Current evidence and challenges of systematic therapies for adult recurrent glioblastoma:Results from clinical trials
Wenlin Chen,Delin Liu,Penghao Liu,Ziren Kong,Yaning Wang,Yu Wang,Wenbin Ma
Department of Neurosurgery,Peking Union Medical College Hospital,Chinese Academy of Medical Sciences and Peking Union Medical College,Beijing 100730,China
Abstract Recurrence is a major concern for adult patients with glioblastomas (GBMs),and the prognosis remains poor.Although several therapies have been assessed,most of them have not achieved satisfactory results.Therefore,there is currently no standard treatment for adult recurrent GBM (rGBM).Here,we review the results of clinical trials for the systematic therapy of rGBM.Regorafenib,rindopepimut and neoadjuvant programmed death 1 (PD-1)inhibitors are promising agents for rGBM,while regorafenib is effective in both O6-methylguanine DNA methyltransferase (MGMT) promoter methylated and unmethylated patients.Temozolomide rechallenge and alkylating agents combined with bevacizumab can be useful for patients with MGMT methylation,and patients with isocitrate dehydrogenase (IDH) mutations or second recurrence can benefit from vocimagene amiretrorepvec (Toca 511).Some phase I trials on targeted therapy and immunotherapy have shown positive results,and results from further studies are expected.In addition to the analysis of existing clinical trial results,forthcoming trials should be well designed,and patients are encouraged to participate in appropriate clinical trials.
Keywords:Recurrent glioblastoma;systematic therapy;clinical trial;targeted therapy;immunotherapy
Introduction
Glioblastoma (GBM) is the most common malignant central nervous system (CNS) primary tumor and shows strong invasiveness (1).GBM is rare but lethal,with an incidence of 3.44/100,000 and a median overall survival(mOS) of 8 months regardless of treatment and a 5-year survival of 7.2% (2).The existence of the blood-brain barrier (BBB),the invasion and aggressive growth,the spatial and temporal heterogeneity,the redundancy of signaling pathways,stem cell resistance and the inactivated immune microenvironment of the tumor are all factors contributing to the poor prognosis of GBM.
Tumor recurrence is the leading cause of death,and GBMs inevitably recur less than 7 months after initial diagnosis (3).Tumors located in the functional area result in a limited extent of resection.Additionally,GBMs have shown a certain degree of treatment resistance.In terms of recurrence patterns,approximately two-thirds of GBMs recur within 2 cm of the primary tumor margin (local recurrence),while one-third recur distantly (distant recurrence) (4,5).
In clinical practice,recurrence and treatment-induced pseudoprogression,in which obvious mass effects are commonly observed,are often confusing.The Response Assessment in Neuro-Oncology (RANO) working group criteria are commonly applied (6).With the development of immunotherapy,immunotherapy-induced pseudoprogression is gradually being recognized,and the Immunotherapy Response Assessment in Neuro-Oncology(iRANO) criteria further improve the evaluation accuracy.It has also been suggested that elevated relative cerebral blood volume indicates a high possibility of recurrence in contrast-enhancing lesions but has not been included in the diagnostic criteria (7).
Progress in clinical trials of systematic therapies for adult recurrent GBMs (rGBMs)
Currently,several systematic therapies are available for recurrent glioma,but the majority have not achieved satisfactory results.The National Comprehensive Cancer Network (NCCN) guidelines and the European Association for Neuro-Oncology (EANO) guidelines have not determined the standard of care for rGBMs.In the EANO guidelines and the 2020 Society of Neuro-Oncology (SNO) and EANO consensus review,the authors stated that “there is no clear standard-of-care salvage therapy” (3).While the NCCN guideline recommendation categories for most regimens are 2A,therapies with more significant adverse events,such as etoposide or platinumbased regimens,have a lower recommendation category of 2B and 3,respectively.
However,possible directions may come from published phase II and III clinical trials exploring possible treatment options and potential benefits in subgroups.The specific conclusions of related clinical trials are classified and described in detail below.The protocol for the research was approved by a suitably constituted Ethics Committee of the institution within which the work was undertaken,and it conforms to the provisions of the Declaration of Helsinki.
