快速老化小鼠模型NgR、磷酸化Tau蛋白表达情况及其关系的研究
温世荣 许杰 赵延峰 郑姣琳 李鹤 赵秀丽 张欣
[摘要] 目的 利用Nogo受體(NgR)拮抗剂NEP1-40研究P8品系快速老化小鼠(SAMP8)鼠脑中NgR与磷酸化Tau蛋白(p-Tau)表达情况,探讨NgR可能作用。 方法 6月龄抗快速老化系1(SAMR1)雄性小鼠为对照组(A组),3月龄SAMP8鼠(B组),6月龄SAMP8鼠为假治疗组(C组)和治疗组(N组),每组6只。C组小鼠腹腔注射0.9%生理盐水0.2 mL、N组小鼠0.2 mL NEP1-40各14 d。治疗前后分别采用Morris水迷宫试验监测小鼠行为学改变,利用免疫组化染色检测神经细胞内p-Tau和NgR表达的变化。 结果 B、C、N组与A组比较,水迷宫试验潜伏期明显延长、穿越平台次数明显减少(P < 0.05);N组第二次与第一次水迷宫实验比较,潜伏期明显缩短、跨越平台次数明显增多(P < 0.05)。神经元胞浆、突起NgR阳性棕黄色颗粒状结构与细胞膜关系密切,C组海马NgR阳性细胞计数和光密度值较A组和N组明显增多(P < 0.05)。C组p-Tau阳性细胞沉积较A组和N组明显增加(P < 0.05)。 结论 SAMP8鼠随月龄增大,学习记忆能力减退,NgR及p-Tau在海马沉积增多明显,与小鼠老化程度相一致。NgR拮抗剂NEP1-40可能是通过下调NgR表达、减少下游p-Tau表达量,改善小鼠学习及记忆能力。
[关键词] 阿尔茨海默病;Tau蛋白;Nogo受体;NEP1-40
[中图分类号] R749.16 [文献标识码] A [文章编号] 1673-7210(2019)09(b)-0022-04
The expression and relationship between NgR and p-Tau in brains of senescence accelerated mice
WEN Shirong1 XU Jie2 ZHAO Yanfeng3 ZHENG Jiaolin3 LI He1 ZHAO Xiuli1 ZHANG Xin1
1.Department of Neurology, the First Affiliated Hospital of Harbin Medical University, Heilongjiang Province, Harbin 150001, China; 2.Department of Neurology, the Second Hospital of Qinhuangdao, Hebei Province, Qinhuangdao 066600, China; 3.Department of Neurology, the Second Affiliated Hospital of Harbin Medical University, Heilongjiang Province, Harbin 150086, China
[Objective] To investigate the expression of NgR and p-tau in senescence accelerated mouse P8 (SAMP8) was studied by using Nogo receptor (NgR) antagonist NEP1-40, explore the possible role of NgR. Methods Six months old senescence accelerated mouse-resistence 1 (SAMR1) male mice were the control group (group A); 3 months old SAMP8 mice (group B). Sham treatment group (group C) and treatment group (group N) of SAMP8 mice at 6 months of age, with 6 mice in each group. Mice in group C were intraperitoneally injected with 0.2 mL 0.9% normal saline, and mice in group N were intraperitoneally injected with 0.2 mL NEP1-40 for 14 days. Morris water maze test was used to monitor behavioral changes in mice before and after treatment, and immunohistochemical staining was used to detect changes in p-Tau and NgR expression in nerve cells. Results Compared with group A, the incubation periods of water maze test in group B, C and N were significantly prolonged, and the times of crossing platform were significantly reduced (P < 0.05). Compared with the first water maze experiment, the incubation period of group N was significantly shortened and the times of crossing platform were significantly increased (P < 0.05). The NgR positive cell count and optical density of the hippocampus in group C were significantly increased compared with those in group A and group N (P < 0.05). The deposition of p-Tau positive cells in group C was significantly reduced compared with that in group A and group N(P < 0.05). Conclusion The learning and memory ability of SAMP8 mice decreased with the aging of the month, and NgR and p-Tau increased significantly in the hippocampus, which was consistent with the aging degree of the mice. NgR antagonist NEP1-40 may improve the learning and memory ability of mice by down-regulating NgR expression and reducing downstream p-Tau expression.
