野百合碱诱导肺动脉高压模型的机制研究及潜在临床应用价值进展
程筱涵 齐靖 白玉华 郑晓东
[摘要] 肺动脉高压(PAH)是以肺动脉压力和肺血管阻力升高为特征的致死性心血管疾病。野百合碱(MCT)引起的PAH病理特征和临床患者十分相似。MCT可以通過炎症反应、内皮途径以及调节肺动脉平滑肌细胞的增殖等因素诱导PAH的发生。因此本文对近几年MCT诱导PAH动物模型的作用机制以及在PAH新治疗药物靶点筛选中的应用进行简要综述。
[关键词] 肺动脉高压;野百合碱;信号通路;药物靶点
[中图分类号] R322.1 [文献标识码] A [文章编号] 1673-7210(2020)09(c)-0040-04
Advanced mechanisms and potential clinical application value of monocrotaline-induced pulmonary artery hypertension animal model
CHENG Xiaohan1 QI Jing2 BAI Yuhua1 ZHENG Xiaodong2
1.College of Pharmacy, Harbin Medical University(Daqing), Heilongjiang Province, Daqing 163319, China; 2.College of Basic Medical Sciences, Harbin Medical University (Daqing), Heilongjiang Province, Daqing 163319, China
[Abstract] Pulmonary artery hypertension (PAH) is a fatal cardiovascular disease characterized by increases in pulmonary artery pressure and pulmonary vascular resistance. The pathological features of PAH induced by monocrotaline (MCT) are similar to those of clinical patients. MCT-induced PAH through multiple factors including inflammatory response, endothelium pathway, and regulation of pulmonary artery smooth muscle cells proliferation, et al. Therefore, this article briefly reviews the advances molecular mechanism of the MCT-induced PAH and the exploration in the novel therapeutic targets by using this animal model in recent years.
[Key words] Pulmonary arterial hypertension; Monocrotaline; Signal pathway; Drug target
肺动脉高压(pulmonary artery hypertension,PAH)是一类由多种病因和不同发病机制引起的以肺血管阻力增加为特点,最终导致右心衰竭的心血管疾病[1]。PAH基本病理生理特征包括急性肺血管收缩和慢性肺血管重构[2]。PAH患者预后较差,死亡率高,根据Koudstaal等[3]研究显示患者5年的平均生存率为57%~59%。PAH常用的治疗药物包括前列环素途径药物(如前列醇)、NO途径(如西地那非)以及内皮素受体拮抗剂(如波生坦)[4],这些药物通过改善患者血管收缩减轻患者症状,但并不能明显降低患者死亡率,主要原因是已经发生的肺血管重构不能被逆转,因此新的有效阻断、逆转肺血管重构的药物靶点是该领域迫切需要解决的问题。
