elution is rough and has rich pore structure.Imprinted sites were left on the IEIIP after eluting template Cd(II).In the preparation process of IEIIP,eluting template ions could improve the adsorption surface area and binding sites to increase the adsorption capacity.
The existence of functional groups in IEIIP could be confirmed by FTIR spectra.Fig.3 showed the FTIR spectra ofIEIIP before and after elution.The absorption peaks of IEIIP before and after elution have the same position.The N--H stretching vibration in functional monomer acrylamide and stretching vibration of rest O--H after the crosslinking reaction between β-cyclodextrin and epichlorohydrin are shown at 3409 cm−1–3330 cm−1.The anti-symmetric stretching vibration of--CH2--is re flected by the peak of 2923 cm−1.The absorption peak at 1641 cm−1is C=O stretching vibration and N--H bending vibration in acrylamide.The peak at 1029 cm−1indicates the presence of Cc--O group on the IEIIP.Results showed that IEIIP have synthesized successfully using the monomers of β-cyclodextrin and acrylamide.

Fig.3.FTIR spectra of Cd(II)imprinted polymers:(a)before and(b)after elution.
Fig.4 presented the thermal mass loss of IEIIP before and after elution.In the first stage,the mass loss of IEIIP before and after elution changed from 100%to 35.67%and 100%to 34.15%at 30 °C to 400 °C.The IEIIP decomposed at this stage.At 850°C,the mass of IEIIP before elution was 22.54%,while the mass of IEIIP after elution was only 21.34%.This is because the IEIIP before elution contains cadmium ion.Cadmium is a kind of heavy metal and does not decompose with increasing temperature.Therefore,the mass of IEIIP before elution is heavier than that of IEIIP after elution at 850°C.The results of the TGA thermogram showed that cadmium was successfully imprinted and eluted.
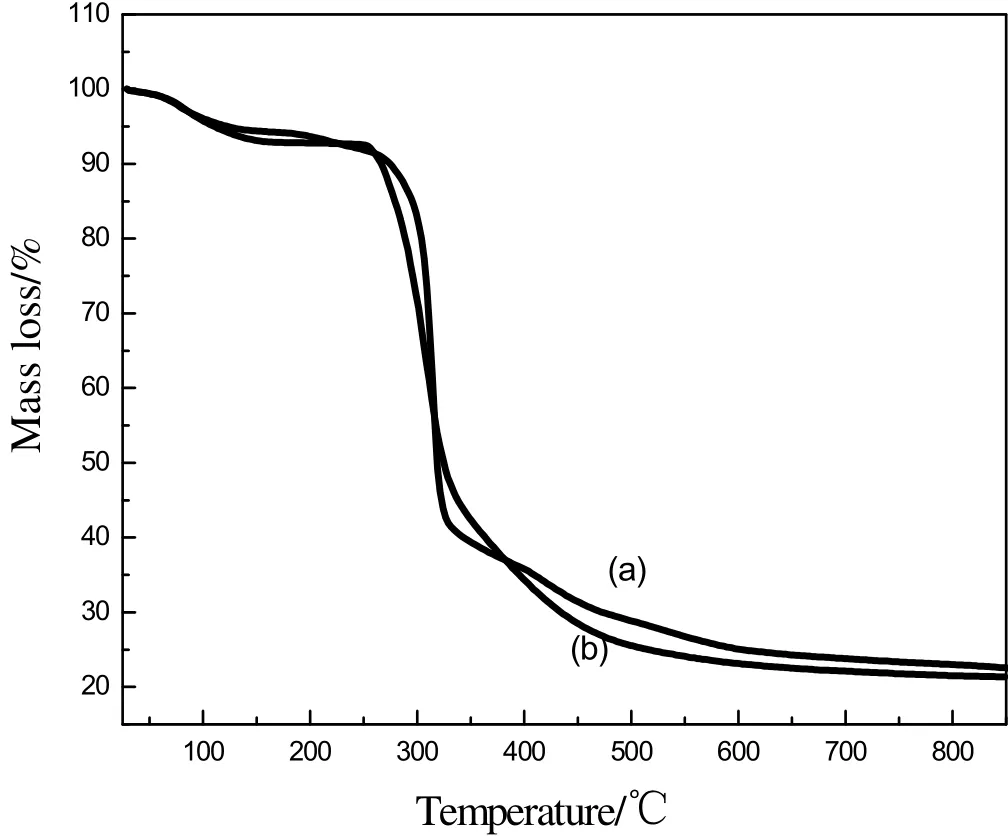
Fig.4.TG curves:(a)IEIIP before elution;(b)IEIIP after elution.
