Discovery of superhard materials via CALYPSO methodology∗
Shuangshuang Zhang(张爽爽),Julong He(何巨龙),Zhisheng Zhao(赵智胜),Dongli Yu(于栋利),and Yongjun Tian(田永君)
Center for High Pressure Science(CHiPS),State Key Laboratory of Metastable Materials Science and Technology,Yanshan University,Qinhuangdao 066004,China
Keywords:CALYPSO,superhard materials,crystal structure,prediction
1.Introduction
Superhard materials have been widely used in industrial operations due to their superior hardness,incompressibility,and wear resistance compared with other materials.The synthesis of diamond and cubic boron nitride(cBN)has led to the development of superhard materials and multiple related disciplines.[1,2]Diamond has been considered as the hardest,stiffest,and most incompressible substance in nature,but it has poor thermal stability and reactivity with ferrous alloys.Although cBN has been considered as the more optimal candidate for cutting ferrous and carbide-forming hard substances compared with diamond,the hardness of cBN single crystal is only half of that of diamond crystal.Therefore,the discovery of novel superhard materials with combined superior mechanical performance and high stability is of great interest.
Superhard materials are exclusively covalent compounds because short and strong covalent bonds highly resist both elastic and plastic deformations.[3]The light elements(LE),including B, C, N, and O, etc., have a strong ability to form covalent bonds, making their compounds potential candidates.[4–6]In the past decades,superhard carbon phases and carbon nitrides,diamond-like boron carbides(d-BCx[7–9])and boron carbonitrides(d-BCxN[10–14]),elemental boron allotropes,[15–17]boron-rich compounds(e.g.,B6O,[18]B13C2,[19]and B4C[8]),boron carbon oxide(e.g.,B2CO[20,21]and B2CxO[22]),and boron nitrogen oxide(e.g.,B3NO[23]),have all been extensively explored both experimentally and theoretically. Outstanding structures among these materials are sp3or sp3-rich carbon phases with extremely high hardness compared with that of diamond crystal.[24–30]It is reasonable to suppose that pure covalent C–C bonds provide such materials with remarkable hardness.Compared with the pure covalent bond in diamond,the significant ionicity(0.256)of the B–N bond limits the hardness of cBN crystal to approximately half that of diamond.Therefore,the material with superior hardness than diamond crystal may be difficult to find in the B–C–N–O(–Si)system due to its existing ionicity.For example,the hardness values of diamond-like BC5,BC2N,and BC4N were reported to be 71,[8]76,[10]and 68 GPa,[31]respectively,which were less than that of diamond but exceeded that of c-BN.Nevertheless,other anticipated electrical and thermal properties were found in these polar covalent crystals.For example,the thermal stabilities of d-BCx[8]and d-BCxN[32]under inert conditions were higher than that of diamond.Moreover,peculiar electronic properties,including semi-conductivity or even superconductivity,were found in d-BCxand B2CN materials.[33,34]
In the search for new superhard materials,researchers have focused on transition-metal light-element(TM-LE)compounds because of their high valence electron densities and strong covalent bonding frameworks. However,these compounds are mostly metallic—a property that has an obvious negative effect on their hardness. For example,transition metal(TM)borides(e.g.,WB4)commonly have hardness values of 20–30 GPa.[35]Past studies have identified that the synthesized c-Zr3N4possessed unique semiconducting property,leading to a hardness value of as high as 36 GPa.[36]In addition,the semiconducting pyrite-type PtN2had been predicted to be a superhard material with theoretical hardness of 60 GPa.[37]Thus,the exploration of semiconducting superhard materials in TM-LE systems is highly promising.
The theoretical design of new materials is gaining broad recognition as an effective means of reducing the number of experiments that can ultimately lead to material discovery.In such a context,several efficient structure prediction methods have been proposed and applied to explore new materials,including simulated annealing,[38]genetic algorithms,[39,40]minima hopping,[41]basin hopping,[42]random sampling,[43]and CALYPSO.[44,45]Their computational structures not only added fresh blood to the material family,but also solved many experimental indeterminate phases. For example,the proposed hypothetical carbon phases of M-carbon,[46]bct-C4,[27]F-carbon,[47]W-carbon,[48]and O-carbon[49]have been considered as the structural solution to cold-compressed graphite phases.[47,50,51]Indeed,it was difficult to determine the exact stoichiometric ratios and atomic positions of LE compounds in experiments because of their low diffraction intensity and close atomic number. Furthermore,the large difference in atomic mass between TM and LE also served as a major obstacle in determining their composition and LE position. In relation to the above mentioned,structure prediction provides a powerful approach for clarifying the structures of experimentally discovered phases.For instance,new compounds of boron carbides,including BC3and BC5,have been claimed to be synthesized,but their exact structures are difficult to determine in the experiments.[8,9,51]Various structures of diamondlike BC5,such as tetragonal BC5,[53]hexagonal BC5-2,[54]and orthorhombic BC5,[55]were predicted to match the experimental XRD and Raman results.For the TM-LE compounds,for example,the structure of experimentally synthesized Re2C was determined by combining the experimental results with first-principle calculations.[56]Noticeably,the current wide applications of structure prediction have helped to understand experimentally discovered novel phases,and CALYPSO as one of the most distinguished structure prediction methods has been proven useful in solving complex crystal structures and in designing novel functional materials,such as superhard,superconducting,and semiconducting materials.[57–65]
Here,we focus on recent advances in the design of superhard materials mainly based on the structure prediction of CALYPSO methodology.Its basic theory and general features are introduced first,followed by examples of recent applications of superhard materials among compounds of LE(B,C,N,O,and Si)and TM borides,carbides,and oxides.Finally,the current challenges and future expectations in designing superhard materials through structure prediction methods are summarize.