[Key words] Alzheimer′s disease; Tau protein; NgR; NEP1-40
阿尔茨海默病(Alzheimer′s disease,AD)患者海马锥体层细胞Nogo受体(NgR)免疫活性高达50%以上,特别是CA1~CA2区有大量依赖磷酸化Tau蛋白单克隆抗体AT-8免疫阳性细胞,绑定和识别磷酸化Tau蛋白丝氨酸199/202位点[1]。NgR阳性神经元与神经纤维缠结样改变及AT-8在AD患者海马CA1区共区域化,提示NgR可能与AD神经纤维缠结相关。但AD者CA1区约2/3NgR免疫活性神经元AT-8阴性[2]。本研究利用快速老化小鼠模型,腹腔注射NgR抑制剂NEP1-40,检测NgR表达情况及对Tau蛋白表达影响,以阐释NgR在AD病理改变中的作用。
1 材料与方法
1.1 实验动物
3、6月龄雄性P8品系快速老化小鼠(SAMP8),6月龄雄性抗快速老化系1(SAMR1)鼠,购自天津中医药大学第一附属医院(合格证书:0004580),实验程序由哈尔滨医科大学附属一院动物使用委员会批准,动物伦理号:2016010,置于SPF环境饲养和护理。
1.2 仪器与试剂
1.2.1 仪器 小鼠Morris水迷宫仪、自动数据采集及处理系统(黑龙江省中医药大学);尼康(Nikon)50i研究型正置显微镜;NIS-ElementsF 3.21图像采集软件(Nikon Inc. Japan);图像分析软件(ImagePro-Plus6,MediaCybernetics,Silver Spring,MD,USA)。
1.2.2 试剂 Rabbit Anti-Nogo receptor、兔二抗(博士德生物),p-Tau(sc-101813,SANTA CRUZ生物),NEP1-40(BOC Sciences,475221-20-6)等。
1.3 实验方法
1.3.1 实验动物分组 小鼠适应性饲养1周分6月龄SAMR1组(A组)、3月龄SAMP8组(B组),6月龄SAMP8小鼠随机分为假治疗组(生理盐水,C组)、治疗组(NEP1-40,N组)。
1.3.2 水迷宫实验(Morris水迷宫) 第1、2天训练(可视平台),第3~5天定向航行(隐蔽平台),第6天空间探索(去平台),图像采集分析系统记录动物游泳轨迹数据。定位航行随机取东、西、南、北4个起始位置,找到水下平台时间(s)即潜伏期;空间搜索撤除平台,动物由原象限对侧入水,记录动物进入目标象限次数。
1.3.3 药物干预及二次水迷宫实验 初次水迷宫后,C组腹腔注射0.9%生理盐水0.2 mL/只,N组12.5 μg/(kg·d)NEP1-40(0.2 mL/只),每日1次,连续2周。A组和B组不干预。按初次方法重复水迷宫实验并采集数据。
1.3.4 灌注取脑及切片 二次水迷宫后,所有小鼠用生理盐水60~100 mL、4%多聚甲醛100~150 mL左心室灌注,取脑,4%甲醛固定、常规梯度酒精脱水、二甲苯透明,石蜡包埋,3 μm连续冠状切片。
1.3.5 免疫组织化学染色 石蜡切片脱蜡,脱水,热抗原修复、室温兔血清封閉。一抗p-Tau(1∶80)、NgR(1∶20) 4°C过夜。兔二抗,室温孵育20 min。DAB染色。苏木精复染,脱水、透明、封片。每标本取5张切片,高倍镜(400×)海马区不重叠5个视野观察拍照,阳性细胞(神经元胞浆、突起接近细胞膜棕黄色颗粒状结构为阳性)计数;image pro-plus 5.0图像采集软件采样、图像分析系统选取阳性细胞测定其积分光密度(IOD值)。