野百合碱(monocrotaline,MCT)诱导大鼠产生PAH模型(MCT-PAH)是研究PAH的常用动物模型[5]。其制备方法是皮下注射MCT,2~3周后即可发生和临床PAH患者相似的血流动力学和病理学特征[5]。常用于PAH新型诊断和治疗靶点的药物研究。本文从MCT的分子机制和在PAH诊断和治疗靶点筛选中的应用两个方面进行文献综述。
1 MCT引起PAH的分子机制
1.1 核因子κB信号通路
促炎症因子、生长因子、抗原受体通过核因子κB(nuclear factor κB,NF-κB)信号通路诱导靶基因表达,参与免疫反应调节、炎症、应激反应,同时参与细胞分化、增殖、凋亡、发育等过程。促炎性细胞因子白介素6(interleukin-6,IL-6)是引起PAH的关键因素之一[6],Pang等[7]实验证明NF-κB可以激活IL-6等炎症因子并促进MCT诱导的PAH大鼠模型中关键信号通路。
Gao等[8]研究显示在慢性间接性低压低氧的条件下可以通过抑制NF-κB/p38信号通路来减弱由MCT引起的PAH。Chen等[9-10]发现热休克蛋白70(HSP70)的表达量在MCT-PAH动物模型中增加,HSP70会增加Iκbα的磷酸化水平,进而激活NF-κB信号通路。此外,Shi等[11]发现黄岑素可以通过抑制NF-κB信号通路,然后减弱MCT-PAH内皮间质化。
1.2 骨形态蛋白受体Ⅱ
骨形态蛋白受体Ⅱ(bone morphogenetic protein receptor type-2,BMPR2)與其配体骨形态发生蛋白(bone morphogenetic protein,BMP)相互结合参与细胞增殖、凋亡和内皮间充质化等与PAH发生密切相关过程[12]。BMPR2的基因突变存在于约75%的家族性PAH患者中,提示BMPR2是与家族性PAH的发病机制有关[13]。Zhang等[14]在MCT诱导的PAH大鼠模型中发现,BMPR2蛋白的表达量亦是降低的。Chen等[15]研究显示恢复BMPR2蛋白的表达,降低Smad1/5的磷酸化,可以减轻MCT引起的PAH病理生理学改变。Cheng等[16]研究显示在体注射腺病毒let-7a感染的间充质干细胞,可以激活STAT3/BMPR2信号通路促进PASMCs的凋亡,进而逆转MCT-PAH。Abdul-Salam等[17]研究发现靶向敲除肺血管内皮细胞中氯化物细胞内通道4,可以上调BMPR2表达上调,减轻PAH内皮损伤。
1.3 PI3K/Akt
蛋白激酶B即Akt,又被称作PKB,在调控细胞增殖和凋亡等过程中起重要作用。PI3K是一种胞内磷脂酰肌醇激酶,是Akt常见的上游蛋白。Yu等[18]研究提示,PI3K/Akt/mTOR信号通路参与调控由MCT诱导的PAH。Chang等[19]和Hsu等[20]研究证实,抑制Akt/ERK1/2/GSK3β/β-catenin可以减弱内皮素-1(ET-1)和其受体ETA受体的表达,进而治疗MCT-PAH。Zhu等[21]发现在MCT-PAH模型中,磷酸酯酶与张力蛋白同源物(phosphatase and tensin homolog,PTEN)因泛素化引起表达水平降低,继而Akt的磷酸化水平增加。抑制PTEN的泛素化,可以降低Akt蛋白的磷酸化水平,减轻肺血管重构现象。Wang等[22]研究显示MCT-PAH大鼠Akt的磷酸化增加,上调脂质运载蛋白2(lipocalin 2,LCN2)的表达。抑制Akt的磷酸化,降低LCN2的表达。
1.4 一氧化氮
一氧化氮(nitric oxide,NO)是一种内皮依赖性血管舒张因子,通过激活平滑肌细胞中可溶性鸟苷酸环化酶增加环磷酸鸟苷(cyclic guanosine monophosphate,cGMP),活化PKG引起平滑肌舒张。血管内皮NO主要由内皮型一氧化氮合酶(endothelial nitric oxide synthase,eNOS)产生。
