Optical coherence tomography guided treatment avoids stenting in an antiphospholipid syndrome patient: A case report
Bei-Bei Du, Xing-Tong Wang, Ya-Liang Tong, Kun Liu, Pei-Pei Li, Xiang-Dong Li, Ping Yang, Ying Wang
Bei-Bei Du, Ya-Liang Tong, Kun Liu, Pei-Pei Li, Xiang-Dong Li, Ping Yang, Ying Wang,Department of Cardiology, The Third Hospital of Jilin University, Jilin Provincial Cardiovascular Research Institute, Jilin Provincial Engineering Laboratory for Endothelial Function and Genetic Diagnosis of Cardiovascular Disease, Changchun 130031, Jilin Province,China
Xing-Tong Wang, Department of Hematology, The First Hospital of Jilin University,Changchun 130021, Jilin Province, China
Abstract
Key words: Anti-phospholipid syndrome; Myocardial infarction; Optical coherence tomography; Coronary intervention; Coronary thrombosis; Case report
INTRODUCTION
The etiology of acute myocardial infarction (AMI) is primarily local thrombosis caused by atherosclerotic plaque rupture, and there is a trend toward a younger onset.However, for young patients, especially females, the diagnosis should be made cautiously and secondary factors should be considered[1]. For patients with AMI who have a primary cause, coronary stenting must be performed prudently and the primary disease treated[2,3]. Anti-phospholipid syndrome (APS) is a non-infectious autoimmune disease that is related to arterial or venous thrombosis. APS is more common in young female patients. For patients with this special type of AMI,different coronary angiography (CAG) findings were reported, leading to different intervention strategies[4,5]. Although different CAG-based strategies were made,interventional successes and failures have both been reported. Thus, in addition to conventional treatment, intracoronary imaging should be employed to provide more supportive information to guide further intervention strategy. Here we present the optical coherence tomography (OCT)-guided treatment of a young female patient with AMI and APS.
CASE PRESENTATION
Chief complaints
A 26-year-old female patient was admitted to the hospital with chest pain,palpitations, and dyspnea.
History of present illness
The patient’s chest pain started 2 h prior when she was working for which a colleague called an ambulance. The pain was not alleviated after she arrived at the hospital.
History of past illness
Four years before this admission, the patient was admitted to the hospital with difficult breathing and diagnosed with APS, pulmonary embolism (Figure 1A), and deep vein thrombosis (DVT) in the left lower limb. An inferior vena cava filter was implanted and warfarin 2.5 mg/d and hydroxychloroquine (HCQ) 10 mg/d were prescribed for anticoagulation and anti-inflammatory treatments, respectively. The patient recovered after 6 mo. Follow-up pulmonary computed tomography angiography showed an obvious regression of thrombus (Figure 1B), and lower extremity vascular ultrasound showed that DVT had no progression. However, the patient stopped the warfarin and HCQ 6 mo before this admission. She also had a 2.5-pack-year smoking history and had stopped smoking for 4 years. The patient had no history of hypertension or type 2 diabetes.
Personal and family history
The patient married at a young age and had no children. Her family had no early onset history of cardiovascular diseases.
Physical examination upon admission
Physical examinations showed no abnormalities, and her blood oxygen saturation was 95%.
Laboratory examinations
A blood gas test showed a slightly decreased partial pressure of oxygen of 78 mmHg(normal range, 83-108 mmHg). D-dimer was negative. Myocardial biomarker testing showed elevated troponin (4.0 ng/mL), myoglobin (430 mg/L), and creatine kinase-MB (46.1 U/L).
Imaging examinations
Electrocardiography (ECG) showed an ST segment elevation in the precordial leads(Figure 2A). Emergency CAG showed acute occlusion of the proximal left anterior descending artery (pLAD) and TIMI thrombus grade 5 but a normal circumflex artery and right coronary artery (Figures 2B and D). A 6-F EBU 3.5 guide catheter was engaged and the pLAD was successfully recanalized with a Runthrough guidewire(Terumo, Tokyo, Japan). There was residual thrombus left in the pLAD. Repeated thrombus aspirations were performed and nitroglycerin 200 µg was administered to irradiate the spasm. Subsequent CAG showed mild to moderate stenosis left (Figures 2C and C1). Considering the patient’s age, sex, and the past history of thrombosis, an intracoronary assessment was performed to collect more evidence to support further interventions. OCT was performed and showed residual red and white thrombi and no atheroma in the pLAD, no culprit lesion, no plaque rupture, and no plaque erosion(Figures 2E-H). The minimal lumen area of the pLAD was 2.19 mm2and the area stenosis was 67.3%; the lumen area was reduced by the residual thrombus around the OCT catheter (Figure 2G).