1.4 统计学方法
采用SPSS 17.0对所得数据进行统计学分析,计量资料采用均数±标准差(x±s)表示,多样本均数比较用方差分析;组间比较采用t检验;不符合正态分布采用秩和检验。以P < 0.05为差异有统计学意义。
2 结果
2.1 Morris水迷宫
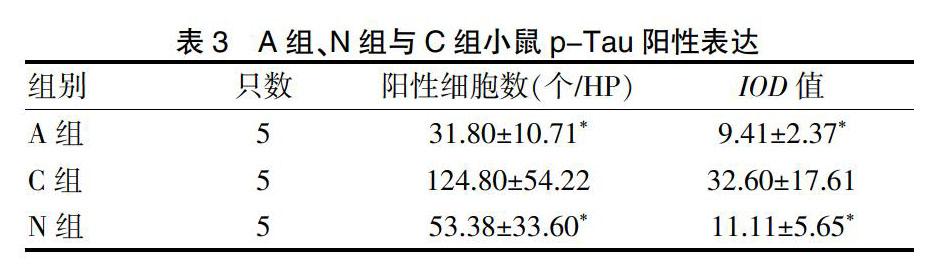
A、B、C三组第二次潜伏期、穿越平台次数与第一次比较,差异无统计学意义(P < 0.05)。N组第二次潜伏期短于第一次、穿越平台次数多于第一次;C组第二次潜伏期明显长于A、B组,穿越平台次数少于A、B组,N组第二次潜伏期短于C组,穿越平台次数多于C组,差异均有统计学意义(均P < 0.05)。表1。
表1 各组动物水迷宫实验结果(x±s)
注:与本组第一次比较,*P < 0.05;与A组同期比较,△P < 0.05;与B组同期比较,◇P < 0.05;与C组同期比较,&P < 0.05
2.2 免疫组化结果
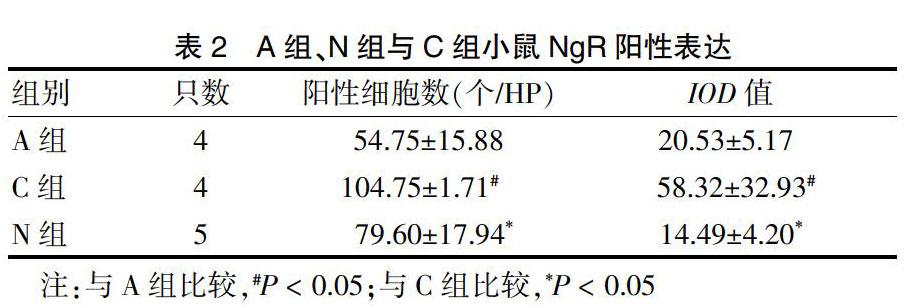
2.2.1 A组、N组与C组小鼠NgR表达情况 C组阳性细胞数和IOD值明显高于A组和N组(P < 0.05)。见表2、图1(封四)。
表2 A组、N组与C组小鼠NgR阳性表达
注:与A组比较,#P < 0.05;与C组比较,*P < 0.05
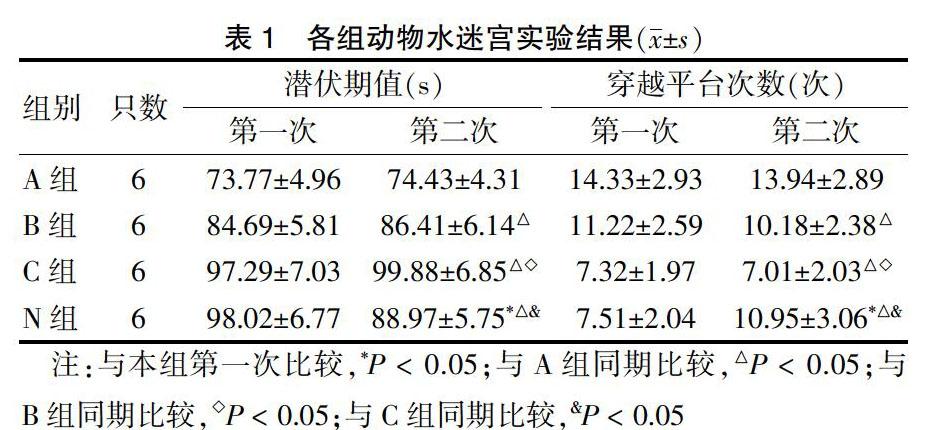
2.2.2 A组、N组与C组小鼠p-Tau表达情况 C组较A组和B组海马阳性细胞数和IOD值明显高(P < 0.05)。见表3、图2(封四)。
表3 A组、N组与C组小鼠p-Tau阳性表达
3 讨论
AD是最常见的老年痴呆,主要病理特征是神经细胞外β淀粉样沉积和神经元细胞内神经元纤维缠结,致神经突触和线粒体结构/功能异常及神经元丢失[3-5]。
水迷宮实验是通过评价空间学习和记忆能力从而进行认知疾病模型验证和治疗评估[6]。SAMP8 2月龄就开始出现学习记忆功能衰退,呈增龄性加速衰退,SAMR1无此类变化[7-8]。本研究结果显示,SAMP8小鼠与同月龄SAMR1比较的确出现空间学习记忆功能障碍,注射NgR抑制剂NEP1-40后,小鼠学习及记忆能力有所改善。
NgR作为髓鞘相关蛋白共同受体,其信号通路限制神经元可塑性,抑制损伤后轴突再生,调节β淀粉样蛋白代谢,可能参与其病理过程[9-10]。另有研究[11]显示,皮质及海马等区域NgR可维持神经元回路稳定性及结构可塑性,促进突触联络和长时程记忆。已有研究[12-13]显示有目的地减少NgR表达,脑内β淀粉样蛋白沉积和营养不良神经炎症细胞增多,提示NgR可能有神经保护作用。本研究中,NgR免疫活性细胞主要分布在海马角和海马齿状回,与以往研究NgR分布区一致。快速老化小鼠大脑海马NgR阳性神经元总数明显高于非老化模型,支持NgR在海马区有促进AD病理过程的作用。
NEP1-40与NgR氨基端40个氨基酸残基结构相同,和NgR下游信号分子竞争性结合,不激活NgR信号通路,一方面阻断Rho信号传导途径,另一方面阻止Nogo-66与NgR结合后介导的生长锥溃变作用,恢复受损中枢神经系统轴突生长和功能,促进神经功能再生。尚可延长NgR低水平表达时程,下调NgR回升时效,进一步发挥神经保护作用[14-15]。本研究中,N组小鼠注射NEP1-40后,水迷宫实验结果明显好于非药物干预组,提示NEP1-40确有保护作用。免疫组化显示N组NgR免疫阳性细胞数量明显减少,提示NEP1-40通过竞争性与NgR底物结合,使NgR活性降低或丧失、表达下调,改变NgR神经抑制作用。
