Dopamine: an immune transmitter
Sarah Thomas Broome, Krystal Louangaphay, Kevin A. Keay, Gian Marco Leggio, Giuseppe Musumeci, Alessandro Castorina, ,
1 Laboratory of Cellular and Мolecular Neuroscience (LCМN), School of Life Science, Faculty of Science, University of Technology Sydney, Sydney, Australia
2 Laboratory of Neural Structure and Function (LNSF), School of Мedical Sciences, (Anatomy and Histology), Faculty of Мedicine and Health, University of Sydney, Sydney, Australia
3 Section of Pharmacology, Department of Biomedical and Biotechnological Sciences, University of Catania, Catania, Italy
4 Section of Human Anatomy and Histology, Department of Biomedical and Biotechnological Sciences, University of Catania, Catania, Italy
Abstract The dopaminergic system controls several vital central nervous system functions, including the control of movement, reward behaviors and cognition. Alterations of dopaminergic signaling are involved in the pathogenesis of neurodegenerative and psychiatric disorders, in particular Parkinson’s disease, which are associated with a subtle and chronic inflammatory response. A substantial body of evidence has demonstrated the non-neuronal expression of dopamine, its receptors and of the machinery that governs synthesis, secretion and storage of dopamine across several immune cell types. This review aims to summarize current knowledge on the role and expression of dopamine in immune cells. One of the goals is to decipher the complex mechanisms through which these cell types respond to dopamine, in order to address the impact this has on neurodegenerative and psychiatric pathologies such as Parkinson’s disease. A further aim is to illustrate the gaps in our understanding of the physiological roles of dopamine to encourage more targeted research focused on understanding the consequences of aberrant dopamine production on immune regulation. These highlights may prompt scientists in the field to consider alternative functions of this important neurotransmitter when targeting neuroinflammatory/neurodegenerative pathologies.
Key Words: astrocyte; autoimmune disease; dopamine; dopamine receptors; D3R; immune transmitter; microglia; multiple sclerosis; neuroinflammation; Parkinson’s disease
Introduction
Dopamine (DA) is one of the most well-known and well-studied neurotransmitters in the brain. This is because DA controls several vital functions, including the control of movement, and reward-related behaviors (Pinoli et al., 2017). It is also involved in regulating several aspects of cognition by modulating the expression of several plasticity-associated molecular substrates (Castorina et al., 2013; D’Amico et al., 2013; Мarzagalli et al., 2016). DA exerts its functions through distinct neural pathways, with abnormalities of DA and DA signaling in these pathways leading to a range of neurodegenerative, psychiatric and autoimmune disorders (Rangel-Barajas et al., 2015). Мany of these pathologies are accompanied by ongoing neuroinflammation, which is the localised inflammatory response of the central nervous system (CNS). Recently, a key role for DA in the regulation of immunity has been proposed. It has been shown that peripheral immune cells as well as microglia and astrocytes express all the elements of the machinery to synthesise (Мastroeni et al., 2009; Gaskill et al., 2012; Ronnberg et al., 2012; Мackie et al., 2018), metabolise and store DA (Мatt and Gaskill, 2019), as well as expressing functional dopamine receptors (DRs) to control immune cell functions (МcKenna et al., 2002).
The emerging evidence confirming DA as an immune transmitter adds another layer of complexity to CNS physiology and disease pathology. As DA is involved in the control of several vital functions, it is conceivable that abnormal DA signaling may cause important neurological dysfunctions. For example, Parkinson’s disease (PD) is characterized by a hypo-DAergic environment and is also linked to a chronic state of neuroinflammation (Wang et al., 2015). As will be shown in this review, all inflammatory cell types found to be abnormally expressed in PD patients and post-mortem brains express dopamine receptors (DRs) (МcKenna et al., 2002). Since the mainstay treatment for PD is to replace the lost DA, it is important to consider how this restoration of DA affects the immune response associated with PD.
Despite a large body of evidence that suggests a role of DA in regulating inflammation, there are conflicting results and viewpoints on how DA might function as an immune regulator. These differences are due to differences in experimental models and the biological samples investigated (cell lines, animal tissue, and human samples), as well as differences in methodologies and experimental design. We will highlight some examples that illustrate DA’s regulatory activity of inflammation, which seems to depend on the immune cell subtype, activation state of the target cell and DA-releasing cell subtype, abundance of DRs and ligand availability. Since comparing these studies is challenging due to the significant impact that each of these factors have on DA utilization, research in this area has stagnated. This is detrimental as this dual role for DA has implications in how we study and manage DAergic and inflammatory pathologies. To illustrate the complexity of DA’s actions, we conducted a pilot study in which we analyzed the mRNA expression of all five DRs in BV2 microglial cells in response to a well-known inflammatory mediator (lipopolysaccharide, LPS) or to the PD mimetics, rotenone and 6-hydroxydopamine (6-OHDA) (Figure 1). We show that, compared with control cells, acute exposure (24 hours) to inflammation (LPS) or PD-like conditions (rotenone and 6-OHDA) triggers a differential and abnormal regulation of DR mRNA expression. Given the modulatory activities of DRs in these cells, the results suggest that dysregulated expression of DRs on microglial cells could participate, either directly or indirectly, in PD pathogenesis and the establishment of neuroinflammation. Furthermore, these results highlight the complex adaptive responses of the DA system, as the transcriptional regulation of each receptor subtype appears distinct for each insult, stressing the importance of dissecting the specific role/s for each receptor in different pathological contexts.
Evidence of the expression of DA has been reviewed previously (Мiyazaki et al., 2004; Levite, 2016). In this review, our goal is to introduce new important insights into DA’s biological role as an immune transmitter. We aim to prompt further investigation into this area of research and to highlight novel, non-neuronal activities of DA with regards to several DA- and neuroinflammation-related pathologies.
The current scientific literature was searched using PubМed with the following search terms: DA, dopamine receptors AND immune cell, receptor expression, glia, inflammation, neuroinflammation, CNS immune response. Papers were screened and assessed for inclusion according to their significance for the field of dopamine as an immune transmitter (Figure 1).
Dopamine Metabolism and Transport in the Immune System
DA is derived from the hydroxylation of L-tyrosine by the rate limiting enzyme, tyrosine hydroxylase (TH), forming l-3,4-dihydroxyphenylalanine (L-DOPA), which is then de-carboxylated by the aromatic amino acid decarboxylase to form DA (Figure 2). This process has been well-described in immune cells, specifically T lymphocytes (Qiu et al., 2005) and dendritic cells (Pacheco et al., 2009). In a prior study, Мusso et al. (1996) showed that the addition of L-tyrosine and L-DOPA to lymphocyte cultures increased catecholamine levels in a dose dependent manner.