3.2.The in fluence of pH values on the adsorption process
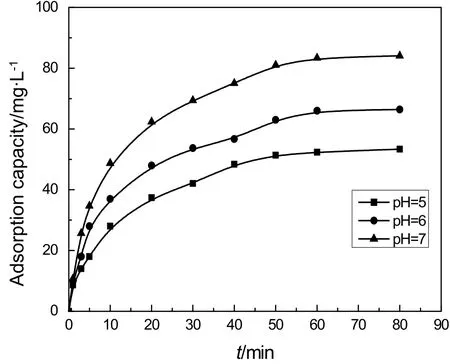
Fig.5.Effect of pH value on the adsorption capacity.
The adsorption capacity of IEIIP is significantly affected by the pH value of the aqueous solution.Results in Fig.5 present the influence of pH values on the adsorption process of IEIIP.As shown in Fig.5,the adsorption capacity for IEIIP is increased with the rise of pH values of Cd(II)aqueous solution.The adsorption capacity is 53 mg·g−1at pH=5.With pH=7,the adsorption capacity could reach 86.7 mg·g−1.The pHvalues of Cd(II)aqueous solution have a significant effect on the adsorption capacity of Cd(II).In acidic conditions,the hydroxyl groups in the functional monomer β-cyclodextrin could be protonated with H+,resulting in electrostatic repulsion with Cd(II)in aqueous solutions.Moreover,Cd(II)and H+could produce competitive adsorption on the surface of IEIIP.Therefore,metal ion is noteasy to beadsorbed on the surface of polymer materials with positive charge,leading to the decrease of adsorption capacity of IEIIP.With the increase of pH,the hydroxyl protonation gradually weakens and the negative charge on the surface of IEIIP increases.The competitive adsorption of Cd(II)with H+is also weakened.The adsorption capacity of IEIIP increases with the increase of pH value.However,Cd(II)and OH−are easy to form hydroxide under alkaline conditions,leading to the reduction of concentration of Cd(II)in Cd(II)aqueous solutions and the adsorption capacity of IEIIP begin to decrease.
3.3.The influence of initial concentration on the adsorption process
As shown in Fig.6,the initial concentration of Cd(II)greatly affects the uptake of IEIIP for Cd(II)in aqueous solution.Fig.6 presented that the maximum adsorption capacity of IEIIP in aqueous solution increased quickly at the low initial concentration.With the increase of the initial concentration of Cd(II),the adsorption capacity of IEIIP reached maximum adsorption capacity at the concentration of Cd(II)120 mg·L−1.Under high initial concentration,more and more Cd(II)gathered on the surface of IEIIP easily,causing the increase of metal ion uptake.When IEIIP reached adsorption saturation,the adsorption capacity kept equilibrium with increasing of initial concentration of Cd(II)in aqueous solutions.
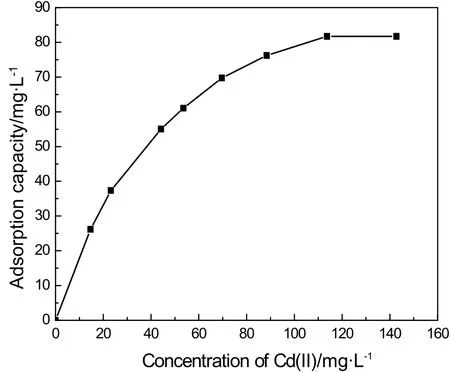
Fig.6.Effect of the concentration of Cd(II)on adsorption capacity.