Lee等[23]研究显示在MCT-PAH模型动物中eNOS表达量降低,利拉鲁肽可以激活eNOS,增加NO的释放,减缓PAH进程。Li等[24]发现中药单体淫羊藿苷通过增加eNOS表达和抑制5型磷酸二酯酶(phosphodiesterase 5,PDE5),增加NO和cGMP的表达含量,预防MCT-PAH。Yu等[25]研究显示,Cav-1F92A基因可以上调eNOS并增加NO的产生,并且通过下调碳酸酐酶1/激肽原信号,上调硒蛋白/14-3-3η蛋白信号逆转MCT-PAH。
1.5 Notch信号通路
Notch信号通路对于调节PASMC增殖和分化至关重要,Guo等[26]研究发现在HIV诱导的PAH中,HIV的反式激活因子TAT可以通过Notch3/血管内皮生长因子(VEGF-A)信号转导进而调节PASMCs的增殖。Chen等[27]研究显示MCT可以激活Notch3通路,且通过其下游蛋白HES5诱导的PAH。
2 MCT-PAH动物模型在药物中的研究
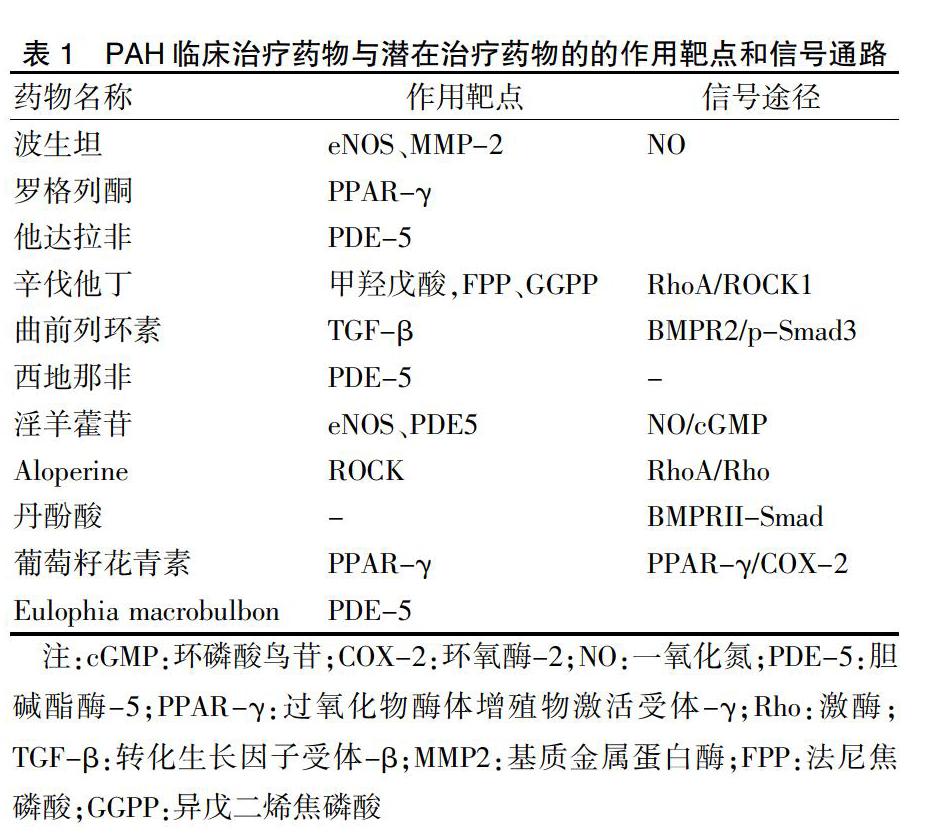
MCT-PAH动物模型被广泛应用于PAH防治药物的研究和开发中。为此,我们将目前市场上用于治疗PAH的药物,以及利用MCT-PAH模型进行临床前研究的药物,和其相应的靶点和信号途径作一综述。表一中列举出已上市的药物和正处于基础研究中药物在该动物模型中的研究情况。其中信号传递途径和作用靶点有很多相似之处,进一步证明了MCT-PAH动物模型在抗PAH药物靶点研究中的潜在应用价值[28-34]。
3结语
MCT-PAH模型只是众多PAH动物模型中的一种,很多研究还利用缺氧以及Sugen缺氧模型来研究PAH[35]。其中缺氧模型也是一种常用的PAH模型,但是在缺氧形成PAH后,经过长时间复氧后,PAH症状易消失,与临床患者表现不符,且模型制备需要设备创造缺氧条件。Sugen缺氧模型是目前认为最接近人类PAH末期的一种模型,形成PAH模型后再复氧2周后,经检测后发现PAH的相关指标并未发生恢复现象[13]。但是缺点是费用相对昂贵,且造模需要时间较长。
虽然我们发现了众多信号途径可以参与MCT诱导的PAH的形成,目前仍未能完全清楚各个通路的具体作用机制以及调控的关键靶蛋白。而且不同信号通路并非单独作用,而是存在交互关系。比如Zheng等[36]研究提示Akt可以作为eNOS的上游对eNOS进行调节,从而影响MCT-PAH的形成。
LncRNA、miRNA等非编码RNA也是治疗PAH比较新的研究热点,目前研究发现一些lncRNA在MCT诱导的PAH中表达发生失调现象[37-38]。Zhu等[39]研究发现miRNA也可以通过PTEN/PI3K/Akt信号通路抑制MCT引起的PAH中内皮细胞的凋亡。随着精准治疗的不断发展,以及测序技术的不断提升,非编码RNA可作为防治PAH的新策略,但因为非编码RNA在不同种属间的保守性问题,在初始实验设计阶段应充分考虑,为后续的转化研究打好基础。总之,与缺氧模型和Sugen模型比较,MCT有着制备简便,价格低廉,发病过程与人PAH接近等特点,在新的PAH的诊断和防治药物的靶点研究中具有较高价值。
[参考文献]
[1] Simonneau G,Montani D,Celermajer DS,et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension [J]. Eur Respir J,2019,53(1):1801913.
[2] Bordenave J,Tu L,Savale L,et al. New insights in the pathogenesis of pulmonary arterial hypertension [J]. Rev Mal Respir,2019,36(4):433-437.
[3] Koudstaal T,Boomars KA,Kool M. Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension: An Immunological Perspective [J]. J Clin Med,2020,9(2):561-582.
[4] 马美玲,劉文东,王芳.肺动脉高压药物的临床应用及市场现状[J].中国新药杂志,2017,26(19):2245-2250.
