Radiotherapy combined with nimotuzumab for elderly esophageal cancer patients:A phase II clinical trial
Department of Radiation Oncology,National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital,Chinese Academy of Medical Sciences and Peking Union Medical College,Beijing 100121,China
Abstract Objective:To investigate the safety and efficacy of nimotuzumab combined with radiotherapy for elderly patients with non-resectable esophageal carcinoma (EC).Methods:Eligible patients were aged 70 years or older and had treatment-naïve,histologically proven inoperable locally advanced EC.Enrolled patients received radiotherapy with a total dose of 50-60 Gy in 25-30 fractions,concurrent with weekly infusion of nimotuzumab.The primary end point was the rate of more than grade 3 toxicities.Results:From June 2011 to July 2016,46 patients with stage II-IV EC with a median age of 76.5 years were enrolled.There were 10,28 and 8 patients with stage II,III and IV disease,respectively.The common acute toxicities included esophagitis (grade 1-2,75.4%;grade 3,8.7%),pneumonitis (grade 1,4.3%;grade 2,6.5%;grade 3,2.2%),leukopenia (grade 1-2,60.9%;grade 3-4,4.4%),gastrointestinal reaction (grade 1-2,17.3%;grade 3,2.2%),thrombocytopenia (grade 1-2,21.7%;grade 3,2.2%),and radiothermitis (grade 1-2,39.2%).The incidence of grade 3-4 adverse effects was 17.4%.No grade 5 toxicities were observed.Clinical complete response,partial response,stable disease,and progressive disease were observed in 1 (2.2%),31 (67.4%),12 (26.1%),and 2 (4.3%)patients,respectively.The median overall survival (OS) and progression-free survival (PFS) were 17 and 10 months,respectively.The 2-,3-,and 5-year OS and PFS rates were 30.4%,21.7%,19.6%,and 26.1%,19.6%,19.6%,respectively.Conclusions:Nimotuzumab combined with radiotherapy is a safe and effective therapy for elderly patients who are not surgical candidates.Further studies are warranted to confirm its therapeutic effects in elderly EC patients.
Keywords:Nimotuzumab;esophageal neoplasm;elderly;radiotherapy;treatment outcome
Introduction
Esophageal carcinoma (EC) is the eighth most common cancer worldwide and the fourth leading cause of cancerrelated deaths in China.Most Chinese male patients with EC are older than 60 years (1,2).At present,concurrent chemoradiotherapy (CCRT) is the standard of care for patients with inoperable EC (3-5).However,many elderly patients cannot tolerate CCRT due to their clinical characteristics.Nonetheless,the treatment outcomes of radiotherapy alone for the management of elderly EC patients are suboptimal.A retrospective study showed that the 1-,3-,and 5-year overall survival (OS) rates of 107 elderly EC patients receiving three-dimensional conformal radiation therapy were 69.7%,10.3% and 1.6%,respectively (6). Therefore,equally tolerable and potentially more effective modalities,including targeted therapy,have shown promise in the management of these clinical scenarios.
The epidermal growth factor receptor (EGFR) is a member of the ERBB transmembrane growth factor receptor family,which can activate the receptor-associated tyrosine kinase,when combined with ligands,such as epidermal growth factor or transforming growth factor(TGF)-α.This process then initiates a series of signal transductions that regulate the growth,proliferation,and differentiation of normal tissue (7).
In esophageal squamous cell carcinoma (ESCC),the overexpression rate ofEGFRgene is approximately 80%(8).Furthermore,EGFRoverexpression is an independent adverse prognostic factor due to its correlation with tumor progression,migration and metastasis (9).
Nimotuzumab is a humanized monoclonal antibody toEGFR.Early preclinical studies have demonstrated that monoclonal antibodies ofEGFRsensitize cells to radiation by promoting radiation-induced apoptosis (10).Recent reports have concluded that nimotuzumab can enhance radiosensitivity and abrogate radio-resistance acquired by EC cell lines (11-13).Previously,we conducted a phase II clinical trial involving 42 patients who received nimotuzumab combined with radiotherapy.The mean survival time (MST) was 14 months and the 2-and 3-year OS rates were 33.3% and 26.2%,respectively.The adverse effects were acceptable with 21.4% of patients suffering from grade 3 toxicities and no grade 4 toxicities (14).Another study also concluded that nimotuzumab combined with radiotherapy was safe,tolerable,and yielded encouraging OS (15).However,it remains unclear whether this promising multimodality therapy is safe and effective for elderly patients.