3.4.The influence of adsorption time and temperature on the adsorption process
Adsorption time and temperature could influence the adsorption behavior of IEIIP.Fig.7 presents the effects of adsorption time and temperature on the adsorption capacity of IEIIP for Cd(II)in the aqueous solutions.In the initial stage of 30 min,adsorption rate is fast.The adsorption reaches equilibrium when the adsorption time is 80 min.Cd(II)in the aqueous solution adsorbed on imprinted sites of IEIIP in the initial stage.As the reaction time goes on,the adsorption process reaches adsorption equilibrium.Fig.7 shows that the equilibrium adsorption capacity values are 69.8,86.7 and 51 mg·g−1when the temperatures are 293 K,303 K and 313 K,respectively.Results suggest that the adsorption capacity for Cd(II)in aqueous solution is increased and then decreased with the increase of temperature.The adsorption capacity achieves maximum value under a temperature of 303 K.High or low temperature go against adsorption process.At low temperature,the mobility of ions is low,which lowers the ion's ability to reach the cavity.At higher temperature,due to high mobility,the stability of template/functional group interaction is lower,making the adsorption lower.Namely,303 K is the optimum temperature for adsorption conditions.

Fig.7.Effect of time and temperature on the adsorption capacity.
3.5.Adsorption kinetics
The mechanism of adsorption is investigated by adsorption kinetic models.Pseudo first and second order kinetic models are used to evaluate the whole adsorption behavior of IEIIP.The regression coefficients and corresponding rate constants are obtained.The pseudo first order kinetic model and pseudo second order model are expressed as follows:

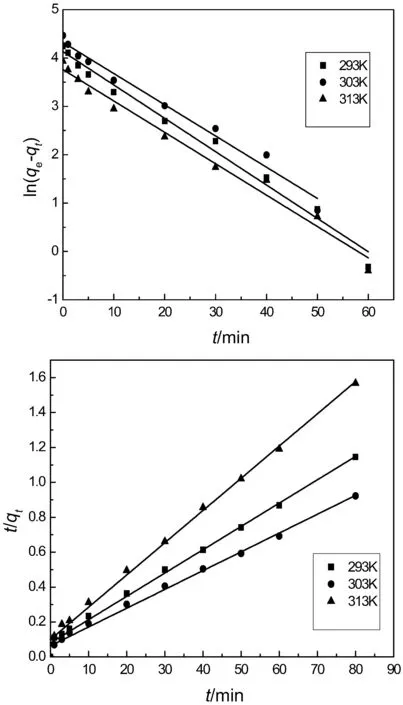
Fig.8.Pseudo first and pseudo second order kinetic models.
where,qtis the adsorption capacity of IEIIP at t min,(mg·g−1);qerepresents the adsorption capacity at equilibrium moment,(mg·g−1);k1(min−1)and k2(g·mg−1·min−1)are the rate constants of the pseudo first and second order kinetic models,respectively.Linear fit of kinetic models are shown in Fig.8.The theoretical adsorption capacity,rate constants and correlation coefficients are listed in Table 1.The correlation coefficients R2of the pseudo first order kinetic model are 0.9856,0.9801 and 0.9818at 293 K,303 K and 313 K,respectively.The pseudo first order kinetic model is inappropriate for describing the whole adsorption process.As shown in Fig.8,the linear of pseudo first order kinetic model has better fitting in the stage of 30 min,suggesting that the pseudo first order kinetic model is more appropriate for describing the initial stage of adsorption behavior.The R2of the pseudo second order kinetic model are 0.9930,0.9910 and 0.9941,respectively.R2obtained from the pseudo second order kinetic model is closer to 1.The theoretical adsorption capacity obtained by the pseudo first order kinetic modelis far from the experimental adsorption capacity.The theoretical adsorption capacity obtained by the pseudo second order kinetic modelis closerto the experimental adsorption capacity.Hence,the pseudo second order kinetic model is more suitable for describing theadsorption process of IEIIP than the pseudo first order kinetic model.The chemical adsorption process is the rate controlling step in the adsorption process.