2.CALYPSO methodology
CALYPSO,one of the advanced structural prediction methods,has been widely applied in the design of various structural materials,including three-dimensional(3D)bulk structures,two-dimensional(2D)surfaces and layers,and isolated clusters or molecules,with a variety of functional properties.[59,60,63–70]The particle swarm optimization(PSO)technique algorithm was adopted as implemented in the CALYPSO code for structure prediction on the basis of an efficient global minimization of free-energy surfaces merging ab initio total-energy calculations. Compared with the previous data mining method,CALYPSO requires only chemical compositions for a given compound to predict structures under given external conditions(e.g.,pressure)without the need of any prior known structural information about unit cell size,shape,and atomic positions.[45,57,71]Additionally,a straightforward way by which to design superhard materials has been developed recently based on CALYPSO;this approach integrated structure searching,total energy calculations,and theoretical hardness models to readily seek the energetically favorable superhard structures.[61]
3.Discovery of superhard materials by using CALYPSO
The search for new superhard materials has been an important research field in material science.Nowadays,these explorations primarily focus on two classes of materials:(i)the LE compounds in the B–C–N–O(–Si)system with 3D networks of strong covalent bonds,including a number of carbon allotropes,and binary and ternary B–C–N–O(–Si)compounds,and(ii)the TM-LE compounds having high valence electron density,such as FeB4,WB3,RhB,Re2C,PtN2,and IrN2.Using structure prediction,plenty of previously undetectable structures of superhard crystals can be created now,and some of them account well for the experimental problems.
3.1.Light-elements compounds
3.1.1.Carbon polymorphs
The carbon element is fascinating because of the diversified polymorphs profiting from its sp-,sp2-,and sp3-hybridized states.For example,graphite,diamond,fullerenes,graphene,carbine,and amorphous carbon exhibit outstanding properties and are of scientific and technological importance.Therefore,exploring new carbon polymorphs has long been a hot topic in scientific communities.
The sp3-and high sp3-hybridized carbons generally have a dense,superhard,and strong nature.Thus,exploring them has become the common way to uncover superhard materials. For example,the predicted all-sp3carbons of Cco-C8,oC32,and F-carbon exhibit extreme hardness values exceeding 90 GPa.[29,30,72]They(Figs.1(a)–1(c))contain(4+8+6)-membered or(5+6+7)-membered rings,can be viewed as solutions to the cold-compressed graphite phases.[47,50,51]The cage-like carbons(also called clathrate carbons),a class of low-density superhard sp3carbons,can be viewed as carbon cages linked by sp3bonds possessing excellent mechanical performance. This is exemplified by the C60clathrate carbon that has a C60cage(Fig.1(d))and a fcc-C32carbon that has a C32cage(Fig.1(e))with hardness values of approximately 82.8 and 79.9 GPa,respectively.[73,74]Significantly,these cage-like structures can also become hosts for superconducting materials. More recent experiments reported a superhydride,LaH10,with a superconducting transition temperature(Tc)close to room temperature[75–77]and a structure reminiscent of the cage-like fcc-C32carbon. The P carbon(Fig.1(f)),another relatively low-density superhard sp3carbon adopting the microporous framework,is a superhard semiconductor with a direct band gap of 3.12 eV.[78]Regardless of density,all-sp3carbons are semiconducting due to the electron localization of sp3-hybridized C–C bonds,as shown in Table 1.

Fig.1.Predicted crystal structures of superhard sp3-hybridized carbon allotropes.Both structures in(a)and(b)consist of(4+6+8)-membered rings,[30,72]and the carbon structure in(c)consists of(5+6+7)-membered rings.[29]These structures were viewed as solutions to the experimentally mysterious cold-compressed graphite or carbon nanotubes.[25,47,48]The carbon structures in(d)and(e)contain the unique C60 and C32 cages,respectively.[73,74]Notably,the predicted fcc-C32(e)has the same structure with recently synthesized LaH10 possessing Tc near room temperature at high pressure.[75–77](f)A type of microporous carbon form.[78]

Table 1.Space group(S.G.),density(g/cm3),and calculated hardness(H,GPa),bulk modulus(B0,GPa),B/G ratio,and band gap(Eg,eV)of predicted sp3-hybridized carbon polymorphs.
Polymerizing various carbon nanotubes is another popular approach to producing superhard carbons. As depicted in Fig.2 and Table 2,3D carbon nanotube polymers exhibit abundant structures[i.e.,3D-(n,0)and 3D-(n,n)],including the sp3(4+6+8)-membered 3D-(2,2),3D-(3,0)(lonsdaleite),and a number of different sp2-sp3porous carbons(Fig.2(a)–2(i))with the junctions composed of diamond-like sp3bonds.[79–81]Further investigations of their mechanical properties show that these polymers have ultrahigh hardness,good ductility,high tensile strength and Young’s modulus,and low density. Contrary to all-sp3carbons,these low-density sp2-sp3carbons have specific electrical properties,from semiconductors with tunable band gaps to conductors.According to the internal arrangement of sp2atoms,the 1D,2D,and 3D metallic carbons can be theoretically designed by compressing carbon nanotubes.
The sp-sp2/sp3carbons,including graphyne,graphdiyne,and yne-diamonds(YDs),have been reported both theoretically and experimentally.The calculations indicated that the acetylenic linkages can assemble these 2D carbons into 3D polymers. As a result,these 3D polymers were expected to be a new kind of superhard materials. Under this assumption,several graphyne,graphdiyne,and yne-diamond polymers were theoretically designed recently(Figs.2(j)–2(l))and were observed to have superior properties,including ultrahigh hardness and tunable band gaps.[82–84]These findings are likely to be useful in guiding the design and synthesis of superhard carbon materials with novel electrical properties.