The aim of this prospective,phase II clinical trial was to assess the efficacy and toxicity of nimotuzumab combined with radiotherapy for elderly patients with non-resectable EC.
Materials and methods
Endpoints
The primary endpoint was the rate of more than grade 3 toxicities. The secondary endpoints included overall response rates (ORR),OS,and progression-free survival(PFS) rates.
Patient selection
The inclusion criteria were as follows:1) patients aged 70 years or older; 2) treatment-naïve patients with histologically proven thoracic-segment EC that was inoperable and could not tolerate CCRT;3) stage II-IV disease (supraclavicular lymph node metastasis only),according to the 6th American Joint Committee on Cancer TNM staging system;4) estimated survival time ≥3 months;5) Karnofsky performance score ≥70;6) adequate bone marrow,as well as hepatic and renal function;and 7)voluntary written consent provided prior to treatment.The definition of inoperable esophageal cancer in our study was as follows:1) T4 tumors with involvement of heart,trachea or adjacent organs including liver,pancreas,lung and spleen;2) stage IVA tumors of the lower esophagus with unresectable celiac nodes,involvement of celiac artery,aorta,or other organs including liver,pancreas,lung and spleen;3) stage IVB tumors with systemic metastasis or non-regional lymph node metastasis;and 4) inoperable for comorbidity. Every subject was discussed in the multidisciplinary team in National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital to determine the resectability.
The exclusion criteria were as follows:1) esophagobronchial or esophagomediastinal fistula;2) patients who had joined other clinical trials prior to this treatment;3)serious heart,liver,and/or kidney insufficiency;4) serious infectious diseases;5) relapse disease or distant metastasis;6) recently diagnosed neoplastic diseases;or 7) previous receipt of surgery,chemotherapy,or radiotherapy for EC.
All patients of this study signed the informed consent.The study protocol was approved by the Institutional Ethical Review Board (National Cancer Center Good Clinical Practice Board) of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital and registered on the ClinicalTrials.gov site(NCT01463605).
Pre-treatment evaluation
All eligible patients underwent pre-treatment work-up,including medical history taking,physical examination,standard laboratory examination,electrocardiogram,esophagoscopy and biopsy,chest and abdominal computed tomography (CT) scans,neck ultrasonography,radionuclide bone scans,and brain magnetic resonance imaging or positron emission tomography (PET)/CT scans.EGFRexpression assessment and endo-esophageal ultrasonography were recommended if possible.The Charlson score system was used to assess patient comorbidities.
Treatment
Radiotherapy
All patients underwent intensity-modulated radiotherapy(IMRT).The gross tumor volume and involved lymph nodes were delineated based on clinical examinations.Elective node irradiation (ENI) was applied.ENI regions depended on the primary tumor location,which generally included the periesophageal,mediastinal,and celiac regions from the thoracic inlet to the superior border of the celiac axis in the case of lower-segment EC,and the supraclavicular,periesophageal,and mediastinal regions ranging from the upper border of the T1 vertebra to the subcarinal region in the case of upper and middle-segment EC.The planning target volume (PTV) was defined as the clinical target volume plus an isotropic 5-mm,3-dimensional margin.The dose prescribed to 95% of PTV was 50-60 Gy,delivered in 2 Gy once daily fractions,with five fractions delivered per week.
Targeted therapy
The patients received weekly infusions of 200 mg of nimotuzumab diluted in 250 mL of normal saline concurrently with radiotherapy for 5-6 weeks (14-16).
Follow-up
The patients were examined weekly during treatment.Follow-up visits were conducted one month after treatment completion,every 3 months in the first 3 years,and 6 months thereafter.Chest and abdominal CT scans,neck ultrasonography,and laboratory examinations were performed for monitoring.Esophagoscope and biopsy were recommended if indicated clinically.
Criteria for evaluation of toxicities and therapeutic effects
The National Cancer Institute’s Common Toxicity Criteria (Version 3.0) were used to assess toxicities (17).The Response Evaluation Criteria in Solid Tumors were used to evaluate treatment response (18). Twodimensionally measurable disease included the longest axis of the largest positive lymph node and the length and width of the primary tumor.