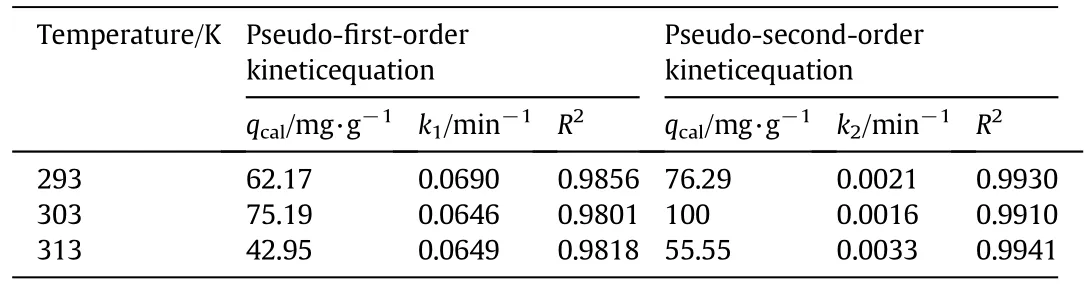
Table 1 Fitting results of Cd(II)adsorption kinetic equations
3.6.Adsorption isotherm
The Langmuir isotherm and Freundlich isotherm models are used to analyze the adsorption property.The relationship between the concentration and adsorption capacity at equilibrium moment could be analyzed by isotherm models.The models are described as follows:

where,Ceis the equilibrium concentration of Cd(II),(mg·L−1);qerepresents the adsorption capacity of IEIIP at equilibrium moment,(mg·g−1);qmis the maximum adsorption capacity,(mg·g−1);k and kFare the Langmuir and Freundlich constants,respectively;1/n is the adsorption index.The values of k and 1/n could be obtained by the slope and intercept of linear equations,respectively.The Langmuir and Freundlich isotherms are applied to describe the homogeneous and heterogeneous adsorption,respectively.The linear plot and isotherm constants of the Langmuir and Freundlich isotherms are showed in Fig.9 and Table 2.The R2and k values of the Langmuir isotherm are 0.993 and 0.2319,respectively.The values of R2,k and 1/n of the Freundlich isotherm are 0.958,7.486 and 0.511,respectively.Asshown in Fig.9,linear fitting of the Freundlich isotherm presents a nonlinear relationship.1/n is 0.511,showing that adsorption reaction is easy to be performed.Therefore,the adsorption behavior could be described by the Langmuir isotherm.Results suggest that the adsorption of IEIIP is in mono molecular type and homogeneous.

Fig.9.Langmuir and Freundlich isotherm models.

Table 2 Fitting results of Cd(II)adsorption isotherm equations
3.7.Adsorption thermodynamics and activation energy
The difficulty degree and spontaneity of the adsorption process could be analyzed by thermodynamic behavior.Thermodynamic parameters including changes of standard free energy ΔG0,changes of enthalpy Δ H0and the changes of entropy Δ S0could be calculated by Eqs.(7)and(8).The Arrhenius Equation(Eq.(9))is used to calculate the reaction activation energy Ea.

where,R(8.314 J·mol−1·K−1)and KDrepresent the universal gas constant and adsorption equilibrium constant,respectively.Changes of ΔH0and ΔS0are calculated based on the slope and interceptby 1/T versus ln KD,respectively.Eaand A0are apparent activation energy(kJ·mol−1)and Gibbs free energy factors,respectively.Results of thermodynamic parameters for adsorption of Cd(II)are presented in Table 3.The values of ln KDincrease first and then decrease with the temperature rising from293 Kto 313 K.The results show that303 K is most beneficial to the adsorption process.Under different temperatures,the values ofΔG0are negative,suggesting the spontaneity of the adsorption process.The standard enthalpyΔH0is positive,indicating the endothermic reaction of IEIIP for Cd(II).The changes of entropy ΔS0are positive,suggesting that the adsorption process for Cd(II)in aqueous solution is an entropy increase process.An entropy increase process could cause the increase of disorder in an adsorption system,re flecting that the interaction between IEIIP and Cd(II)produces the structural changes.The reaction activation energy Eais 37.44 kJ·mol−1,showing the adsorption process could be performed easily.