Chemotherapy
Temozolomide-based chemotherapy is important in newly diagnosed GBM (nGBM) and is also widely applied in recurrent tumors.In the early stage,chemotherapy in rGBMs mainly involves temozolomide,carmustine(BCNU),lomustine (CCNU) and platinum-based regimens.At present,studies generally focus on the combination of chemotherapy and targeted therapy (which will be reviewed in the targeted therapy section) and new regimens.
Platinum-based regimens have a long history in cancer treatment and have been tested repeatedly in rGBMs.In 2019,Villaniet al.examined the effect of weekly carboplatin in a single-arm phase II trial (8).The median progression-free survival (mPFS) was 2.3 months,the mOS was 5.5 months,and patients who showed clinical benefits tended to have a better prognosis,while the inclusion of different grades of glioma,the small sample size and the nature of a single-arm trial made the evidence level weaker.The effect of carboplatin was not improved with RMP-7,a substance that elevates BBB permeability (9).Adjusting the dosage and medication plan of RMP-7 might improve the efficacy,but this result further suggests that platinum-based regimens are not as effective as expected in rGBMs.
As the first-line therapy in nGBMs,the effectiveness of temozolomide has naturally received particular attention and was also tested in rGBMs.Temozolomide achieved satisfactory efficacy and acceptable safety in two phase II trials in 1999 and 2000 (10,11).However,in a 2007 phase II trial in children with CNS tumors,the objective response rate (ORR) of temozolomide did not meet expectations (12).We believe this deviation may be due to the differences in tumor pathology.In addition,the continuous dose-intense temozolomide scheme was suggested as an active option by the RESCUE trial,with a 6-month PFS of 23.9% (13).The methylated O6-methylguanine DNA methyltransferase (MGMT) promoter was identified as a strong beneficial prognostic biomarker for temozolomide rechallenge in both the RESCUE trial and DIRECTOR trial (14).In addition,the phase II,twoarm DIRECTOR trial showed that patients who received their last temozolomide above 2 months responded better to dose-intensified temozolomide rechallenge.O6-benzylguanine,disulfiram and copper have been confirmed as temozolomide sensitizers in preclinical studies.Unfortunately,these agents did not enhance the therapeutic effect of temozolomide in temozolomideresistant rGBMs (15,16).The best strategy for temozolomide-resistant gliomas remains difficult to determine,while temozolomide rechallenge may be used for patients with methylatedMGMTpromoters.
BCNU and CCNU were also considered.The first study of BCNU in rGBM was published in 1989 (17).In this trial,the toxicity was tolerable.However,chemotherapeutics with similar effects and fewer adverse events have been discovered.Therefore,the obvious adverse events resulted in a decline in BCNU usage,which was assessed by Brandeset al.in a phase II trial (18).Combination therapy may reduce the adverse effects of BCNU and enhance its therapeutic effect.
Several well-known chemotherapy agents,including irinotecan (CPT-11) (19),ortataxel (20) and etoposide(VP16) (21),have been examined.Unfortunately,although VP16 showed a modest therapeutic effect,CPT-11 and ortataxel failed to demonstrate efficacy.
In conclusion,several chemotherapies have failed in studies of rGBM,and the combination of targeted therapy or immunotherapy has become a possible direction for chemotherapy.In addition,patients with a methylatedMGMTpromoter can benefit from temozolomide rechallenge.
Targeted therapy
Targeted therapy has become a hot topic in cancer treatment.Antiangiogenic pharmaceuticals have attracted increased attention for richly vascularized GBMs.Several tumor growth-related pathways are also targeted in rGBM treatment.
At present,widely considered therapeutic targets mainly include vascular endothelial growth factor (VEGF),phosphatidylinositol 3-kinase/mammalian target of rapamycin (PI3K/mTOR),epidermal growth factor receptor variant III (EGFRvIII),VEGF receptor(VEGFR),platelet-derived growth factor receptor(PDGFR),mesenchymal-epithelial transition factor(MET),fibroblast growth factor receptor (FGFR) and so forth (Figure 1).Many studies have focused on discovering therapeutic targets and examining targeted agents,and the number of trials of targeted therapy in combination with chemotherapy or immunotherapy has increased significantly.