Statistical analysis
Estimates of OS and PFS were derived using the Kaplan-Meier method.The subgroups were compared using the log-rank test.The independence of prognostic factors was assessed using the Cox proportional hazards model.Differences in the distributions of adverse effects between the subgroups were assessed using Pearson’s Chi-squared test or Fisher’s exact test,as appropriate.The data were analyzed using IBM SPSS Statistics (Version 19.0;IBM Corp.,New York,USA).Cox regression model was used to calculate hazards ratio (HR) and 95% confidence interval(95% CI).Analysis items with two-sided P<0.05 were considered statistically significant.We previously reported a rate of 21.4% for grade 3 toxicities among EC patients who received radiotherapy combined with nimotuzumab(14).Andersonet al.reported that more than grade 3 hematologic toxicity rate was 40% among 25 elderly EC patients who received combined modality chemoradiation(19).The rate of more than grade 3 toxicities was predicted to be decreased from 40% to 21% based on the results of the two studies,with one-sided α=0.05,β=0.2 and 80%power.A total of 42 cases were required.Assuming a dropout rate of 10%,46 cases were needed for this phase II trial.
Results
Patients’ characteristics
Between June 2011 and July 2016,46 patients with a median age of 76.5 (range,70.0-90.0) years were enrolled.The clinical characteristics of the patients are presented inTable 1.The patients were divided into either group A or group B,according to their Charlson score,which was used to assess comorbidity burden.Patients with scores 0 and ≥1 were stratified into group A and group B,respectively.Twelve (26.1%) patients had a Charlson score ≥1 and 2 of them had a Charlson score ≥2.Cerebrovascular disease,diabetes,prior myocardial infarction,and second primary tumors were observed in three,three,one,and three patients,respectively.
Treatment compliance
Three patients did not complete radiotherapy.One refused to receive the full radiotherapy dose and subsequently underwent surgery;this patient died 38 months later due to comorbidities.Two other patients who experienced grade 3 pneumonitis and gastrointestinal reactions did notcomplete treatment due to adverse events.One patient received only 3 cycles of targeted therapy due to fever.Three other patients received 4 cycles: 1 refused subsequent treatment and 2 experienced grade 3 pneumonitis and esophagitis,respectively.
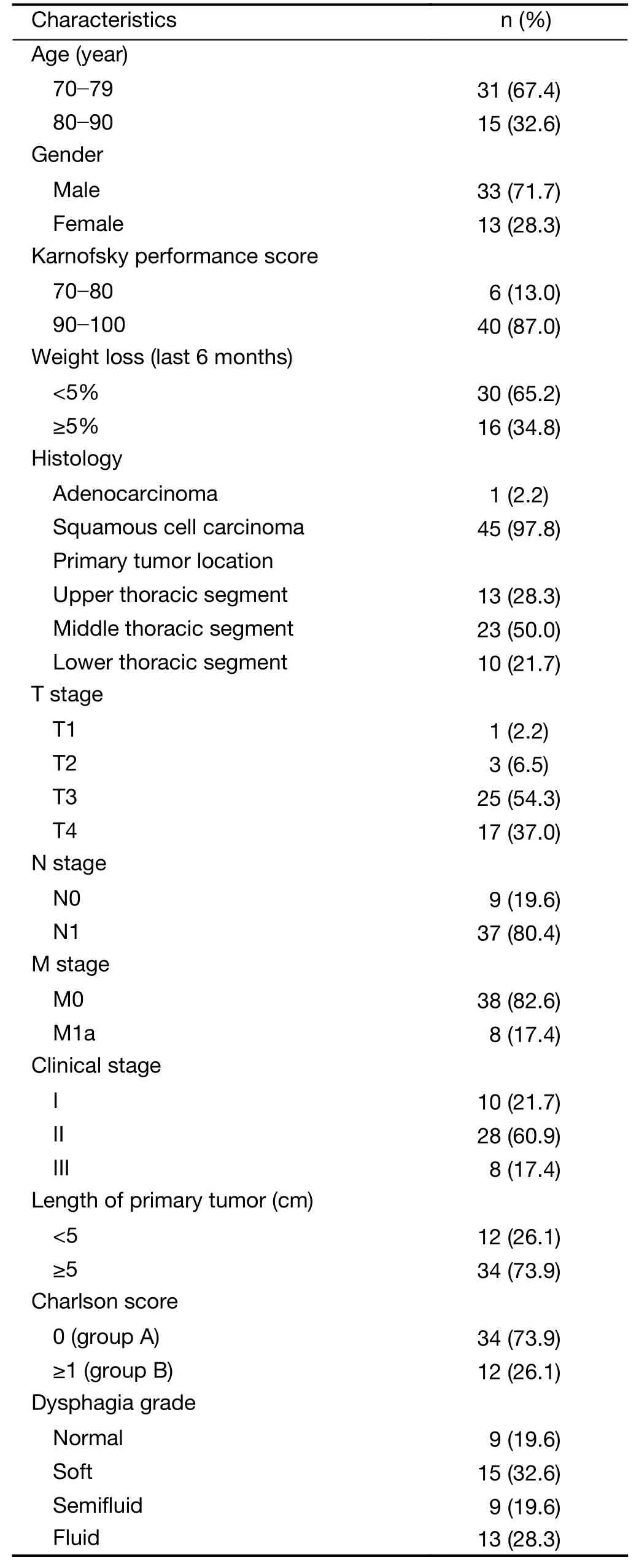
Table 1 Clinical characteristics of study patients (N=46)
Toxicities