Fig.2.Predicted crystal structures of superhard,low-density carbon polymers.(a–i)3D carbon nanotubes polymers.[80](j)and(k)3D graphyne polymers.[83](l)yne-diamond polymer.[82]The structure of 3D-(2,2)carbon(a)is the same as bct-C4,[27]and(b)3D-(3,0)carbon is actually the lonsdaleite structure.(a,b)sp3 carbons;(c–k):sp2-sp3 carbons.(l)sp-sp3 carbon.Due to the bonding difference,the carbon polymers possess distinct conductivity,such as insulating or semi-conducting characteristics with tunable band gaps(a–c)and novel(g)1D,(h–i)2D,and(d–f)3D metallicities.The orange shadows represent insulating sp3-hybridized carbon layers,which interrupt the electronic conduction in 3D.

Table 2.Space group(S.G.),density(g/cm3),and calculated hardness(H,GPa),bulk modulus(B0,GPa),B/G ratio,band gap(Eg,eV),axial tensile strength(σa,GPa),and radial tensile strength(σr,GPa)of various carbon polymers.
3.1.2.Boron nitrides
c-BN has thermal and chemical stabilities considered superior to those of diamond,although its crystal hardness is only half that of diamond.As such,the desirability to uncover novel B–N polymorphs to further increase hardness has not been stopped.Compressing BNNTs is expected to be one of the most promising approaches to obtain superhard B–N polymorphs.Similar to carbon nanotube polymers,a variety of 3D BNNT polymers,such as 3D-(n,0)and 3D-(n,n)polymers,have been proposed.[85,86]Ultrahigh hardness,good ductility,high tensile strength,low density,and tunable band gaps make this class of B–N polymorphs of great interest in the viewpoints of scientific research and practical applications. As depicted in Fig.3 and Table 3,the structures of 3D BNNT polymers highly depend on the size,geometry nanotube orientation,and the pressure and temperature conditions used.[86]At relatively low hydrostatic pressure,low-density 3D BNNT polymers can be produced by the direct linkage of their parent structures(Figs.3(a)–3(c)). Meanwhile,at higher pressure,BNNTs would be further squeezed and deformed into dense 3D BNNT polymers forming sp3-hybridized intratube B–N bonds,as seen from the 3D(4,4)-II structure(Fig.3(e)).The calculated hardness values of the 3D BNNT polymers range from 55.1–66.6 GPa,demonstrating their potential application as superhard materials.According to the estimated Pugh’s(B/G)ratios(Table 3),the 3D(4,4)-I,3D(6,0)-I,and 3D(4,4)-V phases have better ductility than those of diamond.The band gaps also generally narrowed with the increase in sp2/sp3ratios. For example,the structures of 3D(10,0)-I(Fig.3(d))and 3D(10,0)-II(Fig.3(c))contain 1/5 and 3/5 sp2/sp3fractions,respectively,leading to the corresponding band gaps of 3.92 and 2.56 eV.In particular,the full sp3-hybridized 3D(10,0)-III was predicted to be a semiconductor with a direct band gap.

Fig.3. Predicted crystal structures of boron nitrides. (a–g)3D BNNT polymers.[85,86]The light blue shadows emphasize the components of parent BNNTs.For the 3D polymers in(a–c),they can be formed by compressing corresponding BNNTs at relative low pressures,and more dense forms of 3D polymers shown in(d–f)can be obtained at higher pressures.Distinct from the semiconducting 3D polymers in(a–f),the boron nitrides in(g–i)are conductive and considered rare.[85,87,89]The gray regions indicate the conductive units in structures,composed of sp2 or sp2/sp3-hybridized bonding.The B and N atoms are painted in green and white colors,respectively.The hardness calculations indicate that these boron nitrides are all superhard except for M-BN(see detail in Table 3).
Another new sp2-sp3BN structure,M-BN,(Fig.3(h))was proposed based on the CALYPSO algorithm.[87]Its structure comprises puckered h-BN layers interlinked by partial sp3B–N bonds. Taking its 1D metallicity into account,its calculated hardness is 33.7 GPa lower than that of c-BN.[88]In addition,a tetragonal B3N4(t-B3N4,Fig.3(i))containing sp3c-BN blocks and sp2N–N bonds possessing high hardness value of 42.5 GPa was reported and identified as the hardest B3N4polymorph predicted so far.[89]Compared with other proposed B3N4polymorphs,[90]the strong N–N bonds in t-B3N4along the c axis lead to axial ultra-incompressibility,and the insulating boron sheets stacked along the c axis interrupt the conduction,resulting in 2D metallicity.In addition,t-B3N4possesses the combined mechanical and electrical properties of superhardness,ultra-incompressibility,and 2D metallicity in a 3D framework.