Tau促进微管蛋白聚合形成微管并维持稳定性,异常磷酸化不能有效结合并稳定微管,致神经元退化[16]。AD患者异常磷酸化Tau蛋白导致神经纤维缠结和纤维网形成,神经元死亡后形成细胞外缠结,其数量及定位与认知功能下降相关,在AD认知功能缺陷中发挥着关键作用[17-19]。有研究显示,NgR阳性神经元细胞出现神经纤维缠结样改变和大量NgR阳性神经元与p-Tau蛋白在海马CA1区共表达,提示NgR可能与AD的神经纤维缠结相关[20]。NgR侧链含有富含亮氨酸的重复序列,调节蛋白间相互作用。NgR特异生物物理学属性可能使NgR更容易绑定到其他蛋白质[9]。NgR除了以一种未被确定的方式抑制神经轴突生长外,可能还有其他未被发现的功能。小鼠脑中发现NgR配体Nogo-A与α微管蛋白共免疫沉淀,可能与微管稳定性有关。据此推测NgR可能参与Tau蛋白过度磷酸化,与神经骨架相关联并参与AD的神经纤维缠结[21-22]。本研究发现,快速老化小鼠海马可见大量p-Tau蛋白沉积,N组较C组p-Tau蛋白阳性细胞明显减少,与应用NgR抑制剂后NgR表达减少一致,提示NgR可能作为一个上游蛋白直接影响异常p-Tau表达或是发挥作用。
本研究结果显示,在AD病理改变及病情进展方面,异常p-Tau蛋白是一个关键因素,NgR可能通过对该蛋白表达的影响发挥作用,可以考虑将NgR作为AD治疗靶点进一步深入研究。
[参考文献]
[1] Zhu HY,Guo HF,Hou HL,et al. Incresed expression of the Nogo receptor in the hippocampus and its relation to the neuropathology in Alzheimer′s disease [J]. Hum Pathol,2007,38(3):426-434.
[2] ActBraak H,Zetterberg H,Del TK,et al. Intraneuronal tau aggregation precedes diffuse plaque deposition,but amyloid-β changes occur before increases of tau in cerebrospinal fluid [J]. Acta Neuropathol,2013,126(5):631-641.
[3] Dietrich K,Bouter Y,Muller M,et al. Synaptic alterations in mouse models for Alzheimer disease-a special focus on N-truncated abeta 4-42 [J]. Molecules,2018,23(4):718-732.
[4] Zeng Y,Zhang J,Zhu Y,et al. Tripchlorolide improves cognitive deficits by reducing amyloid β and upregulating synapse-related proteins in a transgenic model of Alzheimer's Disease [J]. J Neurochem,2015,133(1):38-52.