The treatment-related acute toxicities included esophagitis,pneumonitis,leukopenia,gastrointestinal reaction,thrombocytopenia,radiothermitis and fever (Table 2).Grade 3-4 adverse effects occurred in 17.4% (8/46) of the patients,including gastrointestinal reaction (n=1),esophagitis (n=4),pneumonitis (n=1),leukopenia (n=2),and thrombocytopenia (n=1) (1 patient experienced concurrent grade 3 gastrointestinal reactions and esophagitis).No grade 5 toxicities were observed.There were no significant differences in the incidences of grade 3-4 adverse effects in group A and B,which were 13.9% (5/36) and 30.0% (3/10),respectively (P=0.344).
Therapeutic response
All 46 patients underwent therapeutic response evaluation one month after the completion of treatment.One patient(2.2%) achieved clinical complete response (cCR),31(67.4%) patients had partial response (PR),12 (26.1%)patients showed stable disease (SD),and only 2 (4.3%)patients suffered progressive disease (PD).The objective response rate (ORR) was 69.6% and the disease control rate (DCR) was 95.7%.The cCR,PR,SD,PD,ORR and DCR rates in group A were 2.8% (1/36),63.9% (23/36),27.8% (10/36),5.6% (2/36),66.7% (24/36),and 94.4%(34/36),respectively.The correspondent rates in group B were 0 (0/10),80.0% (8/10),20.0% (2/10),0 (0/10),80.0%(8/10),and 100% (10/10),respectively.

Table 2 Treatment-related acute toxicities (N=46)
OS and PFS
The last follow-up date was February 20,2020.The median follow-up time was 69.5 (range,51.0-90.0) months.At the time of last follow-up,8 patients were still alive.Of the 37 patients with disease progression,23 (62.2%) had local regional progression,12 (32.4%) developed distant metastasis,and 2 (5.4%) patients showed both local regional and distant failures.Of the 38 patients who died,24 (63.2%) died of local regional progression,13 (34.2%)of distant failure,and 1 (2.6%) of complications.The median OS and PFS time were 17 and 10 months,respectively.The 2-,3-,and 5-year OS rates were 30.4%,21.7% and 19.6%,respectively,while the corresponding PFS rates were 26.1%,19.6% and 19.6% (Figure 1).
Twenty-seven patients’ biopsy specimens obtained via esophagoscope were subjected to an immunochemical assay that testedEGFRexpression status.Expression status was stratified into 4 categories:markedly stronger staining than normal esophageal epithelium as high status,moderately stronger staining than normal esophageal epithelium as medium status,staining identical to that of normal epithelium as low status,and faint staining as negative status.EGFRoverexpression was defined as high and medium EGFR expression (14).In this study,EGFRoverexpression was found in 26 patients (96.3%) (11 with high and 15 with medium statuses) and only 1 patient showed lowEGFRexpression.The median OS of the high and mediumEGFRexpression groups were 23 and 18 months (HR=1.274,95% CI:0.511-3.178,P=0.603),respectively.The 2-,3-,and 5-year OS rates were 45.5%,27.3%,and 18.2%,respectively,in the high expression group,and 26.7%,26.7%,and 26.7%,respectively,in the medium expression group,without significant difference (Figure 2).