Figure 1 Current potential therapeutic targets for rGBM (from published clinical trial results).Created with BioRender.com.VEGF,vascular endothelial growth factor;VEGFR,vascular endothelial growth factor receptor;EGFR,epidermal growth factor receptor;EGFRvIII,epidermal growth factor receptor variant III;PI3K/AKT/mTOR,phosphatidylinositol 3-kinase/mammalian target of rapamycin,epidermal growth factor receptor variant III;AKT,protein kinase B;TRK,tyrosine kinase receptor;ErbB,erythroblastic leukemia viral oncogene homolog 2;HGF,hematopoietic growth factor;MET,mesenchymal-epithelial transition factor;TGF-β2,transforming growth factor-β2;TGF-βR,transforming growth factor-β receptor;FGF,fibroblast growth factor;FGFR,fibroblast growth factor receptor;PARP,poly ADP-ribose polymerase;DRD2,D2 dopamine receptor;PDGF-β,platelet-derived growth factor-β;PDGFR,platelet-derived growth factor receptor.
Due to different molecular pathological characteristics and varied mutation retention rates between primary and recurrent GBM (4,5),a second biopsy is recommended for recurrent patients to determine the molecular pathology status,especially for those who intend to receive targeted therapy (3,22).
Angiogenesis-targeting therapy
Considering the hypervascularity of GBMs,therapies inhibiting angiogenesis are widely accepted.In 2019,the phase II REGOMA trial confirmed the utility of the multikinase inhibitor regorafenib,whose main function is targeting tumor angiogenesis,in rGBMs (23).Compared with patients in the CCNU group,patients who received regorafenib showed a significant improvement in mOS (7.4vs.5.6 months) and a better mPFS (2.0vs.1.9 months) and 6-month PFS (16.9%vs.8.3%).Moreover,patients achieved better OS in the regorafenib group regardless of theirMGMTpromoter methylation status,which provided a life-prolonging option for theMGMTpromoter unmethylated population with poor previous treatment response.Due to the promising results of REGOMA,regorafenib has been listed as a preferred regimen for rGBMs in the NCCN guidelines,and the sequential phase III trial is anticipated.
Bevacizumab inhibits angiogenesis by targeting VEGF.Earlier studies determined that bevacizumab was effective in prolonging PFS and alleviating edema but had no effect on prolonging OS (24).Several dosage regimens have been applied since then;however,the effective dose of bevacizumab prolonging OS has not been found.The 2019 TAMIGA trial evaluated continuous bevacizumab beyond recurrence or progression,which was once considered beneficial,and no survival benefit was observed (25).Biomarkers from blood samples may provide evidence for patients receiving bevacizumab.A prospective trial published in 2019 stated that baseline neutrophil and Treg counts could predict overall survival and that neutrophil count was related to bevacizumab response prediction in steroid-free patients (26).This finding indicates that bevacizumab is effective in increasing OS in certain subgroups,and further identification of benefits in subgroups is advantageous.
As an oral pan-VEGFR tyrosine kinase inhibitor,cediranib was expected to improve PFS (3.9 months) in rGBM,which was indicated in a single-arm phase II trial in 2010 (27).Regrettably,the subsequent phase III REGAL trial did not succeed in increasing PFS or OS compared with that of lomustine monotherapy and combination therapy (3.1vs.2.7vs.4.2 months),while no beneficial effects in subgroups were reported (28).Although a considerable effect on symptom alleviation was observed,studies on cediranib have since stalled.
Combination therapy based on targeted therapy
As the best-known angiogenesis-targeting agent,bevacizumab is widely applied in combination therapies.Combination therapy including bevacizumab is expected to improve both OS and PFS.