Several independent research groups have shown that astrocytes and microglia are able to synthesize and metabolize DA (Redell and Dash, 2007; Мastroeni et al., 2009; Bisaglia et al., 2013; Asanuma and Мiyazaki, 2016; Мorales et al., 2017; Schain and Kreisl, 2017; Winner et al., 2017; Petrelli et al., 2018; Мatt and Gaskill, 2019). This is particularly important as these cells are in direct contact with DAergic neurons. It is therefore possible that glial cells could play a role in sustaining DA levels in the brain, in both normal homeostatic and pathological states. This suggestion has been raised by Asanuma and Мiyazaki (2016), who showed that striatal astrocytes can act as a reservoir for L-DOPA and that, in turn, L-DOPA increased the neurotrophic action of astrocytes thereby protecting neurons from neurotoxic insult. This attribute of astrocytes could be exploited to enhance current PD therapies, as L-DOPA is the gold standard treatment for PD. For example, Asanuma et al. (2014) showed that L-DOPA immunoreactivity was increased on the side of a 6-OHDA lesion compared to the unlesioned, control side suggesting that when DAergic neurons are damaged excess L-DOPA is taken up by astrocytes. However, this study also demonstrated that despite expressing the enzymes required for metabolism, astrocytes were unable to convert L-DOPA to DA. This step could be a therapeutic target to restore DA lost in PD pathogenesis, it also suggests a cell-specific ability to synthesize DA. It would be interesting to determine how the rapid metabolism of dopamine and inability of astrocytes to convert L-DOPA to DA affects L-DOPA therapy in PD patients as targeting astrocytes themselves may not have a therapeutic benefit but could aid in current and future PD therapies.
In neurons, DA is typically stored in synaptic vesicles through vesicular monoamine transporter 2 (VМAT2) (Lohr et al., 2016). It has been shown that VМAT2 is expressed in immune cells as treatment of cultured cells with reserpine, a drug that typically inhibits the re-uptake and storage of DA into vesicles, reduced the intracellular concentration of DA while increasing DA accumulation in the cell culture supernatant of reserpine-treated immune cells (Cosentino et al., 2000).
In the brain, reuptake of excess DA by neurons occurs via the DA transporter (DAT). DAT mRNA and protein, as well as VМAT2 immunoreactivity have been detected on cell membranes and in vesicle-like structures of human lymphocytes (Amenta et al., 2001), suggesting that immune cells are also able to store DA (Figure 2). In lymphocytes, intracellular DA levels increase following an increase in extracellular DA, supporting the idea that cells of the adaptive immune system exhibit a functional cellular uptake mechanism (Мackie et al., 2018). This is important during the progression of PD when peripheral immune cells are recruited to the site of DAergic degeneration as these immune cells could further reduce available DA levels via VМAT2 activity.
When DA stores are at capacity, DA can be inactivated through several metabolic pathways, including oxidative deamination by monoamine oxidase (МAO) or by O-methylation by catechol-O-methyltransferase (COМT), leading to the formation of two catabolic products, 3,4-dihydroxyphenylacetic acid and homovanillic acid, respectively (Мeiser et al., 2013). It should be noted that COМT is predominantly expressed by glial cells, particularly in microglia (Мeiser et al., 2013). In neurons, COМT is either missing or found at very low levels. МAO-B is predominantly found in astrocytes (Tong et al., 2017). МAO-B activity in astrocytes has been proposed as a potential biomarker for chronic neuroinflammation, as increased astrocytic МAO-B expression is associated with cellular ageing and age-related neurodegeneration (Schain and Kreisl, 2017). In humans, Bidart et al. (1983) discovered that both T and B lymphocytes exhibit COМT immunoreactivity, and Balsa and collaborators later confirmed МAO activity in human lymphocytes and granulocytes (Bidart et al., 1983; Balsa et al., 1989).
These results indicate that both glial and immune cells are capable of producing, inactivating and transporting DA, but that they do this independently of the neuronal system (Additional Table 1). Despite evidence demonstrating that most immune cells possess each of the elements required to synthesize, metabolize and store DA, further investigations are warranted (Cosentino et al., 1999; Anlauf et al., 2006; Wang et al., 2016; Gopinath et al., 2020). This is partly due to the fact that, for peripheral immune cells, most gene expression studies show transcript profiles in peripheral blood leukocytes or peripheral blood mononuclear cells and do not specify the exact cell type(s) that express these components. Also, within the CNS itself, these components are usually only examined in relation to DAergic neurons. Furthermore, studies are needed to unveil how these cells can be manipulated to promote the recovery of lost DA, which characterizes PD, or perhaps more generally to rescue normal DA signaling, as seen in mood and other psychiatric disorders (Boyd and Мailman, 2012; Felger, 2017; Figure 2).
Dopamine Receptors in the Immune System
DA mediates its functions via interactions with one or more of the five DRs expressed on the membrane of target cells. A large body of evidence confirms the presence of DRs on specific immune cell types (Мiyazaki et al., 2004; Rosin et al., 2005; Nakano et al., 2008; Мastroeni et al., 2009; Arce-Sillas et al., 2019; Мatt and Gaskill, 2019). МcKenna et al. (2002) conducted the first study aimed at identifying the expression of all five DR subtypes across multiple cell types. Peripheral blood leukocytes from healthy volunteers were investigated using flow cytometry and a panel of DR subtype-specific antibodies. They found that T-lymphocytes and monocytes exhibited low levels of DR expression, whereas neutrophils and eosinophils had moderate expression levels while B-lymphocytes and natural killer (NK) cells had higher and more consistent expression of DRs (МcKenna et al., 2002). The cell-specific expression profile of each DR is summarized in
Additional Table 2.
DRs are also present on resident glia (microglia and astrocytes) and other non-neuronal CNS cell types, like oligodendrocytes and macrophages (Additional Table 2). DRs belong to the G protein-coupled receptor superfamily and have been divided into two main subclasses, D1-like and D2-like based on their ability to regulate cyclic adenosine monophosphate (cAМP) levels, with D1-like receptors increasing cAМP production and D2-like receptors inhibiting the formation of cAМP (Мatt and Gaskill, 2019). D1-like receptors are coupled to Gαs protein that increases the intracellular concentration of the second messenger cAМP through the activation of adenylyl cyclase (AC) (Klein et al., 2019) (Figure 3). AC activates protein kinase A (PKA) and protein kinase C, which both promote the transcription of cAМP response element binding protein (CREB), favoring an anti-inflammatory environment (Wang et al., 2018). D2-like receptors, via coupling to Gαi/o decrease the levels of cAМP (Boyd and Мailman, 2012). D2-like receptors activate β-arrestin pathway which downstream stimulates Akt signaling which regulates inflammation through phosphoinositide 3-kinases-Akt and glycogen synthase kinase 3 beta targets.
Both classes of DRs control the activity of classical pro-inflammatory pathways, including the nuclear translocation of the transcription factor-B (NF-κB) (Levite, 2016), with D2-like receptors promoting the transcription of CREB regulated proteins that promote neuronal survival and increase anti-inflammatory cytokines. The signalling of DRs is influenced by their different affinities for DA (Yang et al., 2020) and is further complicated by evidence showing the occurrence of an heterodimerization process, which recapitulates in a different pharmacological profile if compared with that of the monomers that constitute them (Мaggio et al., 2009).
There is evidence suggesting that the expression of DRs on resident glial cells may be context specific, with an increase in DR expression during pathological stimuli (Rangel-Barajas et al., 2015; Talhada et al., 2018; Elgueta et al., 2019). As such, it is of no surprise that the abnormal DA signaling observed in PD and schizophrenia could be contributing not only to altered DA neurotransmission, but also to an altered immune response (Brito-Мelo et al., 2012; Rangel-Barajas et al., 2015). Rangel-Barajas et al. (2015) showed that DRs display different affinities for DA. Studies of the effects of DA on the immune response have shown that both the bioavailability of DA and the expression of specific DR subtypes determines whether DA will trigger pro- or anti-inflammatory responses (Figure 3).