Figure 1 OS and PFS curves of the entire study cohort.OS,overall survival;PFS,progression-free survival.
The median OS of group A and B were 18 and 16 months (HR=0.858,95% CI:0.403-1.825,P=0.691),respectively.The 2-,3-,and 5-year OS rates were 29.4%,20.6% and 17.6% in group A and 33.3%,25.0% and 25.0% in group B,respectively,without significant difference (Figure 3).
The univariate analysis showed that weight loss,dysphagia grade,and M stage were prognostic factors of OS and PFS.The multivariate analysis showed that M stage was an independent prognostic factor of OS and PFS.
Discussion

Figure 2 OS curves of the high and medium EGFR expression group.OS,overall survival;EGFR,epidermal growth factor receptor.

Figure 3 OS curves of group A (Charlson score=0) and group B(Charlson score ≥1).OS,overall survival.
In this study,we attempted to determine the treatment mode of elderly EC patients using a combination of nimotuzumab and radiotherapy.Eventually,46 elderly EC patients were enrolled and received this combined therapy.In our study,the incidence of grade ≥3 toxicities was 17.4%;no grade 5 adverse effects were observed.These results are comparable to those obtained in our previous study,in which 21.4% of patients developed grade 3 toxicities and no grade ≥4 adverse effects were observed(14).Another retrospective study reported a higher adverse effect incidence with 50% (33/66) of patients suffering from grade 3-4 adverse events.However,79% (52/66) of patients received concurrent nimotuzumab and chemoradiotherapy which explains the evidently higher grade 3-4 toxicity incidence observed in comparison to that observed in our study (15).Katoet al.reported that most cases of grade ≥3 toxicities were hematotoxicity,particularly lymphocytopenia and radiation esophagitis among 10 Japanese EC patients who received a combination of nimotuzumab and concurrent chemoradiotherapy (20).Huanget al.reported that the rate of grade ≥3 toxicities was 25.5% among 271 elderly EC patients who received concurrent chemoradiotherapy (21).Zhaoet al.reported that the rate of grade ≥3 toxicities was 13% among 183 elderly EC patients who received radiotherapy alone or concurrent chemoradiotherapy (22).Genet al.reported that the rate of grade ≥3 toxicities was 50% among 50 elderly EC patients who received definitive radiotherapy (23).Furthermore,two similar recent studies exploring the efficacy and safety of S-1 based concurrent chemoradiotherapy in elderly EC patients reported high incidence of side effects with 28% of grade 3-4 acute toxicities and 55.4% of grade 3-4 leucocytopenia,respectively (24,25).Zhanget al.reported that the rate of grade 3-4 hematological toxicities was 36.9% among elderly EC patients who received concurrent chemoradiotherapy (26).Overall,the comparatively lower treatment-related toxicities observed in our study reflect the potential value of treatment mode of radiotherapy combined with nimotuzumab,especially for elderly EC patients with shorter life expectancy and greater need for better quality of life.
In our study,the proportion of patients in group B(Charlson score ≥1) was approximately 22%.The incidence rate of grade 3-4 acute adverse effects in group B was nearly 20% higher than that in group A.However,Andersonet al.reported that grade 3-4 toxicities were associated with a Charlson score of ≥2 (19).Another study also reported that patients with a Charlson score ≥1 experienced more grade 2 or higher adverse events (27).Several factors may contribute to this discrepancy.First,all of the previous studies used small sample sizes.Second,the previous two studies utilized chemoradiation,which is a more aggressive multimodality therapy compared to ours.Third,differences in patients’ characteristics,including ethnicity,age,clinical disease stage,level of education,etc.,should not be overlooked.Fourth,even patients with equivalent Charlson scores may have different treatment tolerances for multiple comorbidity categories included in the Charlson score system which represents different conditions.Last,the Charlson score system was originally used to estimate the risk of death from disease.Therefore,it may be necessary to establish a specific system to predict adverse effects resulting from the combination of nimotuzumab and radiotherapy for elderly EC patients.
Our treatment response rates are of great similarity to those reported in another retrospective study that aimed to assess the efficacy of nimotuzumab combined with radiotherapy in 16 elderly patients with ESCC,which reported that the cCR,PR,SD,PD,ORR and DCR rates were 6.3%,62.5%,25.0%,6.3%,68.8% and 93.8%,respectively (28).Based on the present data,we think that nimotuzumab combined with radiotherapy can yield optimal DCR in elderly EC patients.