FINAL DIAGNOSIS
According to the symptoms, the past medical history, and the laboratory and imaging findings, the patient was diagnosed with acute anterior wall ST elevation myocardial infarction (acute coronary thrombosis), Killip class IV; APS; pulmonary embolism(old); DVT (old); and post-inferior vena cava filter implantation.
TREATMENT
Considering the past medical history and OCT evidence for this young female patient,deferring or avoiding stenting based on further CAG and intracoronary imaging assessment findings was chosen. Also, coronary thrombosis was believed to be highly likely related to APS. Intensified antithrombotic treatments with dual antiplatelets(aspirin 100 mg/d + clopidogrel 75 mg/d), anticoagulant (low molecular weight heparin 4200 IU/d), and a glycoprotein IIb/IIIa inhibitor (tirofiban 100 mg until 24 h post-procedure) were performed.
After the procedure, the patient’s chest pain and other symptoms were relieved,and the post-procedure ECG findings returned to normal. Echocardiography showed left ventricular anterior wall hypokinesis and no right-to-left shunt. Lower-extremity venous ultrasonography showed right femoral vein and bilateral iliac vein thrombosis. Coagulation test findings were normal. Both total anticardiolipin antibody (aCL) and immunoglobulin G of aCL levels were elevated (55.6 and 32.5 RU/mL, respectively). Other antinuclear antibody tests were negative.
ECG, CAG, and OCT repeated after 1 wk of treatment (Figure 3) showed that no residual thrombus was left in the pLAD and that the intima was intact. No fibrous plaque, lipid plaque, or plaque rupture was detected (Figure 3A-G); the circumflex and right coronary arteries were checked and no obvious atherosclerosis was visible.Bubble study was done and ruled out patent foramen ovale.
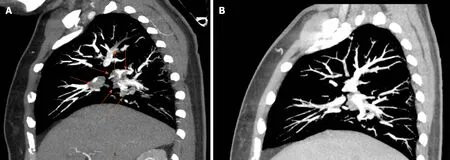
Figure 1 Representative computed tomography angiography evidence of pulmonary embolism and follow-up recovery. A: Four years before this admission,the patient was diagnosed with pulmonary embolism. This is one representative computed tomography angiography image of the pulmonary embolism. Red arrows indicate the thrombi inside the pulmonary arteries; B: Following 6 mo of treatment after the diagnosis of pulmonary embolism, follow-up pulmonary computed tomography angiography showed obvious thrombus regression.
OUTCOME AND FOLLOW-UP
For antithrombosis, heparin was replaced with warfarin (2.5 mg/d) 3 d after the procedure combined with dual antiplatelet treatment for 3 mo, followed by a later warfarin-clopidogrel combination until 1 year. After 1 year, lifelong warfarin was recommended with international normalized ratio monitoring and control at 2.0-3.0.Despite other anti-ischemia therapies, HCQ 10 mg/d was re-prescribed to reduce antiphospholipid antibody (aPL) titer. The patient was discharged 1 wk later and recovered well. A good result at the 3-mo follow-up was confirmed by phone.
DISCUSSION
Here we for the first time present direct evidence of non-atherosclerosis coronary thrombosis and no atheroma in a young female AMI patient with a previous history of APS. Serial OCT examinations demonstrated a good in-hospital clinical outcome after intense antithrombosis treatment without stenting.
APS is reportedly related to various cardiac diseases, such as heart valve disease,coronary artery disease (including AMI), intracardiac thrombosis, and pulmonary hypertension[6].