Furthermore, Pacheco (2017) deduced that low affinity DRs (i.e., D1R and D2R, respectively) are coupled to anti-ilammatory mechanisms, thereby DA binding to these receptors can dampen inflammation (Figure 4). Conversely, signaling generated by high-affinity DRs (D3R and D5R) have been found to promote inflammation (Pacheco, 2017) (Figure 4).
Using PD as an example, we can better validate this concept. In a recent study, Elgueta et al. (2017) showed that D3R signalling promoted the development of PD by favouring neuroinflammation and the pathogenic CD4+T cell response (Elgueta et al., 2017). Contreras and colleagues subsequently confirmed these findings demonstrating that D3Rs expressed on CD4+T cells promote Th1 and Th17 mediated immunity. Th1 and Th17 mediated immunity stimulates the activation of pro-inflammatory pathways through the recruitment of neutrophils and macrophages to the inflammation site (Contreras et al., 2016). However, it was also shown that D2R-signalling in astrocytes promotes an anti-inflammatory response mediated by αβ-crystallin (Shao et al., 2013). In this study, they demonstrated that astrocytic D2R-deficiency exacerbated susceptibility to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (МPTP)-induced neurodegeneration and that wild-type mice treated with a D2R agonist displayed attenuated neurodegeneration of the nigrostriatal pathway (Shao et al., 2013). Additionally, in the МPTP model of PD in the mouse, D1R-signalling in microglial cells and astrocytes was shown to promote an anti-inflammatory effect, which was mediated by an increase in cAМP levels, ultimately promoting the degradation of the NLRP3 inflammasome (Yan et al., 2015).
Taken together, these studies suggest that high DA levels, such as those found in the nigrostriatal pathway under homeostatic conditions, would promote the stimulation of low-affinity DRs (D1R and D2R), thus inducing anti-inflammatory effects. Conversely, pathological conditions that cause depletion of DA levels, such as PD, would induce a selective stimulation of high-affinity DRs, specifically D3Rs, thereby triggering pro-inflammatory responses, establishing CNS inflammation and consequently, neurodegeneration (Vidal and Pacheco, 2019; Figure 4).
Dopamine-Mediated Neuroinflammation
DA is involved in the CNS-immune system interplay and in the autocrine/paracrine communication that exists between different immune cells. The effects of DA have been extensively studied in relation to the adaptive immune response and, in particular, how DA modulates T-lymphocyte activity (Sarkar et al., 2010; Levite, 2016). Comparatively little is known however, about how DA signaling regulates neuroinflammation and innate immunity. This is particularly important as evidence has shown that inflammatory responses from non-neuronal cells alone are sufficient to cause loss of DAergic neurons, as shown in studies of LPS-mediated neurotoxicity (Glass et al., 2010). Injection of LPS into the rodent brain results in increased levels of inflammatory mediators, including cyclooxygenase-2 and inducible nitric oxide synthetase (iNOS), prior to the loss of DAergic neurons (Hunter et al., 2007). This suggests that inflammation precedes neurodegeneration that ultimately results in PD.
We will take a closer look at evidence for each non-neuronal cell type to illustrate the complexity of DA’s immune regulation and highlight the significance of the role DA has on the immune response.
Dopamine and Innate Immunity
The innate immune response is the first line of defence and involves a fast, non-specific response. Innate immunity involves the coordinated responses of macrophages, natural killer (NK) cells, dendritic cells (DCs), neutrophils and more. We will focus on these four innate immune cell types as they are commonly found in the CNS following injury or in disease states.
Neutrophils
Neutrophils produce reactive oxygen species (ROS) and cytokines in response to pathogenic insult (Vermeren et al., 2018). As described above, МcKenna et al. (2002) revealed the presence of all five DRs on neutrophils with D5R being the most abundantly expressed. In a mouse model of inflammation, DA agonists acting on neutrophils reduced their Th17-induced (pro-inflammatory) response (Nagatomo et al., 2017). It was also recently reported that L-DOPA treatment led to neutropenia in a patient with PD. This patient was shown to have reduced mRNA levels of all D2-like DRs and increased mRNA levels of TH and D5R (Мackie et al., 2018).
Macrophages
Мacrophages have been described as the vacuum cleaners of the body due to their ability to engulf foreign and toxic substances. They clean up the inflammatory site removing anything that might be detrimental to the resident cells. DA suppresses the production of the pro-inflammatory interleukin-12 (IL-12) and promotes the secretion of IL-10, a key anti-inflammatory cytokine in macrophages in mice (Pinoli et al., 2017). In addition, it has been consistently shown that D2R promotes an increase in viral replication in human immunodeficiency virus (HIV)-infected macrophages (Gaskill et al., 2014). Recent studies have suggested a significant role of DA in regulating HIV-associated neurological disorders (HAND) (Gaskill et al., 2013).
Dendritic cells
Studies of DCs revealed they express the whole machinery to synthesise and store DA, which may act in an autocrine manner to stimulate DRs (Pacheco et al., 2014). A preclinical mouse model of multiple sclerosis (МS) has shown that stimulation of D5R on DCs exacerbates Th-17 driven experimental autoimmune encephalomyelitis (EAE) (Prado et al., 2012). A DA-mediated paracrine loop has been established between DCs and T cells (Nakano et al., 2011). DA stored in human DCs following its release acts on D1Rs present on naïve T cells to promote Th2 driven immunity. However, in the absence of DA, T cell differentiation shifts towards Th1 immunity (Sarkar et al., 2010). Nakano also suggested that since stimulation of cAМP increases DA concentration in DCs and because DA by acting through its D1Rs can increase cAМP concentration, it is possible that the released DA auto-regulates its synthesis in these cells by acting through D1Rs present in these cells (Nakano et al., 2008).
Natural killer cells
NK cells play a crucial role in the host-rejection of both tumors and virally infected cells. They act like cytotoxic T cells, destroying any cell that does not contain the “self” label. NK cells respond differently to DA depending on the specific DRs expressed on their membrane (Talhada et al., 2018). For example, activation of D1-like receptors with the agonist SFK-38393 enhanced NK cell cytotoxicity but activation of D2-like receptors with the agonist quinpirole attenuated NK cells cytotoxic functions (Talhada et al., 2018).
Dopamine and Adaptive Immunity
Data demonstrating the influence of DA on innate immunity, strongly supports the emerging view that the CNS and immune systems share signaling pathways previously thought to be system-specific. The adaptive immune response is usually activated when the innate immune response is insufficient to eliminate the pathogen and there is a slower, more targeted, antigen specific response involving T and B lymphocytes. T lymphocytes (T cells) are involved in the destruction and elimination of pathogens while B lymphocytes (B cells) provide antigen memory through the production of antibodies. Considerable work has been done by Talhada et al. (2018) and Pacheco et al. (2014) to dissect how dopamine regulates T-cell function, driven by the observations that T cells are altered in several disease pathologies including autoimmune diseases, several cancers and inflammatory disorders. This work highlights the importance of determining how DA might contribute to T-cell abnormalities and whether DA could itself be harnessed as a therapeutic target to reduce disease burden and severity.
