Enhancement of removal efficiency of heavy metal ions by polyaniline deposition on electrospun polyacrylonitrile membranes
Noor Mohammad,Yomen Atassi
Department of Applied Physics,Higher Institute for Applied Sciences and Technology,Damascus,Syria
Abstract This paper describes the preparation of a membrane of polyacrylonitrile(PAN)and its corresponding membrane coated with polyaniline(PANI)for the adsorption of heavy metal ions.Scanning electron microscopy micrographs revealed that all the membranes exhibited nanofibrous morphology.The prepared membranes were characterized by Fourier transform infrared spectroscopy(FTIR).The prepared membranes were used as an adsorbent for hazardous heavy metal ions Pb2+and Cr2O2-7.The adsorption capacity and the removal efficiency of the membranes were examined as function of the initial adsorbate concentration and pH of the medium.Coated membranes with PANI showed better adsorption performance and their direct current(DC)conductivities were correlated to heavy metal ion concentrations.Adsorption isotherms were also performed,and the adsorption process was tested according to the Langmuir and Freundlich models.The regeneration and reuse of the prepared membranes to re-adsorb heavy metal ions were also investigated.The enhancement in adsorption performance and reusability of PANI-coated membranes in comparison with non-coated ones is fully discussed.The results show that the maximum adsorption capacities of lead and chromate ions on the PANI-coated membranes are 290.12 and 1 202.53 mg/g,respectively.
Keywords:Membrane;Electrospinning;Polyaniline;Polyacrylonitrile;Heavy metal ion removal
1.Introduction
Given that heavy metal ions are considered inhibitors of enzymes and metabolic toxins(Charerntanyarak,1999),water contamination resulting from heavy metals is an issue of concern because of its direct impact on human health(Chen et al.,2012).Hydrodynamics is an important factor that affects the transport and adsorption of contaminants(Jin et al.,2010,2020).Water pollution with heavy metals is one of the most dangerous environmental problems that threaten water bodies,as well as different kinds of life,due to the toxic and cumulative effects of heavy metals.In addition to their ability to bond with living fatty and phosphoric tissues,heavy metal ions act as an inhibitor of a broad spectrum of bioactive enzymes(Zhang et al.,2010).Moreover,heavy metals are not biodegradable and increasingly used in many industries including paper,paints,metallurgy,and others(Zhang et al.,2010).Therefore,it is important to find methods that reduce the concentration of these ions and prevent their accumulation in water,especially drinking water(Mwangi and Ngila,2012).
Among all heavy metals,Pb2+and Cr2O2-7ions are very harmful and can cause many diseases,including cardiovascular diseases,nervous system disorders,bone-related diseases,liver cancer,and kidney cancer(Flora et al.,2012).Chromium can be found in two types of positive ions,trivalent Cr3+and hexavalent Cr6+(Deng and Bai,2004).Cr3+ions are important for lipid metabolism,and their deficiency may cause diabetes and cardiovascular diseases(Hummel et al.,2007),whereas Cr6+ions are quite toxic due to their carcinogenic and mutagenic properties(Mamyrbaev et al.,2015).Lead ions are highly poisonous,and they are regarded as the main reason for serious diseases such as renal sickness,irritability,and dizziness(Needleman,2004).Water can be contaminated by lead when it is used in pipes,or as solder for pipe connections or fixtures(Wong and Berrang,1976).High levels of lead in water may cause serious neurological damage and organ failure,and in some cases,it may lead to death(Flora et al.,2012).Recently,researchers have elaborated novel methods and materials to remove chromium and lead ions from wastewater,including electrochemical precipitation,ion exchange,solvent extraction,and membrane filtration and adsorption(Kongsricharoern and Polprasert,1995;Garg et al.,2004).