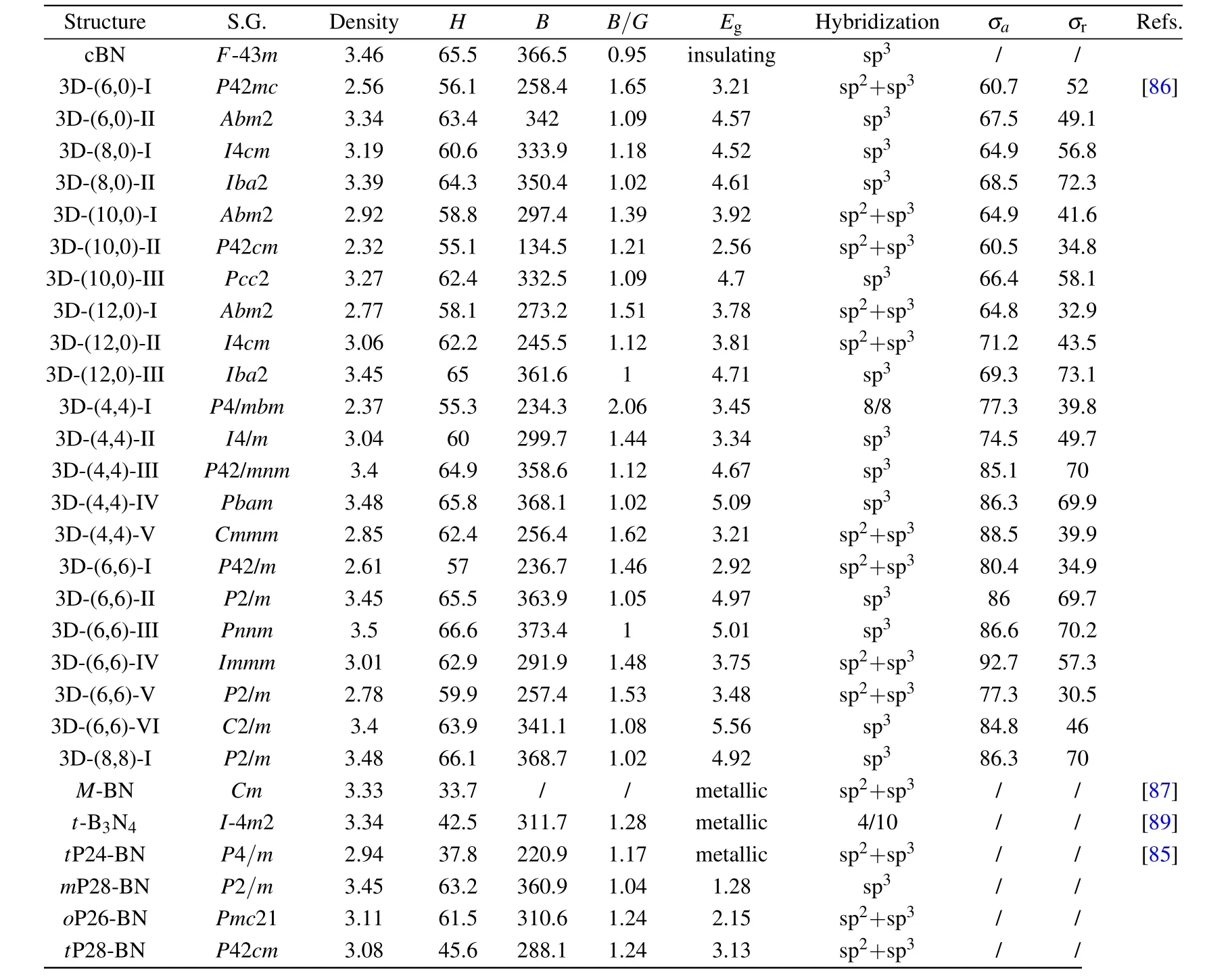
Table 3.Space group(S.G.),density(g/cm3),and calculated hardness(H,GPa),bulk modulus(B0,GPa),B/G ratio,band gap(Eg,eV),sp2/sp3 atomic ratio,axial tensile strength(σa,GPa)and radial tensile strength(σr,GPa)of various boron nitrides.
3.1.3.B–C–N compounds
Diamond and c-BN are two classes of excellent superhard materials,thus,ternary B–C–N compounds are expected to be the best candidates for superhard materials.Many experimental and theoretical studies have focused on BCxN compounds. Particularly,the diamond-like c-BC2N,regarded as the ideal mixture of diamond and c-BN,has been investigated extensively and reported to have a high Vickers hardness(76 GPa).[10,13]BC2N structures are predicted to be superhard through hardness calculations.[13,91–94]In addition,it has been reported that greater C–C bonding in BCxN is beneficial to the enhancement of hardness. Thus,diamond-like BC6N attracted considerable attention from researchers.Two high-density hypothetical BC6N structures,i.e.,t-BC6N and r-BC6N(Figs.4(a)and 4(b)),have been predicted to be superhard with hardness value of approximately 80 GPa.[12]However,the commonly proposed BCxN are classifies as semiconductors or insulators due to their similar isoelectronic properties with diamond. Therefore,the design of superhard and metallic BCxN was anticipated by researchers. Based on first-principle calculations,several superhard BCxN structures(Fig.4(c)and 4(d))have been designed by replacing partial B and N atoms in the o-B3N5structure,[95]and the resulting sandwich-structured o-BC6N,t-BC6N-1,t-BC6N-2,and o-BC2N exhibit intriguing electrical properties,including 1D and/or 2D metallicity due to the presence of sp2-hybridized atoms.[11,12,14]
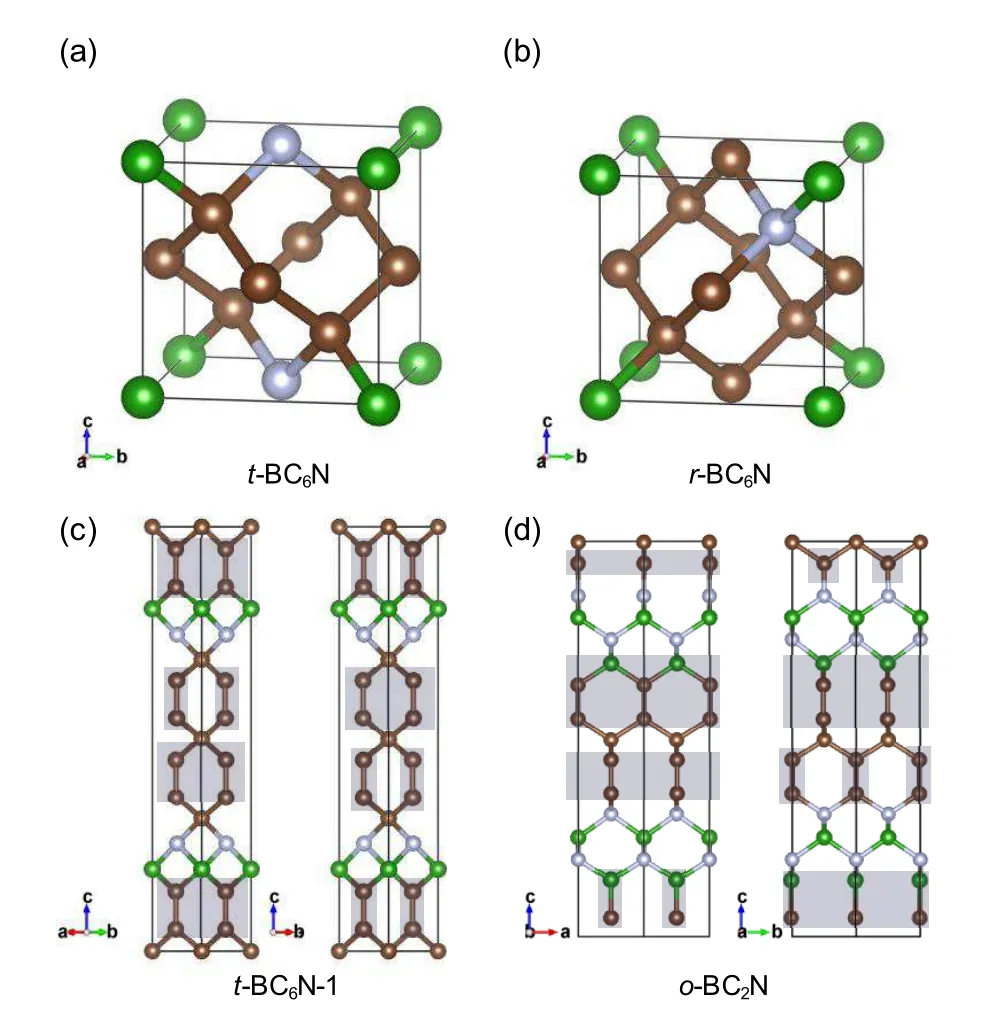
Fig.4. Predicted crystal structures of superhard B–C–N compounds. (a)and(b)are the structures of sp3 BC6N that originated from diamond structure,[12]and(c)and(d)are low-density sp2-sp3 BC2N structures that originated fromC2221-B3N5.[11,14](c)t-BC6N-1 possesses novel 1D metallicity,and(d)o-BC2N possesses both 1D and 2D metallicities.The gray shadows represent the conductive units.The C,B,and N atoms are painted in brown,green,and white colors,respectively.
3.1.4.Carbon nitrides