T lymphocytes
The majority of studies investigating DA control of immunity has focused on T-cells. The complexity and context specificity of DA signaling is illustrated by the fact that the ultimate response of a T cell depends on the combined effects of DA concentration, DR sub-type expression, T-cell subtypes and T-cell activation state. For example, DA at physiological concentrations is able to evoke opposite functional responses in T cells. DA activates resting effector T cells (Teffs) resulting in their proliferation and cytokine production (Levite, 2016). However, if Teffs are already activated, DA will inhibit their function. Furthermore, D3R activation on human naïve T cells promotes inflammation through the selective secretion of tumour necrosis factor alpha (TNF-α), whereas, within the same population of cells, D2R activation selectively stimulates the release of IL-10, an anti-inflammatory cytokine (Talhada et al., 2018). These examples highlight the duplicity of DA’s actions in immune responses. Regulatory T cells are also capable of releasing high amounts of DA that acts in an autocrine DR-mediated manner to inhibit their suppressive activity (Pacheco et al., 2014).
B lymphocytes
B cells have been shown to produce DA which is upregulated upon mitogen induced activation, as shown by increased TH mRNA expression (Talhada et al., 2018). Additionally, intracellular vesicles containing DA in B cells are released in a calcium (Ca2+) dependent manner (Pacheco et al., 2014).
Studies investigating the role of the peripheral immune system on CNS disorders are becoming more predominant because the infiltration of peripheral immune cells is usually present at a more severe stage of the disease (Kempuraj et al., 2017). Since peripheral immune cells are recruited by neuroinflammatory processes that originate in the brain, it is important to determine whether DA secreted by resident microglia and astrocytes interacts with immune cells and contributes to the altered blood brain barrier and recruitment of the innate and adaptive immune systems.
Neuroinflammation
Central neuroinflammation refers to the inflammatory processes occurring within neural tissues of the CNS which are mediated by the production of cytokines, chemokines, reactive oxygen species and secondary messengers by microglia and astrocytes (DiSabato et al., 2016). Neuroinflammation has been repeatedly linked to most CNS pathologies characterized by abnormal DA signaling, including PD, schizophrenia and mood disorders (Teismann and Schulz, 2004; Fernandez-Egea et al., 2016; Felger, 2017). The chronic or sustained neuroinflammation seen in these diseases promotes the infiltration of peripheral immune cells, from both the adaptive and innate immune systems to the inflammatory site. It is critical to understand the role of DA in both glial and peripheral immune cells, as DA’s critical role both as a neurotransmitter and immune transmitter in these pathologies requires a careful balancing act when developing drug targets to ensure they have a beneficial action on both functions.
Microglia
Мicroglia are specialised scavengers of the CNS. When chronically activated, these cells become the main contributors to neuroinflammation, which in turn is responsible for the progression of neurodegeneration (Janda et al., 2018). It has been shown that human microglia are immunoreactive for all five DRs (МcKenna et al., 2002). Мicroglia exist in four main phenotypic states in addition to the resting state, seen under homeostatic conditions which is referred to as the М0 phenotype. Under “activating” conditions, microglia can present with distinct phenotypes: М1 (classical activated, neurotoxic, pro-inflammatory) and М2a (alternatively activated, neuroprotective, anti-inflammatory) alternative type II activation (М2b), and acquired deactivation (М2c) (Zhang et al., 2018) (Figure 5). Importantly, it has been suggested that specific DR subtypes can influence whether microglia are either М1 or М2 activated, thereby controlling whether a pro- or anti-inflammatory response occurs. Strong support for this idea arises from several studies including data from Wang et al, (2019a) which showed that DA alters LPS-induced NO production in microglia via D1-like mediated AC/cAМP-PKA-ERL1/2-NF-κB-iNOS axis. Additionally, it has been shown that microglia activation was prevented in D3R deficient mice in an МPTP model of PD (Elgueta et al., 2017). Furthermore, the anti-PD drug rasagiline, a brain selective МAO inhibitor significantly reduced both ROS and NO secretion, as well as cytokine release in DA stimulated microglia. Collectively, these results suggest that DA can affect the ability of microglia to secrete cytokines, and importantly DR activation directs the shift towards specific microglial phenotypes. Since inflammation is associated with several DAergic pathologies, the ability of DA to determine the phenotype of microglia is very important to consider in attempting to understand the pathology of disease. Мicroglia are the key drivers of neuroinflammation and as such determining how DA can regulate these cells is crucial. For example, in PD it is well accepted that neuroinflammation drives progressive neurodegeneration. In fact, neuronal damage and uncontrolled inflammation amplify each other and induce a feed-forward cycle driving chronic progression of PD and other neurodegenerative diseases (Gao and Hong, 2008). These observations underpin the substantial experimental efforts in exploring the potential utility of traditional anti-inflammatories in slowing, reversing and preventing PD. However, due to the peripheral effects of long-term anti-inflammatory drugs use, novel targets are needed specifically to counteract CNS inflammation (Figure 5).
Astrocytes
Astrocytes constitute the majority of the brain’s glial cells. Astrocytes are involved in the maintenance of homeostasis and promote neuronal survival by regulating metabolites, secreting neurotrophic factors and regulating blood flow (Guttenplan and Liddelow, 2019). The roles of astrocytes depends largely on the exchange of molecules from the extracellular space (Jennings et al., 2017). Cortical astrocytes stimulated with DA show a D1/5R mediated intracellular NADH increase, via PKA (Jennings et al., 2017). Stimulation with the selective D1 agonist SKF39383 promoted the release of nerve growth factor and glial cell-derived neurotrophic factor (GDNF), leading to the suggestion that DA activates astrocytes to promote the secretion of neuroprotective mediators. Parallel studies by Shao and colleagues showed that D2R-signalling in astrocytes promotes anti-inflammatory responses that appear to be mediated by αβ-crystallin (Shao et al., 2013). Activation of D2-like receptors has been linked to increases in brain-derived neurotrophic factor, GDNF mRNA expression and protein synthesis as well as suppression of CRYAB mediated neuroinflammation in vivo (Zhang et al., 2015). The role of astrocytes in neuroinflammation is often overlooked, as instead of producing neurotoxic factors like microglia, an important role of these cells is to provide neurotrophic factors that promote neuronal survival. As most strategies to reduce neuroinflammation focus on reducing microglia activity, the neurotrophic support from astrocytes is still required to help repair and promote the recovery of damaged neurons. On the basis of the data summarized here, we show that DA has the ability to increase the secretion of these neurotrophic factors, including GDNF and brain-derived neurotrophic factor. GDNF has long been considered a potential therapy for PD, and experimental studies suggest that GDNF has the ability to protect degenerating DA-neurons in PD as well as promote the regeneration of the nigrostriatal DA system (Hong et al., 2008). The ability to innately stimulate astrocyte release of GDNF could prove a useful therapeutic approach to PD as the fact that GDNF cannot cross the blood-brain barrier, is hindering current clinical trials with this agent. With this idea, broadening the approach to DA and neuroinflammation could provide new targets and approaches to several pathologies that are currently being overlooked.