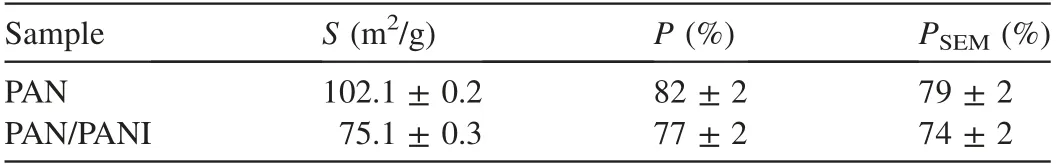
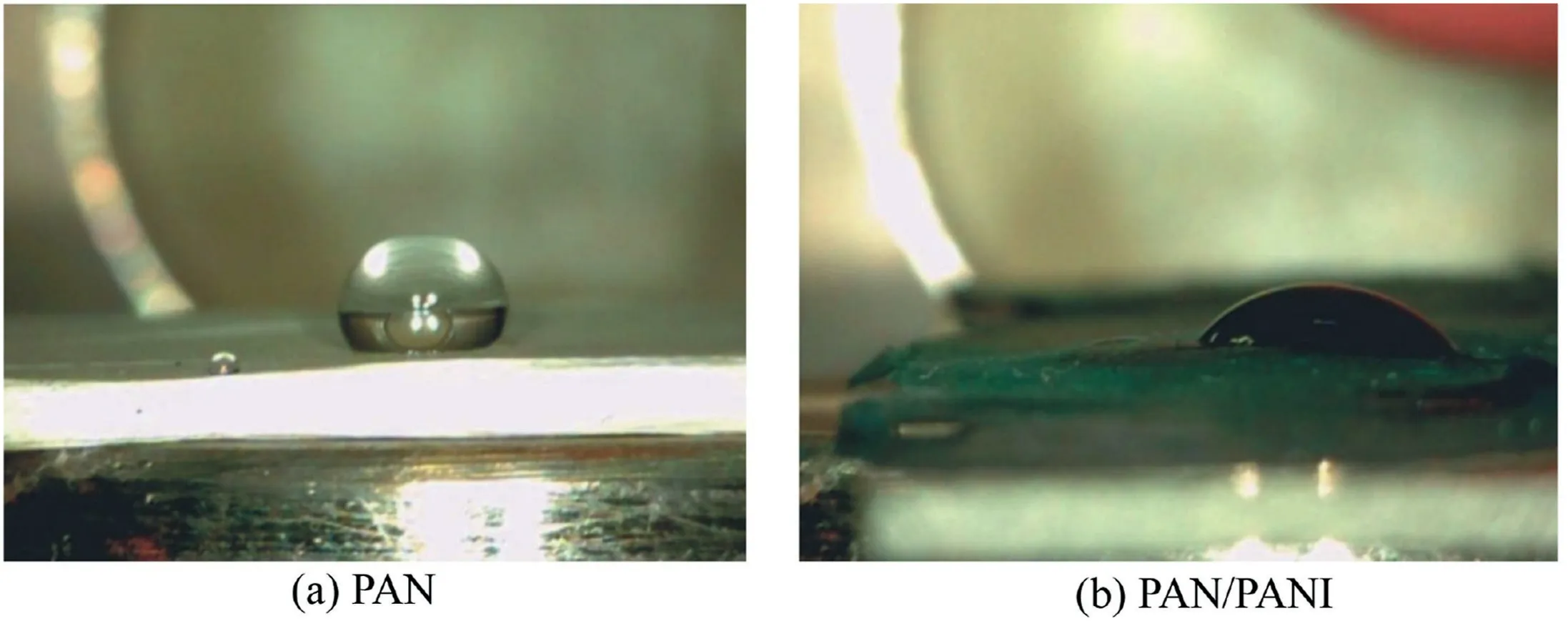
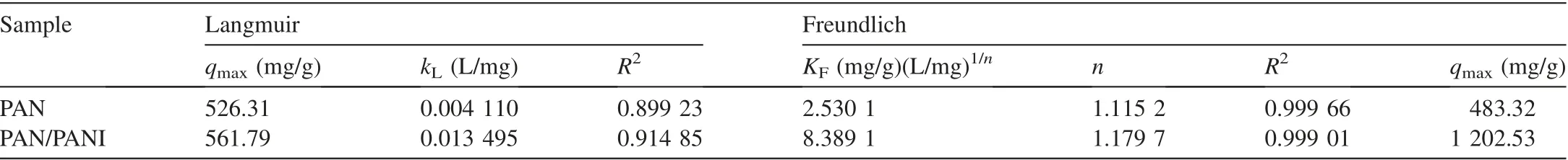
Polymers have been utilized in this field due to their ability to remove heavy ions through complexation or ion exchange mechanisms(Deng and Bai,2004).Bayrakci et al.(2017)prepared electrospun nanofibrous polyacrylonitrile(PAN)/calixarene mats and found that this material was an excellent adsorbent for the removal of chromate ions from aqueous solutions(Bayrakci et al.,2017).Feng et al.(2018)prepared PAN-based porous carbon to absorb hexavalent chromium(Feng et al.,2018).Thamer et al.(2019)developed functionalized electrospun carbon nanofibers to remove lead ions from aqueous solutions with the adsorption capacity of approximately 200 mg/g at a pH value of 2.Jabur et al.(2016)presented a Chitosan/Nylon 6 nanofibrous membrane to remove lead and sodium ions by 87%and 75%,respectively.Aliabadi et al.(2014)prepared a chitosan/hydroxyapatite composite nanofiber membrane for the removal of heavy metal ions from aqueous solution.When the concentration of metal ions was 100 mg/L,the adsorption capacities for lead,nickel,and cobalt were 296.7,213.8,and 180.2 mg/g,respectively.Zang et al.(2019)presented electrospun super hydrophilic membranes for effective removal of Pb2+from water.The maximum adsorption capacity reached 146.21 mg/g according to the Langmuir model.Malik et al.(2018)prepared PAN/magnetite nanofibers to remove lead ions from aqueous solution.With the concentration of 50 mg/L for lead(Pb),copper(Cu),zinc(Zn),and manganese(Mn),the adsorption capacities for different metals were in the following order:Mn PANI is mostly used in its bulk form(Mahanta et al.,2008).However,rare works reported the use of PANI in membrane technology for water treatment.In this respect,many studies have recently investigated the deposition of PANI on electrospun membranes for water remediation by adsorption of heavy metal ions or organic pollutants on the prepared membrane(Alcaraz-Espinoza et