[5] Furotani K,Kamimura K,Yajima T,et al. Suppression of the synaptic localization of a subset of proteins including APP partially ameliorates phenotypes of the Drosophila Alzheimer's disease model [J]. PLoS One,2018,13(9):1-17.
[6] Bromley-Brits K,Deng Y,Song W. Morris water maze test for learning and memory deficits in Alzheimer's disease model mice [J]. J Vis Exp,2011,20(53):2920-2924.
[7] Susan AF,Elizabeth R,Michael L,et al. Metformin improves learning and memory in the SAMP8 mouse model of Alzheimer′s disease [J]. J Alzheimer Dis,2019,68(4):1699-1710.
[8] Lam V,Takechi R,Albrecht MA,et al. Longitudinal Performance of Senescence Accelerated Mouse Prone-Strain 8 (SAMP8) Mice in an Olfactory-Visual Water Maze Challenge [J]. Front Behav Neurosci,2018,12:174-181.
[9] Barton WA,Liu BP,Tzvetkova D,et al. Structure and axon outgrowth inhibitor binding of the Nogo-66 receptor and related proteins[J]. EMBO J,2003,22(13):3291-3302.
[10] Ziebell JM,Ray-Jones H,Lifshitz J. Nogo presence is inversely associated with shifts in cortical microglial morphology following experimental diffuse brain injury [J]. Neuroscience,2017,359:209-223.
[11] Thomas RA,Gibon J,Chen CXQ,et al. The nogo receptor ligand LGI1 regulates synapse number and synaptic activity in hippocampal and cortical neurons [J]. eNeuro,2018,5(4):1-15.
[12] Fang Y,Wang J,Yao L,et al. The adhesion and migration of microglia to β-amyloid (Aβ) is decreased with aging and inhibited by Nogo/NgR pathway [J]. J Neuroinflammation,2018,15(1):210-225.
[13] Fang Y,Yao L,Li C,et al. The blockage of the Nogo/NgR signal pathway in microglia alleviates the formation of Aβ plaques and tau phosphorylation in APP/PS1 transgenic mice [J]. J Neuroinflammation,2016,13(1):56-72.
[14] Xu J,He J,He H,et al. Comparison of RNAi NgR and NEP1-40 in Acting on Axonal Regeneration After Spinal Cord Injury in Rat Models [J]. Mol Neurobiol,2017,54(10):8321-8331.
[15] Cao Y,Shumsky JS,Sabol MA,et al. Nogo-66 receptor antagonist peptide (NEP1-40) administration promotes functional recovery and axonal growth after lateral funiculus injury in the adult rat [J]. Neurorehabil Neural Repair,2008,22(3):262-278.
[16] Obulesu M,Venu R,Somashekhar R. Tau mediated neurodegeneration:an insight into Alzheimer′s disease pathology [J]. Neurochemical research,2011,36(8):1329-1335.
[17] Villemagne VL,Doré V,Bourgeat P,et al. Aβ-amyloid and Tau Imaging in Dementia [J]. Semin Nucl Med,2017, 47(1):75-88.
[18] Kurbatskaya K,Phillips EC,Croft CL,et al. Upregulation of calpain activity precedes tau phosphorylation and loss of synaptic proteins in Alzheimer′s disease brain [J]. Acta Neuropathol Commun,2016,4:34-48.
[19] Zuo YC,Li HL,Xiong NX,et al. Overexpression of tau rescues Nogo-66-induced neurite outgrowth inhibition in vitro [J]. Neurosci Bull,2016,32(6):577-584.
[20] Petrasek T,Prokopova I,Sladek M,et al. Nogo-A-deficient transgenic rats show deficits in higher cognitive functions,decreased anxiety,and altered circadian activity patterns [J]. Front Behav Neurosci,2014,8(90):1-15.
[21] Mehta NR,Lopez PH,Vyas AA,et al. Gangliosides and Nogo receptors independently mediate myelin-associated glycoprotein inhibition of neurite outgrowth in different nerve cells [J]. J Biol Chem,2007,282(38):27875-27886.
[22] Zuo YC,Xiong NX,Shen JY,et al. MARK2 rescues Nogo-66-induced inhibition of neurite outgrowth via regulating microtubule-associated proteins in neurons in vitro [J]. Neurochem Res,2016,41(11):2958-2968.
(收稿日期:2019-04-04 本文編辑:封 华)