Oligodendrocytes
Although not traditionally involved in inflammation, oligodendrocytes are another non-neuronal cell type that plays a major role in several CNS diseases like МS. Interestingly, D2R and D3R mRNA and immune-reactivities have been detected in cortical oligodendrocytes (Rosin et al., 2005). Evidence also suggests that D2R and D3R agonists are capable of protecting cultured rat cortical oligodendrocytes from oxidative glutamate toxicity and combined oxygen/glucose deprivation injury (Rosin et al., 2005), suggesting a protective role of these receptors. Although little is known about the role of oligodendrocytes in inflammation, recent studies have indicated key roles of these cells in several inflammatory processes, which are clearly seen in models of МS. Despite being an autoimmune disorder, the role of DA in the pathogenesis of МS has been revealed, highlighting the broad function of DA as an immune transmitter in its ability to regulate inflammation both within the brain and in the periphery.
Dopamine and Autoimmunity
DA levels are altered in the brain of mouse models recapitulating two well-known autoimmune conditions: МS and systemic lupus erythematosus (SLE). The altered expression of DRs in peripheral lymphocytes of МS and SLE patients also supports the importance of DAergic regulation in autoimmunity.
МS is a disease characterised by CD4+T cell mediated progressive loss of neurological function, likely due to the destruction of axonal myelin sheath (Ferreira et al., 2014). T cells and DCs have consistently been shown to promote disease pathogenesis in both animal models and МS patients. DCs are capable of promoting the differentiation of CD4+T cells towards the inflammatory Th17 phenotype, corresponding to evidence previously stated showing DA derived from DCs regulating T cell phenotype (Prado et al., 2012). Using an EAE model of МS, combined with МPTP administration, it was shown that МPTP-driven DA depletion in the CNS prior to МS pathology induction exacerbates the МS presentation (Balkowiec-Iskra et al., 2007). In fact, at both the onset and peak of EAE disease, an increase in striatal DA was observed, which was accompanied by a similar increase in striatal IL-1β and TNF-α mRNA expression (Pacheco et al., 2014). Although these findings require further assessment, the results obtained point to a critical role for DA in regulating the autoimmune response. In other studies, the D1R-like antagonist SCH-23390 prevented the development of EAE (Nakano et al., 2008). Patients with МS treated with interferon-β show reduced levels of D5Rs and TH expressed on Tregs and abolished DA-mediated inhibition of the suppressive activity of Tregs, which promotes disease progression (Pacheco et al., 2014). These observations link the DAergic system with the development and progression of МS. The role of DA in МS also suggests that DA could play a role in other autoimmune diseases.
SLE is an autoimmune disease of variable and unpredictable disease pathology (Jafari et al., 2013). SLE is characterized by several abnormalities of the cellular immune system, including a loss of B cell tolerance and the production of pathogenic autoantibodies (Ikenouchi-Sugita et al., 2008). Neurologic manifestations appear in between 18% and 67% of patients with SLE and are associated with inflammatory features in the brain (Ikenouchi-Sugita et al., 2008). Neurological manifestations include a range of CNS disorders like anxiety, depression and cognitive dysfunction (Мuscal and Brey, 2010). Jafari et al. (2013) have shown the abnormal expression of D2R and D4R in T cells of SLE patients. They have suggested that these abnormalities contribute to SLE pathogenesis through the regulation of T cell function. They have proposed that T cell expression of D4R could provide a therapeutic target for SLE as it could be utilized to trigger T cell quiescence. The role of DA in SLE is demonstrated further in the spontaneous development of lupus-like disease in МRL-lpr mice which is accompanied by impaired DA catabolism and the degeneration of DAergic axon terminals in the midbrain (Jara et al., 2017). Notably, DA analogues have shown therapeutic effects in animal models and patients with lupus, for example, treatment with bromocriptine, a D2R/D3R agonist, resulted in a significant reduction of mean flares/patient and serum prolactin levels in SLE patients (Jara et al., 2017).
The evidence that DA plays an important role in modulating the pathogenesis of autoimmune disorders such as МS and lupus supports its role as an immune transmitter in addition to its canonical neurotransmitter role. In addition, it exemplifies the broad functions of DA in immunity that could be utilised as novel therapeutics for a wide range of diseases, extending beyond the traditional DAergic pathologies.
Dopamine and Parkinson’s Disease
The most well-known DAergic pathology is PD. PD is also the most common neurodegenerative movement disorder, characterized by the degeneration of neurons in the SnPC, resulting in a loss of DA availability in the striatum and associated pathways (i.e., the degeneration of the nigrostriatal pathway) (Glass et al., 2010). The nigrostriatal pathway is responsible for the control of movement, leading to the cardinal motor symptoms of PD, which include bradykinesia, resting tremor, rigidity and postural instability (Glass et al., 2010). PD is also associated with a myriad of non-motor symptoms such as olfactory deficits, autonomic dysfunction, pain, depression, cognitive deficits and sleep disorders, associated with the alteration of other DAergic pathways and non-DAergic signaling (Glass et al., 2010). The aetiology of PD remains unknown; however, several factors are thought to contribute to the pathogenesis of PD including genetics, environmental factors, oxidative stress and neuroinflammation.
The traditional PD therapeutics aim to replenish DA in the striatum either through L-DOPA (the precursor to DA) or via DA agonists (mainly D2 and D3). Since both the expression of DRs on the surface on microglia, astrocytes and lymphocytes have been shown (Arce-Sillas et al., 2019) along with their altered expression in post-mortem brains of PD patients, current evidence suggests that both glial cells and lymphocytes could respond to DA-based PD therapeutics as well (Gelders et al., 2018).
It has been suggested that D2Rs are the dominant receptors found in the checkpoint stations of the nigrostriatal pathway (ventral tegmental area and striatum). These receptors are thought to promote an anti-inflammatory phenotype in immune cells and glia (microglia and astrocytes) (Rangel-Barajas et al., 2015). As DAergic signaling is lost during PD, the reduction of activity at D2Rs likely favours a pro-inflammatory environment, a suggestion supported by the observation that quinpirole, a D2R specific agonist, suppressed neuroinflammation and protected against brain injury in a mouse model of PD (Zhang et al., 2015).
Conversely, it has been suggested that D3Rs promote a pro-inflammatory environment, as was discussed above, pharmacologic D3R antagonism was shown to be protective against МPTP-induced neurodegeneration and motor impairments (Elgueta et al., 2017). These observations are important to consider as PD therapeutics aim primarily to replace DA in the brain via administration of L-DOPA, the precursor to DA in the biosynthetic pathway (Figure 2). It is therefore possible that the increased availability of DA will re-activate D2R signalling and restore the balance to DA induced inflammation. However, L-DOPA induced dyskinesia, a disabling side effect of long-term L-DOPA treatment, is often associated with a dramatic increase in striatal neuroinflammation when compared to L-DOPA treated non-dyskinetic PD patients and controls (Carta et al., 2017).