Combination bevacizumab and CCNU demonstrated efficacy in the 2014 phase II BELOB trial (29),in which rGBM patients who received combination therapy showed a better mOS (12vs.8vs.8 months) and mPFS (4vs.1vs.3 months) than those who received two monotherapies,and patients with isocitrate dehydrogenase (IDH) mutation orMGMTmethylation showed longer PFS and OS.However,this result was not observed for rGBM patients in the subsequent phase III European Organisation for Research and Treatment of Cancer (EORTC) 26101 trial conducted by Wicket al(30).Although an improvement in mPFS (4.2vs.1.5 months) was observed,the mOS (9.1vs.8.6 months)of the combination group was not different from that of the CCNU monotherapy group.In the subgroup analysis,MGMTpromoter methylation was also a positive prognostic marker,while no therapeutic predictive effect was reported.In addition,subsequent translational imaging analysis in 2019 indicated that temporal muscle thickness above 7.2 mm is independently correlated with better OS and PFS(31).Because temporal muscles can be completely displayed on magnetic resonance imaging (MRI) and correlate with skeletal muscle mass,patients with higher skeletal muscle tend to have a better prognosis.
Fotemustine is a nitrosourea,and fotemustine monotherapy and combination with bevacizumab have been studied.The phase II AVAREG trial indicated an effect of fotemustine monotherapy (mOS:8.7 months,mPFS:3.45 months) for rGBMs (32),and the efficacy of the combination therapy was examined in an open-label phase II trial (mOS:9.1 months,mPFS:5.2 months) (33).A higher performance score,younger age and methylatedMGMTpromoter were related to better survival.Unfortunately,when compared with historical data of monotherapies,combination therapy has not shown a benefit.
The combination of temozolomide and bevacizumab is also interesting.Gilbertet al.conducted a randomized phase II trial in patients with rGBM using either CPT-11 or temozolomide combined with bevacizumab (34).Encouragingly,both combination groups surpassed the presupposed threshold of 35% in elevating 6-month PFS.However,other studies at the same time period drew contrasting conclusions,suggesting that the effect of combination therapy is unclear (35).Subsequent studies have shown that CPT-11 has a limited ability to cross the BBB,and CPT-11 has been removed from the NCCN guidelines.These trials did not conduct an analysis of benefits in subgroups or any molecular markers;thus,we have no evidence to recommend temozolomide and bevacizumab combination therapy to a specific group of patients.Further subgroup analysis focusing onMGMTpromoter methylation status,IDHmutation status and other clinical or molecular features may bring this therapy back into the spotlight.
In addition,the combination of bevacizumab with other drugs is of interest.For example,carboplatin,rilotumumab,vorinostat (histone deacetylase inhibitor) and dasatinib (Src family kinase inhibitor) are combined with bevacizumab(36-39).Unfortunately,none of the phase II trials achieved their primary endpoints or showed significantly improved survival,and there was no significant effect on ORR and PFS in either combination group.No difference in posttreatment quality of life or cognitive competence was reported between the dasatinib and bevacizumab combination therapy group and the bevacizumab monotherapy group.Neither benefits in subgroups nor prognostic biomarkers were reported.Moreover,patients receiving both carboplatin and rilotumumab combined with bevacizumab showed an increased risk of adverse events (36,37).
Targeted therapy aimed at other targets
Phosphatidylinositol 3-kinase/mammalian target of rapamycin (PI3K/mTOR) is a frequently activated pathway and plays an important role in tumor proliferation.Wicket al.conducted a phase III trial comparing the effect and toxicity of enzastaurin (a PI3K pathway inhibitor) and CCNU on rGBM (40) based on the positive results from a phase I/II trial (41).Although enzastaurin therapy showed more acceptable toxicity,its efficacy did not exceed that of CCNU (mOS:6.6vs.7.1 months,mPFS:1.5vs.1.6 months),and there was no conclusive evidence on the possible benefits to subgroups of enzastaurin.After the failure of efficacy and discovery of a biomarker for another PI3K inhibitor,PX-866 (42),a new candidate emerged.Buparlisib monotherapy and combination therapy with bevacizumab and INC280 (a MET inhibitor) were explored in three phase II trials studying rGBM in 2019 (43-45).Again,neither monotherapy nor the combinations showed efficacy,while the combination therapy with bevacizumab induced more adverse events.This poor efficacy is believed to be caused by incomplete blockage of the PI3K pathway,and a more stringent molecular selection scheme might improve patient survival.However,contrary to previous conclusions,patients withIDHmutations had a shorter mPFS than those with wild-typeIDH(0.9vs.1.8 months).Despite these setbacks,trials on promising new agents are ongoing.GDC-0084 is a selective PI3K/mTOR pathway multi-targeted inhibitor that demonstrated tumor inhibition and favorable BBB-penetrating ability in a phase I trial (46).In a two-arm phase II trial (NCT03522298),the efficacy of GDC-0084 was compared with that of temozolomide in nGBMs,and the interim report yielded positive results.In the exploration of therapies targeting the PI3K pathway,GDC-0084 is currently the most promising agent,and the above-mentioned phase II trial may provide a specific therapeutic effect for patients with PI3K pathway activation.