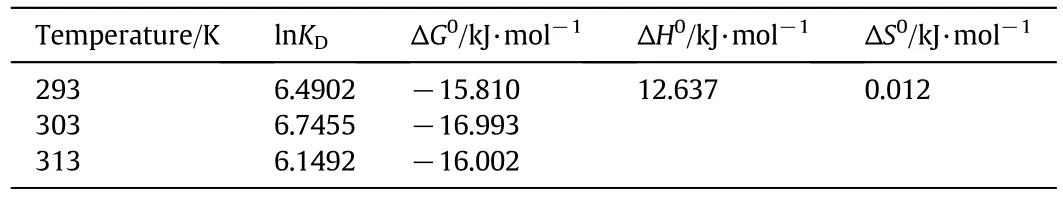
Table 3 Thermodynamic parameters at different temperatures
3.8.Adsorptive selectivity
The selective property of IEIIP was verified by competition adsorption experiments.Zn(II),Cu(II)and Pb(II)that have similar ionic radius and same charge with Cd(II)were used as competing ions.The study of selectivity was carried outin binary solutions ofCd2+/Pb2+,Cd2+/Cu2+and Cd2+/Zn2+under 303 K for 80 min.The concentrations of Cd(II)and other metal ions were measured using FAAS after adsorption reached equilibrium.The distribution coefficient kdand selective coefficient k were obtained according to the following formulas:

where,kdis the distribution coefficient(ml·g−1);k is the selective coefficient;and kd(Cd(II))and kd(X(II))are the selective coefficients of Cd(II)and disturbing ions,respectively.The distribution coefficients and selective coefficients are presented in Table 4.The selectivity coefficients k of Cd2+/Pb2+,Cd2+/Zn2+and Cd2+/Cu2+are 2.4998,1.2437 and 4.6882,respectively.From the selectivity coefficient,IEIIP has better adsorption performance for Cd(II)than that of Pb(II),Cu(II)and Zn(II)in binary solutions.Namely,IEIIP has high selectivity for target ion.The recognition sites and specific binding sites are formed in the synthesis process of IEIIP.The size and shape of the imprinted sites could be matched with cadmium ions.While,the shape of competing ions who have similar ionic radius and same charge with Cd(II)could not match the recognition sites well.Hence,IEIIP has high selectivity for Cd(II)in binary solution(Fig.10).

Table 4 Distribution coefficient and selectivity coefficient of selective adsorption

Fig.10.The adsorption capacity of IEIIP for different ions.
4.Conclusions
In this paper,IEIIP using acrylamide and β-cyclodextrin as functional monomers,epichlorohydrin as cross linking agent and ammonium persulfate as initiator were prepared by inverse emulsion polymerization.The IEIIP were used to absorb Cd(II)in aqueous solutions selectively.The adsorption data suggested that the adsorption capacity of IEIIP could reach 86.7 mg·g−1under the optimum adsorption conditions.The pseudo second order kinetic model(0.990–0.9941)and Langmuir isotherm model(R2>0.99)could evaluate the adsorption process well.Thermodynamic parameters(ΔG0<0,ΔH0>0,ΔS0>0)showed the adsorption reaction was spontaneous and endothermic.The selectivity coefficients k of Cd2+/Pb2+,Cd2+/Zn2+and Cd2+/Cu2+were 2.4998,1.2437 and 4.6882,respectively,showing the good selectivity for Cd(II)in aqueous solutions.Hence,the IEIIP could be used to treat Cd(II)in waste water.
[1]T.S.Nawrot,J.A.Staessen,H.A.Roels,E.Munters,A.Cuypers,T.Richart,A.Ruttens,K.Smeets,H.Clijsters,J.Vangronsveld,Cadmium exposure in the population:From health risks to strategies of prevention,Biometals 23(2010)769–782.
[2]S.Mauchauffée,E.Meux,M.Schneider,Selective precipitation of cadmium from nickel cadmium sulphate solutions using sodium decanoate,Sep.Purif.Technol.62(2008)394–400.
[3]A.Sari,M.Tuzen,Cd(II)adsorption from aqueous solution by raw and modified kaolinite,Appl.Clay Sci.88–89(2014)63–72.
[4]C.W.Wong,J.P.Barford,G.H.Chen,G.McKay,Kinetics and equilibrium studies for the removal of cadmium ions by ion exchange resin,J.Environ.Chem.Eng.2(2014)698–707.
[5]L.B.Chaudhari,Z.V.P.Murthy,Separation of Cd and Ni from multicomponent aqueous solutions by nano filtration and characterization of membrane using IT model,J.Hazard.Mater.180(2010)309–315.