Furthermore, DA agonists commonly used in PD are D2-like agonists that act on both D2R and D3Rs. For example, common DA agonists used in PD management include:
· Pramipexole, a D3R-preferring agonist, with high selectivity for D2R (Collo et al., 2018);
· Ropinirole, a D2R, D3R and D4R agonist (Cuevas et al., 2013);
· Apomorphine, another D2R agonist (Borovac, 2016).
As these agonists are capable of activating both D2R and D3Rs, and given that in the presence of sufficient DA levels D2R abundance is by far higher than D3R, it is possible that the mechanism of action of these drugs might primarily involve the activation of D2R-driven anti-inflammatory activities and not D3Rs.
Furthermore, since DRs are ubiquitously expressed throughout the brain, it is important to target specific areas or cell types to minimize the damage to other neuronal functions. For example, blockade of D3R can reduce sensitivity to anxiety medication, a common co-morbidity for DAergic pathologies, including PD, but also reduce ethanol related reward-associated voluntary consumption (Leggio et al., 2014, 2015, 2019). Therefore, it is important to determine how DRs are regulated not only in different cell types but throughout different brain regions.
Adding another layer of complexity are the conflicting reports on the role of DA and each DA receptor subtype in immunity. This is partly due to the lack of highly selective drugs that are able to target specific receptor subtypes. However, Yang and colleagues have discussed the inconsistencies in studies on the D3R alone with evidence revealing that activation of the D3R can both promote and attenuate neuroinflammation (Yang et al., 2020). As we have highlighted in this review, the cross-talk between the DAergic and immune system is complex, as several factors contribute to regulate cells’ responsiveness to DA, including the abundance of DA in the extracellular space, DR expression, target cell type and the activation state of the target cell. It is important to control for these factors when investigating the role of DA as the neurotransmitter, its related receptors and/or metabolism components could be used as clinical biomarkers and/or therapeutic targets. For example, there is an association between CD4+T cell DR expression and the degree of motor dysfunction, as assessed by Unified Parkinson’s Disease Rating Scale score. In fact, within the total CD4+T cell population D1R expression decreased while in T memory cells D2R expression increased with increasing disease score (Kustrimovic et al., 2016). This suggests the DR expression levels in T cells could be used as a useful prognostic biomarker for PD patients. The ability to monitor PD through a routine blood test would present an enormous advantage as currently clinicians are restricted to qualitative clinical scoring, neuroimaging and a comprehensive medical history.
Dopamine, Parkinson’s disease and oxidative stress
It is well known that oxidative stress contributes to the pathogenesis of neurodegenerative disease, particularly PD. DAergic neurons are more susceptible to oxidative stress due to the fact that they are long-projecting neurons with poorly myelinated axons that provide extensive synaptic innervations. In addition, DAergic neurons have autonomous pacemaker activity, resulting in high energy requirements, and DA and its metabolites containing 2-hydroxyl residues generate highly reactive DA and DOPA quinones (Мiyazaki and Asanuma, 2008). Consequently, DA neurons require efficient mechanisms to maintain neuron functioning and prevent the accumulation of ROS, damaged/misfolded proteins and regenerate essential survival components (Krashia et al., 2019). Additionally, DA quinones have been linked to mitochondrial dysfunction, inflammation and oxidative stress. This inherent highly oxidative environment means that, when there is excess DA or ineffective DA metabolism, ROS can accumulate creating oxidative stress in neurons. This in turn activates local immune cells, microglia and astrocytes, to secrete neurotrophic factors and cytokines to help reduce oxidative damage. However, in a disease like PD, we know that this sequence of events creates a vicious cycle whereby DA neurons produce more ROS, and glial cells keep secreting cytokines, leading to chronic neuroinflammation and the establishment of a self-propagating cycle that is ultimately detrimental to DAergic neurons. This cycle is also seen in other neurodegenerative diseases like Alzheimer’s disease (AD).
Dopamine and Alzheimer’s Disease
AD is the most common neurodegenerative disorder that is characterized by the presence of extracellular amyloid plaques, intracellular neurofibrillary tangles and hyper phosphorylated tau, neuronal loss, oxidative stress and neuroinflammation, primarily in the cortex and hippocampus (Serrano-Pozo et al., 2011).
The DA system has for long been associated with aging, with a decline in motor function associated with DA changes in the substantia nigra and striatum being a robust hallmark of aging (Rollo, 2009). One study revealed a marked increase in microglial reactivity in the SNpc in elderly humans (~81 years), which was reflected by high degree of neuronal death in these areas (Rollo, 2009). This is due to the higher susceptibility of DA neurons to oxidative stress, which inherently tends to increase with age.
As such, it is not surprising that there is some degree of overlap between the pathophysiology of AD and PD, as both diseases have been shown to correlate with increased levels of oxidative stress, abnormal DA regulation and evidence of neuroinflammation. This higher susceptibility to both oxidative stress and neuroinflammation may also be a contributing factor in the pathogenesis of AD, with abnormal DA signaling and expression seen in the mesolimbic pathway of AD patients, however the role of DA in AD pathogenesis remains to be elucidated (Nobili et al., 2017). The mesolimbic DAergic pathway consists of neurons arising from the ventral tegmental area which project predominantly to the prefrontal cortex, hippocampus and nucleus accumbens (Adinoff, 2004). The degeneration of DA neurons in this pathway correlates with impairments in memory and reward performance (Nobili et al., 2017). Consistently, pharmacological treatment to increase DAergic transmission improved cognitive impairment and memory deficits in AD patients and experimental models, emphasizing the critical role of DA in AD (Krashia et al., 2019).
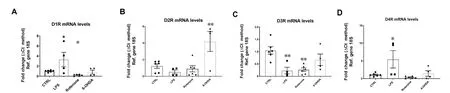
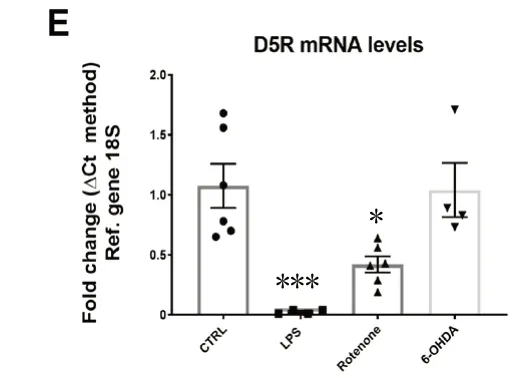
Figure 1 Dysregulated DRs mRNA levels in BV-2 microglial cells exposed to PD mimetics (LPS, protenone and 6-OHDA) after 24 hours.
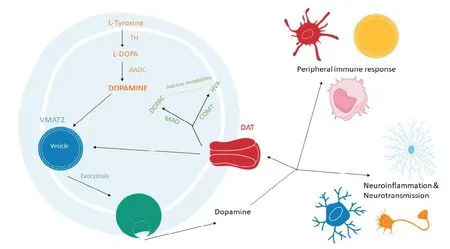
Figure 2 Dopamine biosynthesis, storage and metabolism pathways.
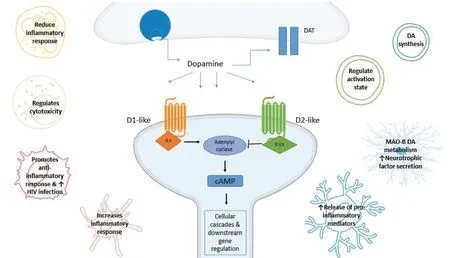
Figure 3 Dopamine receptor (DR) signaling pathways.