The 2-and 3-year OS rates of our previous study were approximately 30% and 25%,respectively (14).Maet al.also reported that the 2-year OS and PFS rates were 54%and 37%,respectively,which are higher than those identified in ours (15).However,79% of patients in their study received concurrent nimotuzumab and chemoradiation and 19% of patients had N0 stage disease,which may partially explain the differences in terms of OS and PFS.Compared with radiotherapy alone,our 3-year OS rates were overwhelmingly better than that reported in a retrospective study in which 10.3% of 107 elderly EC patients received three-dimensional radiotherapy alone (6).Zhang reported that the 3-year OS rate was 36.1% for the concurrent chemoradiotherapy group and 28.5% for the radiotherapy alone group among elderly patients (26).Luet al.reported that the 3-year OS was 13.2% among elderly EC patients who received radiotherapy (29).Meanwhile,the MST identified in our study (17 months) is even superior to that identified in the RTOG8501 trial (12.5 months) (3).However,this survival benefit may contribute to improvements in radiotherapy technology.In another retrospective study of 109 elderly EC patients who received concurrent chemoradiotherapy,the MST was 15.2 months and the 2-year OS rate was 35.5% (27).Huanget al.reported an MST of 23.6 months and a median PFS of 13.6 months among 271 elderly patients (21).Zhaoet al.reported an MST of 18.6 months and 2-and 3-year OS rates of 43.5% and 35.2%,respectively,among 183 elderly patients (22).Recently,two studies published the efficacy of S-1 based chemoradiotherapy in elderly patients with ESCC.The MST were 22.6 and 18.2 months,respectively(24,25).The aformentioned survival data demonstrated that radiotherapy combined with nimotuzumab achieved acceptable therapeutic effects and more importantly with lower toxicities.
In our study,the highEGFRexpression subgroup showed a longer MST than the medium expression subgroup.Interestingly,our prior study presented similar results (14).Both of the results were inevitably limited by a small sample size and potential bias.Wanget al.found that highEGFRexpression and low p-Akt expression may predict a clinical benefit of nimotuzumab combined with radiotherapy or chemoradiotherapy among ESCC patients(30).Thus,certain subgroups of elderly EC patients may derive greater clinical benefits from nimotuzumab combined with radiotherapy.It is thus important to optimize anti-EGFR therapy by exploring and identifying predictive biomarkers.
Besides having a small sample size,our study had some other limitations.First,the cohort included in this study was selective.All the patients included had generally good performance statuses and had relatively fewer complications.Our results show that most toxicities were mild and no grade 5 toxicities were observed.These demonstrated that the toxicities of this treatment mode was well tolerated.Second,the test of biomarker evaluation was not consistently administered to all patients,which made it impossible to perform a univariate or multivariate analysis.Having this information would have helped with patient selection.Finally,we did not administer questionnaire surveys to evaluate the quality of life and mental health of participants,which is more important for elderly patients due to their shorter life expectancy.
Conclusions
Our results showed that nimotuzumab combined with radiotherapy was well tolerated and yielded acceptable treatment outcomes among elderly patients with nonresectable EC.Further studies are warranted to pursue biomarkers for patient selection.
Acknowledgements
This study was supported by Wu Jieping Medical Foundation,the National Key Projects of Research and Development of China (No.2016YFC0904600).
Footnote
Conflicts of Interest:The authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2021年1期
Chinese Journal of Cancer Research2021年1期
- Chinese Journal of Cancer Research的其它文章
- Colorectal cancer burden and trends:Comparison between China and major burden countries in the world
- Changing trends of disease burden of gastric cancer in China from 1990 to 2019 and its predictions:Findings from Global Burden of Disease Study
- Clinical characteristics and clinicopathological correlations of bilateral breast cancer in China:A multicenter study from Chinese Society of Breast Surgery (CSBrS-006)
- Multi-center investigation of breast reconstruction after mastectomy from Chinese Society of Breast Surgery:A survey based on 31 tertiary hospitals (CSBrS-004)
- Laparoscopic vs. open surgery for gastrointestinal stromal tumors of esophagogastric junction:A multicenter,retrospective cohort analysis with propensity score weighting
- Pattern of No.12a lymph node metastasis in gastric cancer