Since the first prediction of low-compressibility C3N4in 1996,[96]the search for carbon nitrides with hardness that is higher than diamond has become the holy grail in the field of superhard materials.Considerable experimental and theoretical studies have attempted to search for such high-hardness compounds. In early predictions,a number of hypothetical C3N4structures were proposed by atomic substitution based on the known A3B4structural types,[97–99]but their existence was still ambiguous because of the limited quantity and heterogeneity of the synthesized samples in experiments.[93]Although the C3N4polymorphs have a very high predicted bulk moduli exceeding that of diamond,the most stable composition of C–N compounds is still inconclusive.[97,101]Therefore,several attempts were made to explore carbon nitrides with different stoichiometric ratios.
One prior theoretical study proposed a diamond-like C11N4(d-CN) through the possible phase transition of graphite-like C11N4(g-CN)under pressure.[102]As shown in Fig.5(b),d-cn involves polyhedral hollow C–N cages with a theoretical hardness value of 58 GPa and indirect band gap of 1.86 eV.A body-centered tetragonal bct-CN2(Fig.5(c))has also been predicted at high pressure based on CALYPSO search.[103]Its structure contains strong covalent C–N and N–N bonds,which leads to its high bulk(407 GPa)and shear(386 GPa)moduli as well as estimated hardness(77 GPa).However,the flexibility and mobility of the non-bonding lonepair states in structure also leads to a relatively low ideal shear strength of 47 GPa.For 1:1 CN compounds,a sp2-sp3Pnnm CN(Fig.5(d))with hardness of 62.3 GPa has been proposed theoretically.[104]Subsequently,the experimental synthesis of a β-InS-type CN phase,which is identical to the theoretically predicted Pnnm CN,has been reported to be synthesized at high pressure and temperature conditions implemented in the diamond anvil cell(DAC).[105]This study indicates that Pnnm CN is semiconducting with a wide band gap of 3.7 eV and agrees well with the experimental observation of the transparency of sample during laser heating. This work pointed out new directions in the search for superhard carbon nitride materials with different stoichiometric ratios for future technological applications.

Fig.5.Predicted crystal structures of carbon nitrides.The compression of graphite-like C11N4(a)can obtain the superhard diamond-like C11N4(b),and the inset in(b)represents a cage-like part structure.[138](c)Superhard sp2-sp3 CN2 with body-centered tetragonal structure and calculated hardness of 77.4 GPa.[103](d)β-InS-type CN structure recently synthesized at HPHT with calculated hardness of 62.3 GPa.[105,139]The C and N atoms are painted in brown and white colors,respectively.