Although APS is believed to be related to accelerated atherosclerosis due to immunopathological and inflammatory status[6], it is generally accepted that APSrelated AMI is caused byin situcoronary thrombosis as a result of a high coagulation condition[7,8]. An increased serum aPL level (including lupus anticoagulant, aCL, and anti-β2-glycoprotein-1 antibody) is considered the main factor leading to thrombosis,but its specific mechanism is unclear[9]. Here we demonstrated direct evidence of nonatherosclerosis coronary thrombosis using serial OCT examinations in a patient with a high aPL level. In fact, based on other traditional risk factors that a patient might have before AMI, there have been reports of severe coronary stenosis and concomitant coronary thrombosis in CAG findings[5,7].
There are limited cases of failed or successful intervention for patients with APS and AMI, which in turn leads to different clinical results and prognosis[10,11]. One major concern related to stenting in these patients is the high risk of stent thrombosis,especially for patients with aPL positivity or poor anticoagulation compliance[7]. To reduce the possibility of thrombosis and recurrent AMI, initial attempt with bare metal stent implantation was made. In one study of 19 APS patients with stent (12 bare metal stents and 7 drug eluting stents) implantation, with up to 3 years of followup, higher targeted vessel revascularization (42.1%vs7.8%) and major adverse cardiac event rate (52.6%vs18.1%) were found compared to patients without APS[8]. Still there have been only limited data up to now reporting drug eluting stent implantation and the prognosis in APS and AMI patients. OCT evidence for this patient proved the high risk of thrombosis but no signs of intima injury; accordingly, avoiding stenting and an intensive antithrombosis strategy were chosen. One-week follow-up CAG and OCT had proven recovery and safety. However, no standard interventional treatment is available for patients with APS combined with myocardial infarction; thus, further research is needed.