EGFRvIII is the most common variant that specifically exists in gliomas,making it an ideal therapeutic target (47).ABT-414 is a newer-generation antibody-drug conjugate targeting EGFRvIII.With encouraging results in phase I trials (48,49),a randomized phase II trial in rGBM patients was conducted (50).Although no significant difference in OS was observed among the ABT-414 and temozolomide combination group,ABT-414 monotherapy group and chemotherapy control group,the combination group had a longer survival rate (28.6%) than the ABT-414 monotherapy group (11.1%) and the chemotherapy group(3.9%) in long-term follow-up.The non-inferiority and long-term survival benefits suggest a promising future for ABT-414 and chemotherapeutic combination therapy.However,we need to consider the failure of ABT-414 in nGBM in a phase III trial (NCT02573324) and be cautious when interpreting these conflicting conclusions.Additionally,the relationship between patient survival and biomarkers,including EGFRvIII andMGMTpromoter methylation status,needs further clarification.
Many clinical trials for different targeted therapies,including the cyclin-dependent kinase (CDK) 4/6 inhibitor ribociclib (51),the D2 dopamine receptor (DRD2)inhibitor ONC201 (52),the tyrosine kinase receptor(TRK) inhibitor larotrectinib (53),the multitarget MET and VEGFR2 inhibitor cabozantinib (54) and the 26S proteasome and the NF-κB pathway inhibitor bortezomib(55),have been conducted.ONC201 and larotrectinib showed efficacy,and ONC201 had possible antitumor activity,especially for patients with H3.3 K27M mutation.Moreover,most of the other trials obtained negative results,and the details are supplemented and summarized inTable 1(56-77).
We mainly included phase II and III clinical trials above,but some drugs have only finished phase I clinical trials and achieved exciting results,including the abovementioned GDC-0084.We have summarized these trials inTable 2(46,78-81).



In summary,patients with a methylatedMGMTpromoter show better survival status after combination therapy with alkylating agents and bevacizumab.The multitarget agent regorafenib showed therapeutic efficacy in both patients with and withoutMGMTpromoter methylation,and it has become a promising option,especially for patients with unmethylatedMGMTpromoters.The initial results of the therapeutic effects of GDC-0084,ABT-414,ONC201 and larotrectinib have been reported,and their efficacy in further trials is of concern.
Immunotherapy
As GBM is a “cold tumor” with less immune cell infiltration,activating the immune system and remodeling the microenvironment have become primary issues in GBM immunotherapies.Researchers have increasingly realized the importance of the immune environment in tumor progression.Currently,clinical trials in recurrent glioma mainly focus on viral therapies,vaccines and immune checkpoint inhibitors (ICIs) (Figure 2).
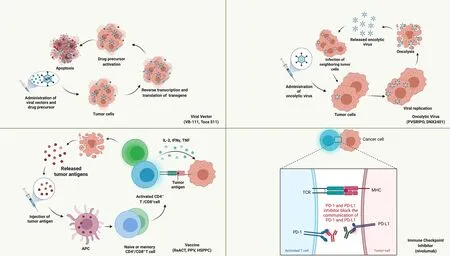
Figure 2 Current potential immunotherapies for rGBM (from published clinical trial results).Created with BioRender.com.PD-1,programmed death 1;PD-L1,programmed death ligand 1.