3.1.5.Boron carbides
For decades,many experimental and theoretical efforts have been made to study boron carbides,typically B4C[8]and B13C2,[19]due to their high hardness and strength at high temperature.In addition,d-BCxpossesses higher stability and antioxidant ability compared with diamond as well as peculiar metallicity and superconductivity.The unique combination of mechanical,electronic,and thermal properties has promoted the studies of electric devices under extreme conditions.The high superconductive transition temperature(Tc)is expected to be obtained by the heavy boron doping in diamond. Unfortunately,it was difficult to achieve d-BCxwhen the doped B content exceeded approximately 5%in experiments.Nevertheless,d-BCx(x=2.8±0.7 or 5)has been synthesized from high pressure and temperature experiments by directly compressing graphite-like BCx(g-BCx).[8,9,52]In view of the small nanocrystalline size in microstructure,the composition and atomic positions in d-BCxcannot be easily determined by common experimental methods.
Theoretically,a series of candidate d-BCx(x=2,3,5,7)structures have been proposed.[53–55,106–112]Comparing experimental and theoretical data(e.g.,XRD and Raman spectra),some reasonable structures can explain the experimental observations.For instance,an orthorhombic Pmma-b BC3[108](Fig.6(b))has been considered as a good candidate structure for synthesized d-BC3in previous reports,[52]and subsequent studies illuminated that monoclinic M-BC3(Fig.6(c))may also be the coexistent phase.[109]Another cubic I-43m BC3(Fig.6(d))structure has been proposed to match the experimentally reported c-BC3.[9,107]In addition,several d-BC5(Figs.6(e)–6(h))structures have been proposed to account for the superhard BC5synthesized in experiments.[55]Considering that the composition of synthetic d-BCxhave been determined by energy dispersive X-ray spectrum(EDS)with large error,studies of d-BCxwith other chemical compositions are also necessary.Potential BC7(Figs.6(i)–6(l))structures have been uncovered by performing variable-cell structure simulations.[111,112]The estimated hardness of these lowenergy BC7structures are approximately 80 GPa,indicating their superhard nature.As depicted in Table 4,the proposed BCxstructures exhibit metallicity and are considered as potential superhard materials.The conductivity may have originated from the introduction of B atoms lacking an electron compared with C atoms,especially because no obvious dependence has been observed between Tcand B concentration.However,hardness significantly increased with increased C content due to the increased fraction of covalent C–C bonds.
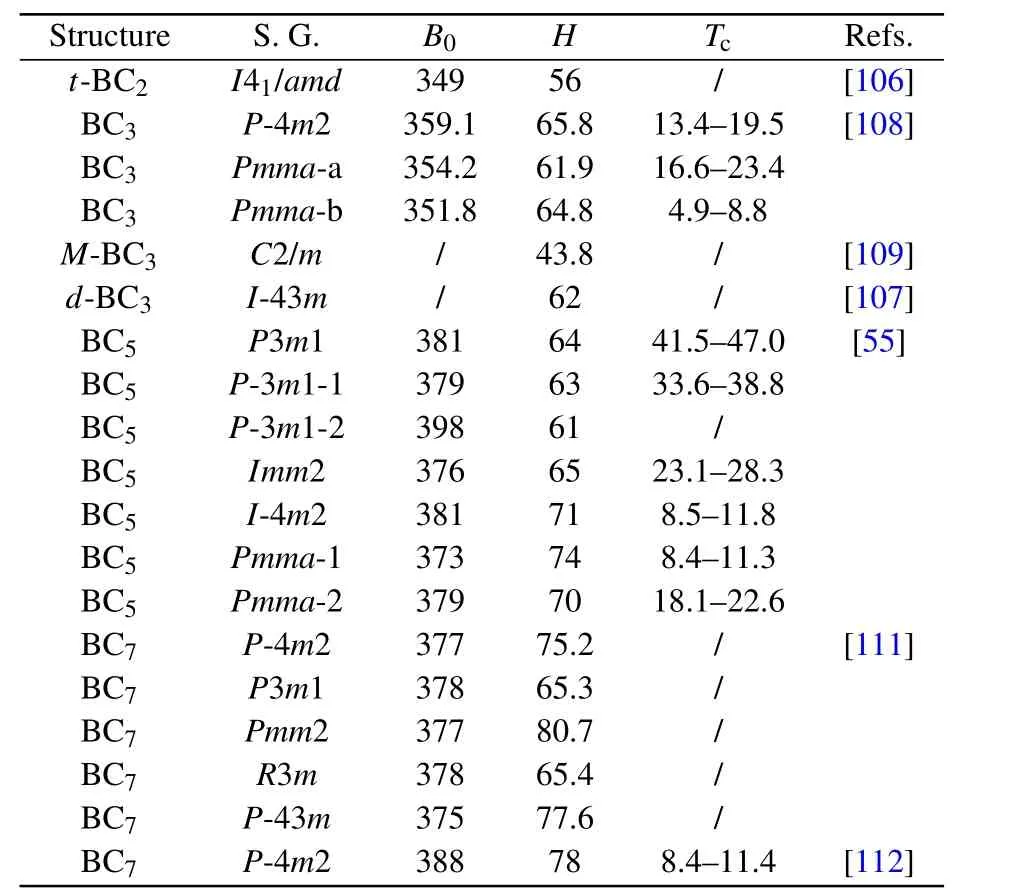
Table 4.Space group(S.G.),and calculated bulk modulus(B0,GPa),hardness(H,GPa),and superconducting transition temperature(Tc,K)of predicted BCx structures.

Fig.6.Predicted crystal structures of superhard diamond-like boron carbides.(a)Tetragonal BC2.[106](b–d)BC3,(e–h)BC5,and(i–l)BC7 structures,respectively.[54,100–102,104,105]The BC3 and BC5 structures have been suggested as reasonable solutions for the experimentally synthesized superhard boron carbide phases.[7,9,52]As seen from Table 4,these structures have high theoretical hardness values,which are above 40 GPa,and are commonly metallic due to the addition or doping of boron.Notably,the structures in(b),(e),(f),(h),and(i)are also superconductive.There is no obvious dependence between Tc and the concentration of B;however,hardness has significantly increased with increased C content.C and B atoms are painted in brown and green colors,respectively.