[5] Bueno-Beti C,Sassi Y,Hajjar RJ,et al. Pulmonary Artery Hypertension Model in Rats by Monocrotaline Administration [J]. Methods Mol Biol,2018,1816:233-241.
[6] Durham GA,Palmer TM. Is there a role for prostanoid-mediated inhibition of IL-6 trans-signalling in the management of pulmonary arterial hypertension?[J]. Biochem Soc Trans,2019,47(4):1143-1156.
[7] Pang Y,Liang MT,Gong Y,et al. HGF Reduces Disease Severity and Inflammation by Attenuating the NF-kappaB Signaling in a Rat Model of Pulmonary Artery Hypertension [J]. Inflammation,2018,41(3):924-931.
[8] Gao L,Liu J,Hao Y,et al. Chronic intermittent hypobaric hypoxia attenuates monocrotaline-induced pulmonary arterial hypertension via modulating inflammation and suppressing NF-κB/p38 pathway [J]. Iran J Basic Med Sci,2018,21(3):244-252.
[9] Chen F,Wang H,Zhao J,et al. Grape seed proanthocyanidin inhibits monocrotaline-induced pulmonary arterial hypertension via attenuating inflammation:in vivo and in vitro studies [J]. J Nutr Biochem,2019,67:72-77.
[10] Chen F,Wang H,Yan J,et al. Grape seed proanthocyanidin reverses pulmonary vascular remodeling in monocrotaline-induced pulmonary arterial hypertension by down-regulating HSP70 [J]. Biomed Pharmacother,2018, 101:123-128.
[11] Shi R,Zhu D,Wei Z,et al. Baicalein attenuates monocrotaline-induced pulmonary arterial hypertension by inhibiting endothelial-to-mesenchymal transition [J]. Life Sci,2018,207:442-450.
[12] Avecilla V. Effect of Transcriptional Regulator ID3 on Pulmonary Arterial Hypertension and Hereditary Hemorrhagic Telangiectasia [J]. Int J Vasc Med,2019,2019:2123906.
[13] Dannewitz Prosseda S,Tian X,Kuramoto K,et al. FHIT,a Novel Modifier Gene in Pulmonary Arterial Hypertension [J]. Am J Respir Crit Care Med,2019,199(1):83-98.
[14] Zhang C,Wang P,Mohammed A,et al. Function of Adipose-Derived Mesenchymal Stem Cells in Monocrotaline-Induced Pulmonary Arterial Hypertension through miR-191 via Regulation of BMPR2 [J]. Biomed Res Int,2019,2019:2858750.
[15] Chen YC,Yuan TY,Zhang HF,et al. Salvianolic acid A attenuates vascular remodeling in a pulmonary arterial hypertension rat model [J]. Acta Pharmacol Sin,2016,37(6):772-782.
[16] Cheng G,Wang X,Li Y,et al. Let-7a-transfected mesenchymal stem cells ameliorate monocrotaline-induced pulmonary hypertension by suppressing pulmonary artery smooth muscle cell growth through STAT3-BMPR2 signaling [J]. Stem Cell Res Ther,2017,8(1):34-44.
[17] Abdul-Salam VB,Russomanno G,Chien-Nien C,et al. PDE5/Arf6 Pathway [J]. Circ Res,2019,124(1):52-65.
[18] Yu X,Zhao X,Zhang J,et al. Dacomitinib,a new pan-EGFR inhibitor, is effective in attenuating pulmonary vascular remodeling and pulmonary hypertension [J]. Eur J Pharmacol,2019,850:97-108.
[19] Chang H,Chang CY,Lee HJ,et al. Magnolol ameliorates pneumonectomy and monocrotaline-induced pulmonary arterial hypertension in rats through inhibition of angiotensinⅡand endothelin-1 expression [J]. Phytomedicine,2018,51:205-213.
[20] Hsu WL,Lin YC,Jeng JR,et al. Baicalein Ameliorates Pulmonary Arterial Hypertension Caused by Monocrotaline through Downregulation of ET-1 and ETAR in Pneumonectomized Rats [J]. Am J Chin Med,2018,46(4):769-783.
[21] Zhu Y,Wu Y,Shi W,et al. Inhibition of ubiquitin proteasome function prevents monocrotaline-induced pulmonary arterial remodeling [J]. Life Sci,2017,173(Complete):36-42.
[22] Wang G,Ma N,Meng L,et al. Activation of the phosphatidylinositol 3-kinase/Akt pathway is involved in lipocalin-2-promoted human pulmonary artery smooth muscle cell proliferation [J]. Mol Cell Biochem,2015, 410(1/2):207-213.