Viral therapy
Viral therapy generally includes replication-deficient viral vectors and replication-competent oncolytic viruses.The viral vector mediates the “suicide gene” to enter the tumor cells and transforms the drug precursor into tumor-killing agents,causing apoptosis of the tumor and surrounding cells.As a replication-deficient adenovirus carrying a transgene fusion,VB-111 inhibits angiogenesis and elevates the immune response.In a recent phase I/II trial,the safety and efficacy of VB-111 were proven in rGBMs (82),and a subsequent randomized phase III trial (GLOBE trial) on VB-111 and bevacizumab combination therapy was conducted (83).Compared to bevacizumab monotherapy,the combination therapy showed no advantages in mOS(6.8vs.7.9 months),mPFS (3.4vs.3.7 months) or ORR.Patients with smaller tumor size,more significant tumor volumetric response and fever during treatment showed a better OS,while age,performance score andMGMTmethylation status were not related to patient prognosis.Adverse events also occurred more often in the combination group. The different trial designs and protocols between the phase I/II and phase III trials and the possible inhibitory effect of bevacizumab on VB-111 may be partly responsible for the failure of the trial.
Vocimagene amiretrorepvec (Toca 511) is another retroviral replicating vector encoding cytosine deaminase and converts Toca fluorouracil (FC) to 5-FC inside the BBB.A phase I trial involving patients with recurrent highgrade glioma achieved promising results in mOS and ORR(84),especially for patients with low genomic mutation burden.These promising results prompted a subsequent randomized phase III trial comparing Toca 511/FC therapy and defined a single approved choice (85).The treatment failed to meet expectations;there were no significant differences in survival or adverse events between the Toca 511/FC group and the active control group(mOS:11.10vs.12.22 months).However,it is worth noting that patients experiencing a second recurrence andIDHmutation tended to have a better OS,which might be related to susceptibility to viral vectors.
Oncolytic viruses infect cancer cells and directly destroy tumor cells by self-replication.The results of phase II/III clinical trials of oncolytic viruses are still absent,while several phase I trials with worldwide attention have reported positive outcomes of oncolytic viruses and viral vectors,including PVSRIPO,DNX-2401 and Ad-RTShIL-12 (86-88).In addition,the following phase II trials are ongoing.
Vaccine
Peptide vaccines have been a research hotspot in rGBMs.As an active immunotherapy strategy,polypeptides of tumor-specific antigen sequences were constructed and sent into circulation,activated the immune response and attacked the tumor cells.The best-known peptide vaccine is rindopepimut (aimed at EGFRvIII).In a phase II trial(ReACT),rindopepimut and bevacizumab combination therapy was applied in EGFRvIII-positive rGBM (89),and patients who received rindopepimut had a higher 6-month PFS (28%vs.16%),mOS (HR 0.53) and 24-month OS(20%vs.3%).However,this study has some drawbacks,such as a small sample size,lack of molecular pathology and lack of subgroup analysis.Notably,a phase III trial for rindopepimut in nGBM (ACT IV) obtained a negative outcome (22).In ACT IV,patients with significant residual disease obtained a survival benefit,which may be due to different combination regimens and EGFRvIII expression loss in recurrence and can be analogous to the positive results in ReACT.
Personalized peptide vaccination (PPV) represents the implementation of precision medicine in rGBMs.Naritaet al.conducted a phase III trial and included 88 patients with rGBM,but neither OS (8.4vs.8.0 months) nor PFS was elevated in the PPV group compared to the best supportive care control group (90).Further studies discovered that patients who received PPV containing SART2-93,were older than 70 years,were heavier than 70 kg or had a performance score equal to 3,had a poor immune response and survival status,and a significant survival benefit was observed excluding patients with SART2-93 and those over 70 years old,which indicated that PPVS may only be effective in certain subgroups.
In addition to the above-mentioned high-profile methods,heat-shock protein (HSP) peptide complex-96 vaccination (HSPPC-96) is an interesting option for rGBM.HSP is recognized as a widely existing protein that responds to temperature changes and is related to tumor proliferation,differentiation,infiltration and metastasis.Furthermore,HSPs can combine with tumor antigen peptides,form HSPPCs and then activate immune cells and pathways after endocytosis and antigen presentation.In the phase II trial,Blochet al.showed considerable efficacy in terms of OS (10.65 months) and PFS (4.78 months) (91).Patients who received more vaccine doses showed better PFS and OS,as expected,and a higher absolute lymphocyte count level was correlated with the outcome,which can be explained by immune activation.However,there are currently no ongoing or completed phase III trials on HSPPC-96.