3.1.6.B–N/C–O compounds
As a result of the reported synthesis of B2O,[113]the ternary compounds in the B–C/N–O system are considered as candidate superhard materials. Using crystal structure prediction,the structures of B2CO were explored in past studies.[20,21]Three hypothetical structures(Figs.7(a)–7(c)),tI16-B2CO,tP4-B2CO,and oP8-B2CO,were assembled by sp3B–C and B–O bonds.Compared with diamond-like B2O,the lengths of B–O bonds are similar,but the B–C bonds are much shorter in B2CO.As a result,B2CO,with a calculated Vickers hardness of 50 GPa,is harder than B2O(38 GPa).[18,20,21]B3NO,like B2CO,is isoelectronic with diamond. A search for superhard materials based on CALYPSO method has been used to explore new B3NO structures. As shown in Figs.7(s)–7(f),two orthorhombic structures,oI20-B3NO and oP20-B3NO,with calculated Vickers hardness values of 45.9 and 47.4 GPa,respectively,have been proposed.[23]The present results reveal that B3NO structures can stabilize in a 3D network with typically strong covalent B–B,B–N,and B–O bonds.The B–N bonds in B3NO are shorter than those in B2O,resulting in higher hardness.A high calculated elastic modulus indicates that these structures are highly incompressible.
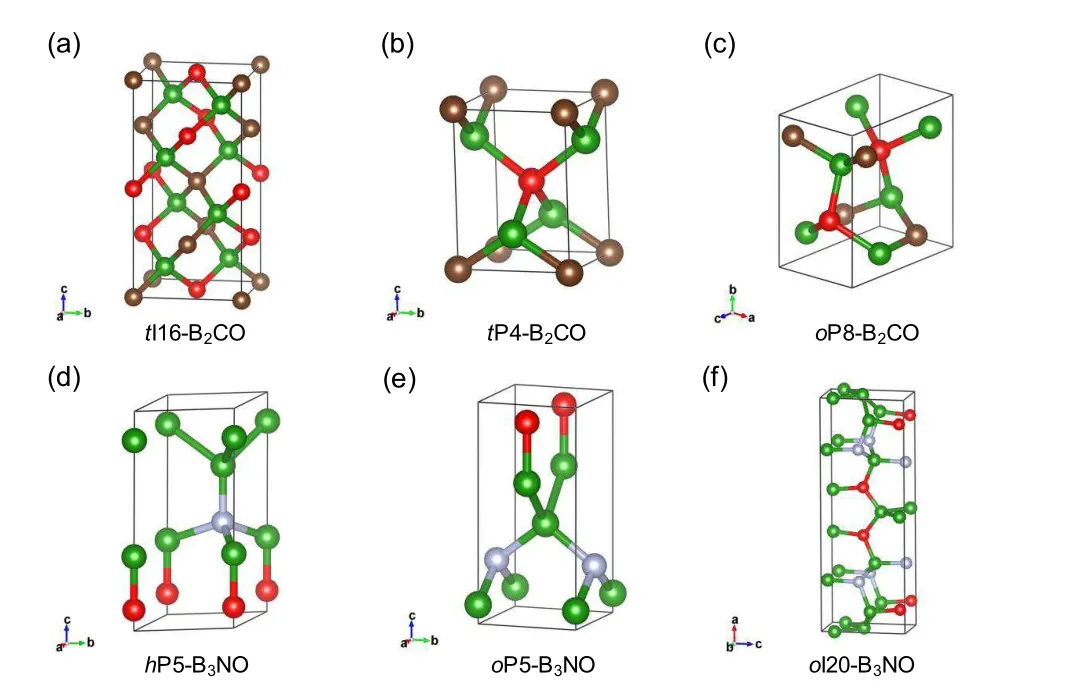
Fig.7.Predicted crystal structures of superhard B–C/N–O compounds.(a–c)Hypothetical diamond-like B2CO structures with covalent sp3 B–C and B–O bonds.[20,21](d–f)Hypothetical B3NO structures with covalent B–B,B–N,and B–O bonds.[23]All structures are isoelectronic with diamond,indicating their semiconducting characteristics.Their hardness value is approximately 50 GPa.The B,C,N,and O atoms are painted in green,brown,white,and red colors,respectively.
3.1.7.Si–C–N compounds
The binary compounds of silicon carbide(SiC)and silicon nitride(Si3N4)have received much research attention in the past due to their wide applications as structural materials.Their composites have also been studied for a long time.[114]However,the synthesis of hard or superhard Si–C–N compounds has yet to be achieved.[115]Structure prediction was performed by first-principle calculations,[116]and three lowenergy SiCN were reported(Fig.8).t-SiCN(Fig.8(a))is a superhard semiconductor with a narrow band gap of 0.89 eV and hardness of 41.5 GPa.In addition,the long bonds in Si–C–N compounds tend to limit their hardness.Through a comparison of their thermodynamic stability,h-SiCN(Fig.8(b))and o-SiCN(Fig.8(c))may be considered as possible highpressure phases of t-SiCN,and their phase transitions may occur at 21.6 and 21.9 GPa,respectively.

Fig.8. Predicted crystal structures of SiCN compounds. The calculation of total enthalpy with pressure indicates that(a)t-SiCN may change into(b)h-SiCN and(c)o-SiCN under high pressures of 21.9 and 21.6 GPa,respectively.[116](a)t-SiCN was a narrow gap semiconductor with a band gap of 0.89 eV,and(b)h-SiCN and(c)o-SiCN are metallic.The calculated hardness of(a)t-SiCN is 41.5 GPa.The Si,C,and N atoms are painted in blue,brown and white colors,respectively.