[23] Lee MY,Tsai KB,Hsu JH,et al. Liraglutide prevents and reverses monocrotaline-induced pulmonary arterial hypertension by suppressing ET-1 and enhancing eNOS/sGC/PKG pathways [J]. Sci Rep,2016,6:31788.
[24] Li LS,Luo YM,Liu J,et al. Icariin Inhibits Pulmonary Hypertension Induced by Monocrotaline through Enhancement of NO/cGMP Signaling Pathway in Rats [J]. Evid Based Complement Alternat Med,2016,2016:7915415.
[25] Yu WC,Chen HY,Yang HL,et al. rBMSC/Cav-1(F92A) Mediates Oxidative Stress in PAH Rat by Regulating SelW/14-3-3eta and CA1/Kininogen Signal Transduction [J]. Stem Cells Int,2019,2019:6768571.
[26] Guo ML,Kook YH,Shannon CE,et al. Notch3/VEGF-A axis is involved in TAT-mediated proliferation of pulmonary artery smooth muscle cells: Implications for HIV-associated PAH [J]. Cell Death Discov,2019,5:22-34.
[27] Chen X,Zhou W,Hu Q,et al. Exploration of the Notch3-HES5 signal pathway in monocrotaline-induced pulmonary hypertension using rat model [J]. Congenit Heart Dis,2019,14(3):396-402.
[28] Rashid J,Alobaida A,Al-Hilal TA,et al. Repurposing rosiglitazone, a PPAR-gamma agonist and oral antidiabetic, as an inhaled formulation, for the treatment of PAH [J]. J Control Release,2018,280:113-123.
[29] Liu WH,Xu XH,Luo Q,et al. Inhibition of the RhoA/Rho-associated,coiled-coil-containing protein kinase-1 pathway is involved in the therapeutic effects of simvastatin on pulmonary arterial hypertension [J]. Clin Exp Hypertens,2018,40(3):224-230.
[30] Nikitopoulou I,Manitsopoulos N,Kotanidou A,et al. Orotracheal treprostinil administration attenuates bleomycin-induced lung injury, vascular remodeling,and fibrosis in mice [J]. Pulm Circ,2019,9(4):2045894019881954.
[31] Lee H,Kim KC,Cho MS,et al. Modafinil improves monocrotaline-induced pulmonary hypertension rat model [J]. Pediatr Res,2016,80(1):119-127.
[32] Wu F,Yao W,Yang J,et al. Protective effects of aloperin on monocroline-induced pulmonary hypertension via regulation of Rho A/Rho kinsase pathway in rats [J]. Biomed Pharmacother,2017,95:1161-1168.
[33] Liu J,Hu S,Zhu B,et al. Grape seed procyanidin suppresses inflammation in cigarette smoke-exposed pulmonary arterial hypertension rats by the PPAR-gamma/COX-2 pathway [J]. Nutr Metab Cardiovasc Dis,2020, 30(2):347-354.
[34] Wisutthathum S,Demougeot C,Totoson P,et al. Eulophia macrobulbon extract relaxes rat isolated pulmonary artery and protects against monocrotaline-induced pulmonary arterial hypertension [J]. Phytomedicine,2018,50:157-165.
[35] Budas GR,Boehm M,Kojonazarov B,et al. ASK1 Inhibition Halts Disease Progression in Preclinical Models of Pulmonary Arterial Hypertension [J]. Am J Respir Crit Care Med,2018,197(3):373-385.
[36] Zheng Z,Yu S,Zhang W,et al. Genistein attenuates monocrotaline-induced pulmonary arterial hypertension in rats by activating PI3K/Akt/eNOS signaling [J]. Histol Histopathol,2016,32(1):11768.
[37] Su H,Xu X,Yan C,et al. LncRNA H19 promotes the proliferation of pulmonary artery smooth muscle cells through AT1R via sponging let-7b in monocrotaline-induced pulmonary arterial hypertension [J]. Respir Res,2018,19(1):254.
[38] Cao Y,Yang Y,Wang L,et al. Analyses of long non-coding RNA and mRNA profiles in right ventricle myocardium of acute right heart failure in pulmonary arterial hypertension rats [J]. Biomed Pharmacother,2018, 106:1108-1115.
[39] Zhu G,Zhang W,Liu Y,et al. miR371b5p inhibits endothelial cell apoptosis in monocrotaline induced pulmonary arterial hypertension via PTEN/PI3K/Akt signaling pathways [J]. Mol Med Rep,2018,18(6):5489-5501.
(收稿日期:2020-01-15)