ICI
Programmed death 1 (PD-1) inhibitors are currently the most researched ICIs.Checkmate 143 serial trials were first reported in 2018. In a phase I trial,nivolumab monotherapy showed acceptable toxicity and considerable efficacy on rGBM (92).However,nivolumab showed no advantage over bevacizumab in a further phase III trial on mOS (9.8vs.10.0 months),mPFS (1.5vs.3.5 months) and ORR (7.8%vs.23.1%) (93).Subgroup analysis indicated that corticosteroid naïveness andMGMTpromoter methylation were related to a better response to nivolumab.The difference could be attributed to the suppressive effect on the immune system,which led to a poor response to immunotherapy. Additionally,patients who require corticosteroid usage tend to have faster disease progression and might not have enough time to benefit from immunotherapy.As the first phase III clinical trial for ICIs in rGBMs,researchers adopted RANO criteria instead of iRANO criteria to evaluate disease progression,which might underestimate the mPFS of nivolumab and overestimate the mPFS of bevacizumab.
After the failure of Checkmate 143,several studies explaining the reason for failure and exploring new regimens were conducted.In a phase II trial,the immune microenvironment after pembrolizumab treatment was analyzed (94).T cell numbers declined while CD68+macrophage numbers increased,indicating an immunosuppressive microenvironment.
In 2019,two studies proposed a new use for neoadjuvant PD-1 inhibitors (95,96).Patients with rGBM who received neoadjuvant therapy (nivolumab administration pre-and post-surgery) showed increased levels of T cells and interferon-γ-related genes and downregulation of cell cycle-related gene expression,while this phenomenon was not observed in the adjuvant group (96).These promising results indicate the value of neoadjuvant PD-1 inhibitors,and further controlled trials including more patients are widely anticipated.
Thus,patients who experienced a second recurrence and haveIDHmutations might benefit from TOCA 511,while some phase I trials on other viral therapies showed promising results.Rindopepimut effectively improved the survival of EGFRvIII-positive rGBM patients.Adjuvant PD-1 inhibitors failed in phase III trials,but neoadjuvant PD-1 inhibitors have shown promise to date.
Conclusions
Solid studies have shown the promise of regorafenib,rindopepimut and neoadjuvant PD-1 inhibitors as treatments for rGBM treatment,and regorafenib is effective in bothMGMTpromoter methylated and unmethylated groups,which has provided hope forMGMTmethylation-negative patients. Patients withMGMTpromoter methylation can benefit from temozolomide rechallenge and alkylating agents combined with bevacizumab in the subgroup analyses,while TOCA 511 may be effective for patients withIDHmutation or second recurrence.Although most regimens fail to prolong OS,positive conclusions from phase I targeted therapy and immunotherapy studies are expected.New drugs and therapies should be well designed,and patients are encouraged to participate in clinical trials.In addition,the establishment of multidisciplinary teams should be promoted to improve the quality of life and prognosis of patients.
Acknowledgements
This study was supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences(No.2016-I2M2-001),the Beijing Municipal Natural Science Foundation [No. 7202150 and 19JCZDJC 64200(Z)],and the Tsinghua University-Peking Union Medical College Hospital Initiative Scientific Research Program (No.2019ZLH101).
Footnote
Conflicts of Interest:The authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2021年3期
Chinese Journal of Cancer Research2021年3期
- Chinese Journal of Cancer Research的其它文章
- Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines for malignant lymphoma 2021 (English version)
- Mitochondrial malic enzyme 2 promotes breast cancer metastasis via stabilizing HIF-1α under hypoxia
- Psychosomatic symptoms affect radiotherapy setup errors in early breast cancer patients
- Reappraise role of lymph node status in patterns of recurrence following curative resection of gastric adenocarcinoma
- Pain threshold,anxiety and other factors affect intensity of postoperative pain in gastric cancer patients:A prospective cohort study
- A male-ABCD algorithm for hepatocellular carcinoma risk prediction in HBsAg carriers