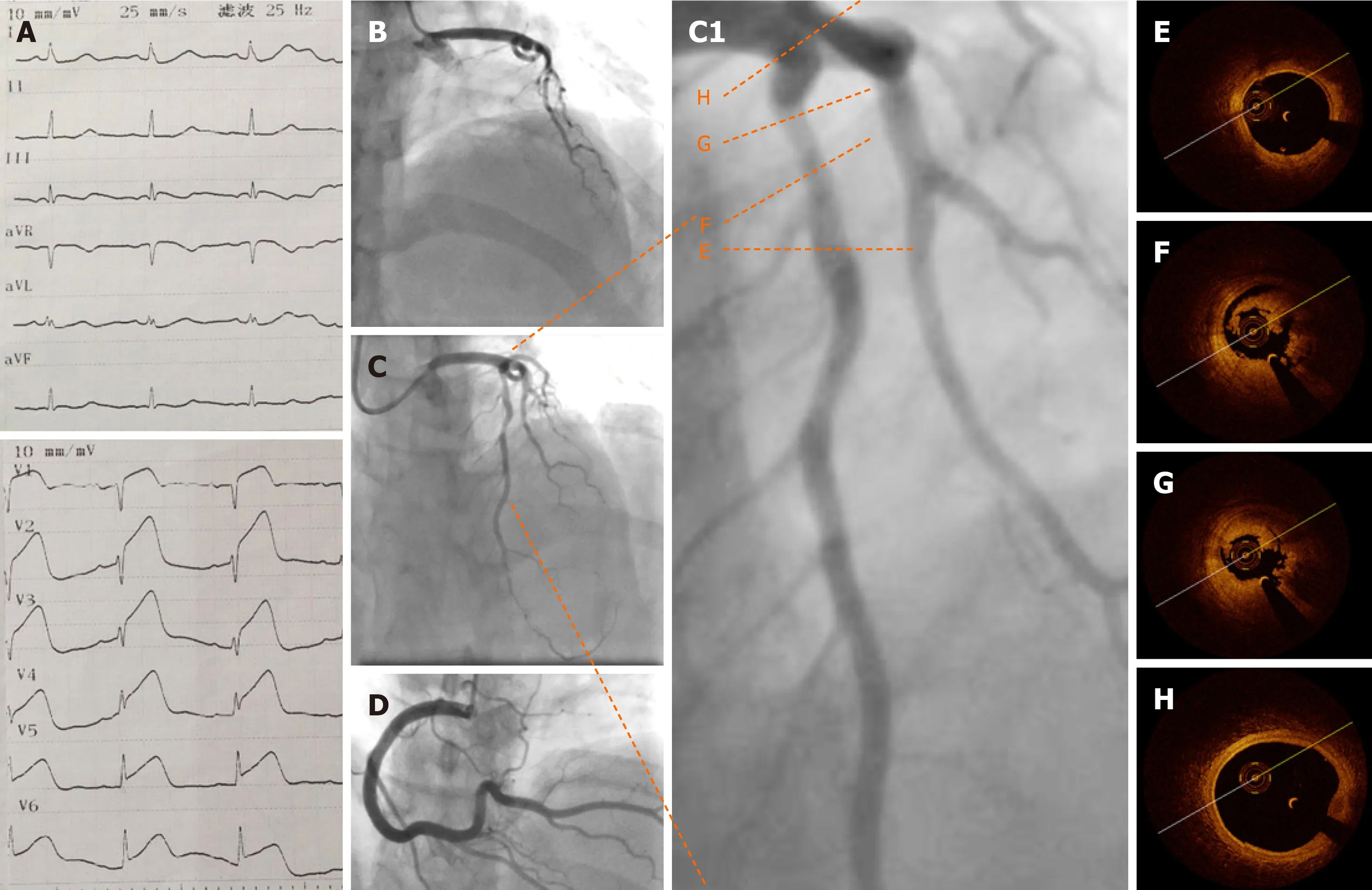
Figure 2 Baseline electrocardiography, coronary angiography, and optical coherence tomography. A: Emergency electrocardiography showing acute anterior wall ST elevation myocardial infarction; B: Emergency coronary angiography showing acute total occlusion and thrombus of the proximal left anterior descending artery (pLAD); C: pLAD after repeated thrombus aspirations; C1: Zoom-in image of pLAD; D: Normal right coronary artery; E-G: Representative cross-sectional optical coherence tomography images of the pLAD. Distal reference image of the pLAD stenosis (E), residual red and white thrombi around the optical coherence tomography catheter (F), and minimal lumen area of pLAD (2.19 mm2), area stenosis (67.3%), and residual thrombus (G, 3 o’clock to 9 o’clock) are shown; H: Normal left anterior descending artery ostium.
For antithrombotic treatment, no consensus has been achieved in this special clinical setting. We adopted the triple antithrombotic strategy to maximally reduce the risk of thrombosis for this patient with thrombophilia[12]. The use of direct oral anticoagulants has not shown efficacy in the management of this kind of case.
This case demonstrates that, during the diagnosis and treatment of young female patients with AMI, clinicians should be vigilant about the possibility of APS-caused non-atherosclerosis coronary thrombosis. For this special young patient cohort,intracoronary imaging can provide more information and may avoid stenting, which promises a better clinical prognosis.
CONCLUSION
In young female patients, APS can cause acute non-atherosclerosis coronary thrombosis which presents as an AMI. Intracoronary OCT findings can guide interventional strategies and guarantee a better prognosis in this special clinical scenario.