3.2.Transition-metal light-element compounds
TM-LE compounds, such as transition-metal borides(TMBs),carbides(TMCs),and nitrides(TMNs),have outstanding mechanical properties and have been considered as promising candidates for superhard materials. However,because of the large differences in atomic number and mass between TMs and LEs,their structures are difficult to determine in experiments and are often controversial.[117]Consequently,studies of TM-LE compounds have been intensified for decades. On many prior attempts,a variety of TM-LE compounds have been studied,for example,Fe2B,FeB4,RhB,WBx,Na2B30,RuC,Re2C,Zr3N4,PtN2,and IrN2.[36,37,56,117–126]The synthesized oP10-FeB4(Fig.9(a))was initially considered as a candidate superhard material with hardness exceeding 40 GPa,[125,127]but later studies suggested that it was unlikely to be superhard because of the weak Fe–B bonding and because the measured hardness of polycrystalline FeB4bulk material was only 10–30 GPa.[128–130]Using structure prediction,a new form of FeB4,tP10-FeB4(Fig.9(b)),was proposed and was obtained by compressing oP10-FeB4above 65.9 GPa.[122]It is semiconducting,which is rare in metal borides,and enhances the electron locations,resulting in an estimated hardness value of as high as 45 GPa. For WBxcompounds,structure predictions were carried out in a wide range of chemical compositions.[35,131–134]The synthesized γ-W2B,α-WB,and ε-WB2,were well matched with the predicted structures of I4/m-4u,I41/amd-8u,and P63/mmc-4u,respectively.[131,132]The structural identification of syntheyized WB4has generated great interest and controversy.Initially,it was assumed to be in a hexagonal P63/mmc-4u WB4,[135]and subsequent analysis of thermodynamic and lattice stabilities suggested that it should be described as P63/mmc-4u WB3.[112,115]Further calculations of stress-strain relations demonstrated that the rising boron concentrations in WBx(x=1,2,3,4)result in little improvement or even trend downward with rising boron content in most cases,for example,the indentation stress peaks under(1-10)[110]-orientation largely decrease going from WB2(∼50 GPa)to WB3(∼30 GPa),and then recover for WB4(∼35 GPa).[131]In addition,the high-pressure phase transitions of B1-structured stoichiometric TMCs(TM=Ti,Zr,Hf,V,Nb,and Ta)were investigated using ab initio calculations.[137]The study found two intriguing phase-transition routes and a high-pressure phase of T1I’-type,namely,B1 →distorted T1I(T1I’)→TT1I and/or B1 →and/or TiB(TiB’)→BTiB.This suggests that TiB-type phase is a high-pressure phase and T1I’-type phase may be recovered at ambient pressure. These findings provide crucial insights for understanding the rich and complex crystal structures of TM-LE compounds,which have broad implications for further exploration.
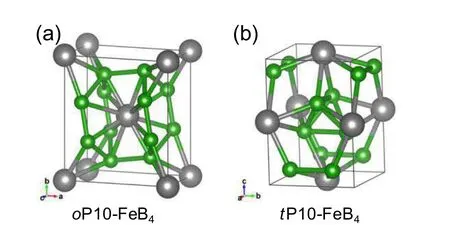
Fig.9. Crystal structures of FeB4. (a)oP10-FeB4 with an orthorhombic structure,which was first predicted by Kolmogorov et al.,[127]and was later successfully synthesized at HPHT conditions.[125]This type of FeB4 is a superconductor with measured Tc below 2.9 K;its hardness was first measured to be 62 GPa[125]and later proven to be ∼10–30 GPa.[128–130](b)tP10-FeB4 as a predicted high-pressure phase.This phase is a superhard semiconductor with band gap of approximately 1.85 eV and calculated hardness of 45 GPa.[122]The B and Fe atoms are painted in gray and green colors,respectively.
4.Conclusion and perspectives
Exploring superhard materials is an important topic due to their scientific interest and potential application.Here,we present a short review of recent advances in the discovery of superhard materials in LE compounds and TM-LE compounds using advanced CALYPSO structure prediction. A large number of promising,predicted crystal structures have excellent properties,including ultrahigh hardness,semiconductivity,and superconductivity,and some of them are very helpful in understanding the phases that cannot be completely determined experimentally.Nevertheless,current methodologies are mainly effective for structure predictions of singlecrystal materials.The properties of polycrystalline materials,including hardness,are dependent not only on intrinsic crystal structures,but also on their microstructures such as defects,dislocation,grain size,and boundary.The investigations have proven that nanostructuring or nanotwinning can significantly enhance the hardness of polycrystalline covalent materials.For example,the synthesized nanotwinned diamond exhibits extremely high hardness,which is twice that of a single crystal of diamond.[1,2]Therefore,developing a general approach of structure prediction for both single-crystal and polycrystalline materials is highly anticipated,and this will lead to new directions in searching for superhard materials among promising polycrystalline systems.
- Chinese Physics B的其它文章
- Compact finite difference schemes for the backward fractional Feynman–Kac equation with fractional substantial derivative*
- Exact solutions of a(2+1)-dimensional extended shallow water wave equation∗
- Lump-type solutions of a generalized Kadomtsev–Petviashvili equation in(3+1)-dimensions∗
- Time evolution of angular momentum coherent state derived by virtue of entangled state representation and a new binomial theorem∗
- Boundary states for entanglement robustness under dephasing and bit flip channels*
- Manipulating transition of a two-component Bose–Einstein condensate with a weak δ-shaped laser∗