[6]S.L.Wei,Y.Liu,M.D.Shao,L.Liu,H.W.Wang,Y.Q.Liu,Preparation of magnetic Pb(II)and Cd(II)ion-imprinted microspheres and their application in determining the Pb(II)and Cd(II)contents of environmental and food samples,RSC Adv.4(2014)29715–29723.
[7]Y.Jiang,D.Kim,Synthesis and selective adsorption behavior of Pd(II)-imprinted porous polymer particles,Chem.Eng.J.232(2013)503–509.
[8]J.J.Wang,F.Liu,Synthesis and application of ion-imprinted interpenetrating polymer network gel for selective solid phase extraction of Cd2+,Chem.Eng.J.242(2014)117–126.
[9]N.Zhang,B.Hu,Cadmium(II)imprinted 3-mer captopropyltrimethoxysilane coated stir bar for selective extraction of trace cadmium from environmental water samples followed by inductively coupled plasma mass spectrometry detection,Anal.Chim.Acta 723(2012)54–60.
[10]Y.Xi,Y.T.Luo,J.M.Luo,X.B.Luo,Removal of cadmium(II)from wastewater using novel cadmium ion-imprinted polymers,J.Chem.Eng.Data 60(2015)3253–3261.
[11]M.Li,C.G.Feng,M.Y.Li,Q.X.Zeng,Q.Gan,H.Y.Yang,Synthesis and characterization of a surface-grafted Cd(II)ion-imprinted polymer for selective separation of Cd(II)ion from aqueous solution,Appl.Surf.Sci.332(2015)463–472.
[12]T.Alizadeh,An imprinted polymer for removal of Cd2+from water samples:Optimization of adsorption and recovery steps by experimental design,Chin.J.Polym.Sci.29(6)(2011)658–669.
[13]W.M.Li,R.He,L.Tan,S.Y.Xu,C.C.Kang,C.H.Wei,Y.W.Tang,One-step synthesis of periodic ion imprinted mesoporous silica particles for highly specific removal of Cd2+from mine wastewater,J.Sol-Gel Sci.Technol.78(2016)632–640.
[14]M.Ornelas,D.Loureiro,M.J.Araújo,E.Marques,C.Dias-Cabral,M.Azenha,F.Silva,Synthesis of glycylglycine-imprinted silica microspheres through different waterin-oil emulsion techniques,J.Chromatogr.A 1297(2013)138–145.
[15]M.Monier,N.H.Elsayed,Selective extraction of uranyl ions using ionimprintedchelating microspheres,J.Colloid Interface Sci.423(2014)113–122.
[16]M.J.Shin,Y.J.Shin,S.W.Hwang,J.S.Shin,Recognizing amino acid chirality with surface-imprinted polymers prepared in W/O emulsions,Int.J.Polym.Sci.2013(2013)1–5.
[17]X.B.Luo,L.L.Liu,F.Deng,S.L.Luo,Novelion-imprinted polymer using crown ether as a functional monomer for selective removal of Pb(II)ions in real environmental water samples,J.Mater.Chem.A 1(2013)8280–8286.
[18]M.R.Younis,S.Z.Bajwa,P.A.Lieberzeit,W.S.Khan,A.Mujahid,A.Ihsan,A.Rehman,Molecularly imprinted porous beads for the selective removal of copper ions,J.Sep.Sci.39(2016)793–798.
 Chinese Journal of Chemical Engineering2018年3期
Chinese Journal of Chemical Engineering2018年3期
- Chinese Journal of Chemical Engineering的其它文章
- Numerical investigation on flow and heat transfer characteristics of corrugated tubes with non-uniform corrugation in turbulent flow
- Investigations on pool boiling critical heat flux,transient characteristics and bonding strength of heater wire with aqua based reduced graphene oxide nano fluids
- Heavy metals adsorption by banana peels micro-powder:Equilibrium modeling by non-linear models
- Potential aspect of rice husk biomass in Australia for nanocrystalline cellulose production
- Fouling evaluation on membrane distillation used for reducing solvent in polyphenol rich propolis extract
- Investigation on a vertical radial flow adsorber designed by a novel parallel connection method☆