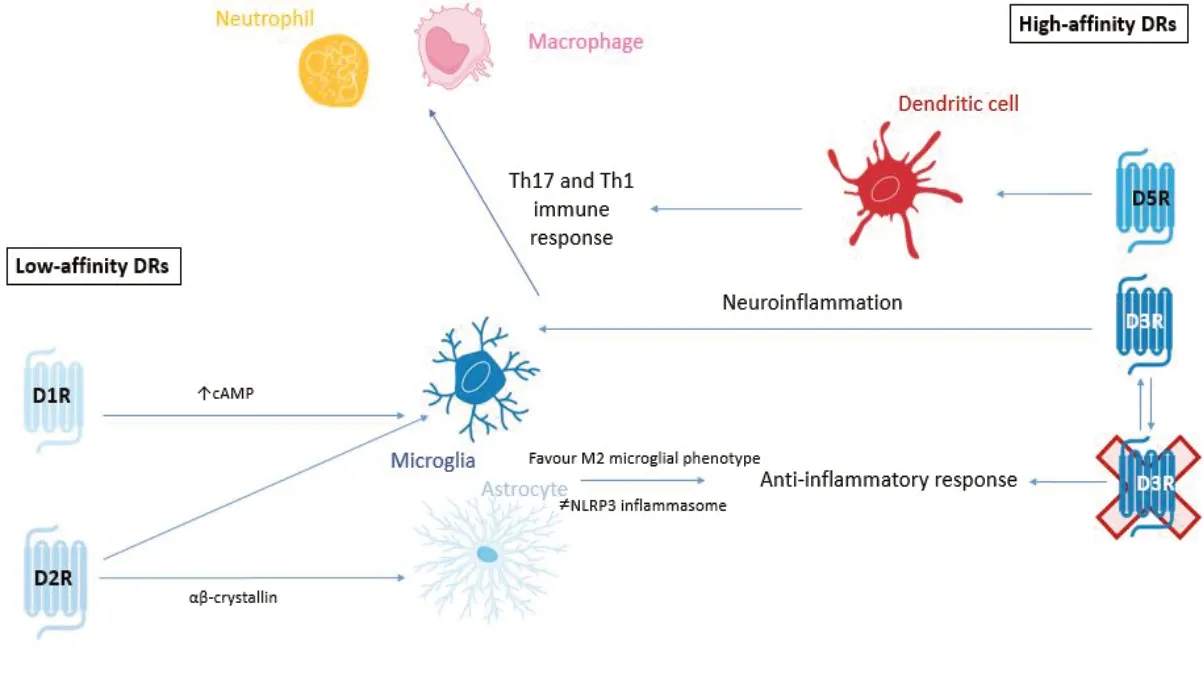
Figure 4 Summary of low-affinity and high-affinity actions of DRs.
Figure 5 Microglia phenotypes.
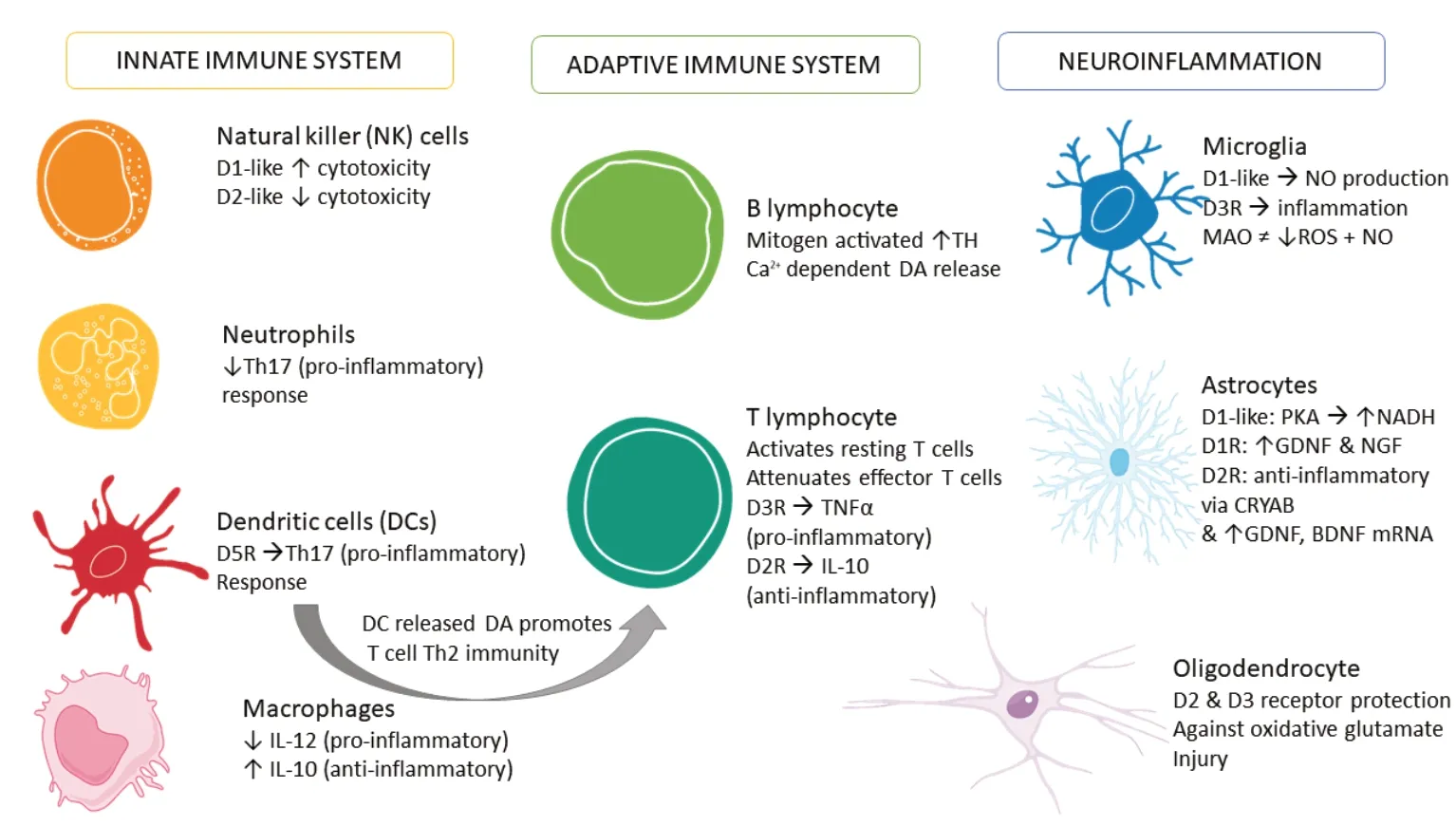
Figure 6 Summary of immune cells responses to dopamine.
For example, it has been shown that D2R in the hippocampus correlates with memory functioning in AD (Donthamsetti et al., 2018). Koch et al. (2014) also demonstrated that D2R/D3R agonists may restore cortical plasticity in AD patients, which is consistent with findings in post-mortem brains of AD patients showing that there is a significant decrease in D2-like receptors in the hippocampus and prefrontal cortex.