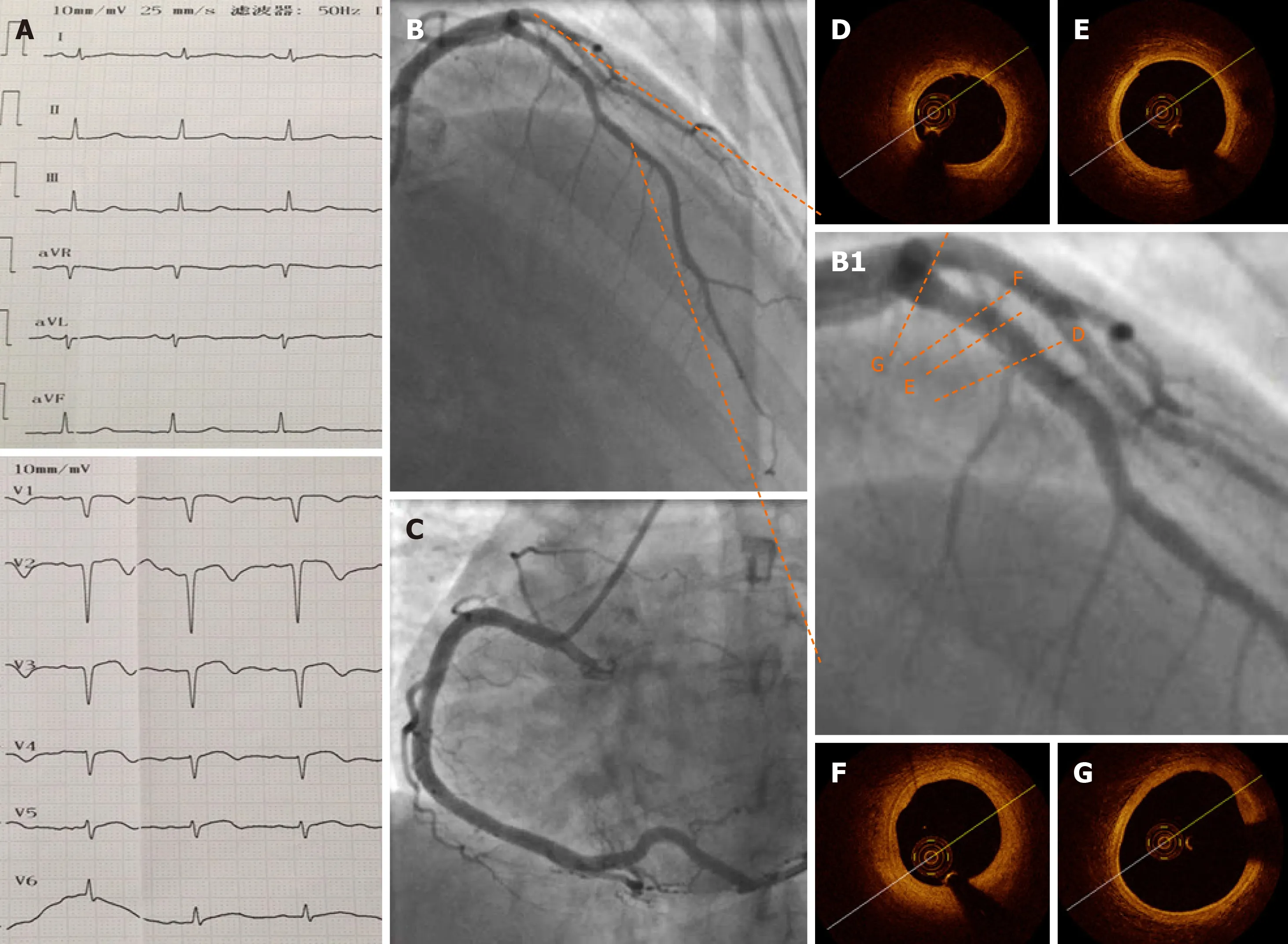
Figure 3 Electrocardiography, coronary angiography, and optical coherence tomography at 1 wk after the procedure. A: Pathological Q waves were visible on electrocardiography in leads V1–V4 at 1 wk after the procedure; B and C: One week later, coronary angiography showed a normal proximal left anterior descending artery (pLAD) and normal repeated thrombus aspirations; B1: Zoomed-in image of pLAD; D-G: Representative images of the pLAD showing a nearly normal coronary artery with an intact intima and no residual thrombus. F: Focal segment of a stable thick-cap plaque. Minimal lumen area was 5.57 mm2, and the percentage of area stenosis was 16.6%.
 World Journal of Clinical Cases2020年11期
World Journal of Clinical Cases2020年11期
- World Journal of Clinical Cases的其它文章
- Tumor circulome in the liquid biopsies for digestive tract cancer diagnosis and prognosis
- Isoflavones and inflammatory bowel disease
- Cytapheresis for pyoderma gangrenosum associated with inflammatory bowel disease: A review of current status
- Altered physiology of mesenchymal stem cells in the pathogenesis of adolescent idiopathic scoliosis
- Association between liver targeted antiviral therapy in colorectal cancer and survival benefits: An appraisal
- Peroral endoscopic myotomy for management of gastrointestinal motility disorder