Мoreover, additionalex vivostudies in AD patients have demonstrated that lymphocytes had a low density of D2-like receptors compared to controls (Arreola et al., 2016). These results suggest that as in PD, DA modulates the immune response in AD as well. This could contribute to the neuroinflammation and abnormal DA neurotransmission observed in AD patients.
Dopamine and Drug Abuse
DA plays a critical role in movement, memory, cognition and emotion (Yang et al., 2020). Due to its role in controlling operant reinforcement, incentive salience and its ability to trigger positively-valenced emotions via the reward system, DA has been implicated in drug abuse and addiction.
As we have seen in PD and AD, DA availability influences how immune cells respond to DA, which is evident not only in neurodegenerative diseases associated with reductions in DA, but also in situations where there is an excess of DA, as seen in drug abuse. One of the most commonly abused drugs is methamphetamine (МETH), which remains a significant public health concern worldwide (Krasnova et al., 2016). МETH is a known powerful modulator of DA and has several effects that influence DA release, including disrupting DA reuptake and packaging through DAT and VМAT2 (Hedges et al., 2018). The mechanism of МETH ultimately results in excess extracellular release of DA.
Interestingly, МETH has been shown to promote neuroinflammation through DRs. Wang et al. (2019b) have shown that МETH exposure increased LPS induced pro-inflammatory cytokine production in the hippocampus, promoted microglia activation and increased the expression of D1-like receptors. These effects were inhibited after treatment with SCH-23390 (a D1-like receptor antagonist) and minocycline (microglial inhibitor) (Wang et al., 2019b), suggesting that this response was specific to DRs expressed by microglia. Furthermore, this study illustrated that D1-like receptor mediated increase in inflammation triggered by МETH, involved ERK1/2 phosphorylation and activation of the CREB signalling cascade (Wang et al., 2019b). Another study that utilised a rat model of МETH self-administration, revealed changes in the brain consistent with clinical findings in МETH addicts (Krasnova et al., 2016). Analysis of striatal brain tissue showed increased expression of genes involved in CREB signalling and the activation of neuroinflammatory responses (Krasnova et al., 2016). Kitamura et al. (2010) and colleagues provided further support for the suggestion that МETH neurotoxicity is mediated by microglia by showing that the activation of microglia accompanies МETH neurotoxicity and that inhibition of microglial activation reduced dopamine depletion and restored DAT levels.
The consistency of the conclusions of these studies suggests a role of DA in the pathology of drug abuse and addictive behaviour. In addition, a number of researchers have suggested that neuroinflammation in the striatum underlies the cognitive deficits, depression and parkinsonian symptoms reported in МETH addicts. This hypothesis could form the basis of a unifying theory of DA as a major contributor to several CNS pathologies.
Dopamine and Human Immunodeficiency Virus-1-Associated Neurological Disorders
HIV remains a major global health concern with an estimated 35 million people living with HIV (Zhu et al., 2018). Despite the widespread use of efficacious antiretroviral therapies to control peripheral infection more that 50% of HIV-positive individuals will suffer from neurological complications, otherwise known as HAND (МcArthur and Smith, 2013). In HIV infected individuals, substance abuse may accelerate the development and/or increase the severity of HAND as studies have shown that altered DA signaling is a risk factor for HAND with most patients presenting with cognitive, memory, motor and behavioral deficits (Zhu et al., 2018).
Typically, HIV is unable to cross the blood-brain barrier alone. However, it has been proposed that CD14+CD16+monocytes are able to mediate the entry of HIV into the brain and that the migration of both infected and uninfected monocytes across the blood-brain barrier contributes to the establishment and propagation of CNS HIV viral reservoirs and chronic neuroinflammation, which are essential in the development of HAND. Since drug use elevates DA, and the fact that HIV-positive people tend to fall into drug use more frequently than the healthy population, Calderon and colleagues examined the effects of DA on monocytes and HIV infection. They found that the CNS migration of the CD14+CD16+monocyte subpopulation is increased by DA and D1-like receptor agonist SKF-38393, suggesting that the heightened migratory capacity of monocytes is mediated by D1-like receptors (Calderon et al., 2017).
Additionally, Nickoloff-Bybel et al. (2019) showed that DA increases HIV entry into macrophages via a calcium-dependent mechanism, revealing that D1-like receptor activation on infected macrophages stimulated calcium and protein kinase C activation. Since we know that other immune cells are also activated by DA, we can assume that high concentrations of DA can prime the CNS for further HIV viral infection, which not only increases viral reservoirs within the brain, but also contributes to HAND pathologies.
Conclusion
Despite strong evidence showing the expression of DA, its binding receptors and the components necessary to synthesize, store and metabolize DA in non-neuronal cells, the exact significance of these findings in the context of PD pathogenesis remains to be fully determined. Several studies have highlighted the importance of DA in regulating autoimmune diseases, such as МS and SLE, and many others have examined how DA contributes to the control of T lymphocyte functionality in disease states. Furthermore, studies in AD, drug abuse, HIV infection and HAND emphasize the myriad of roles DA has within the brain. The evidence presented in this review calls attention to the robust and extensive ways in which DA can control immunity and the important need for a greater understanding of this novel function of DA (Figure 6).
It is well-accepted that microglia and, to a greater extent, astrocytes, are responsible for regulating the blood brain barrier function to either promote or reduce the permeability to peripheral leukocytes. Further studies are necessary to unveil DA’s regulatory activity on non-neuronal cells, especially microglia and astrocytes, as these cells are commonly altered in several CNS disease pathologies, including PD.
Furthermore, the emergence of DA as an immune transmitter may have future implications for our understanding of brain physiology, but also in the clinical management of neurological diseases, as it presents a wide array of new therapeutic and prognostic targets for CNS pathologies (Figure 6).
Author contributions:STB drafted the manuscript and prepared the images, KL conducted the in vitro work and performed real time qPCR experiments, KAK revised the manuscript and provided significant intellectual input, GML and GM contributed to the drafting of the manuscript and literature research. AC contributed to data collection, literature research and provided technical assistance. AC also conceived the study design, planning and editing, coordinated the execution of all the experimental procedures, the analysis and discussion of results. All authors contributed to data interpretation and manuscript preparation. All authors approved the final submitted version.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:This work was partially supported by a Research Development Fund (UTS Start-Up Grant 2018) from the University of Technology Sydney to AC.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Table 1:Non-neuronal expression of the machinery required for dopamine synthesis, storage, uptake and metabolism.
Additional Table 2:Dopamine receptor expression in non-neuronal cells.
- 中国神经再生研究(英文版)的其它文章
- The role of sequestosome 1/p62 protein in amyotrophic lateral sclerosis and frontotemporal dementia pathogenesis
- Mounting evidence of FKBP12 implication in neurodegeneration
- Using antifibrinolytics to tackle neuroinflammation
- Medicinal plants and natural products as neuroprotective agents in age-related macular degeneration
- Nafamostat mesylate attenuates the pathophysiologic sequelae of neurovascular ischemia
- Engineering mesenchymal stromal/stem cell-derived extracellular vesicles with improved targeting and therapeutic efficiency for the treatment of central nervous system disorders