al.,2015;Mohammad and Atassi,2020).For example,electrospinning membranes doped with PANI were used to remove organic dyes(cationic or anionic),and the PANI coated membranes outperformed those without coating,and the maximum removal capacities of methylene blue and methyl orange were approximately 400 mg/g and 333 mg/g,respectively(Mohammad and Atassi,2020;Al-Qassar Bani Al-Marjeh et al.,2019). To the best of our knowledge,the use of PAN nanofiber membrane coated with PANI to remediate water with heavy metal pollutants has not been reported.In this study,PANI was deposited on a PAN membrane prepared with the electrospinning technique and then was used to treat wastewater polluted by heavy metal ions.In this respect,lead(Pb2+)and hexavalent chromium(Cr6+)were used as an example of heavy metal ions.Firstly,the PAN membrane was prepared from its own solutions with the electrospinning technique.Afterwards,the PAN membrane was coated with chloridedoped PANI with the in situ oxidative polymerization method to form PAN/PANI.The removal efficiency and the adsorption capacity of the membranes were evaluated in terms of lead and chromium ions.Meanwhile,the effects of ion concentration and pH of the solution on the adsorption capacity and removal efficiency of the membranes were investigated.In addition,adsorption isotherms were studied.Electrical characterization of the membranes was also performed,and the correlation between the electrical conductivity and ion concentration was analyzed.The enhancement in adsorption performance and regeneration ability of PANIcoated membrane was fully investigated. PAN(molecular weight of 120 000 g/mol),anilinium chloride(99%purity),acetone,ammonium peroxydisulfate(APS),dimethylformamide,HCl,lead(II)nitrate(PbNO3),and potassium dichromate(K2Cr2O7)were purchased from Sigma-Aldrich and used without purification.All other reagents were analytical and used without any further purification. A programmable syringe pump(TOP-5300,Japan)was used to prepare the electrospun membranes.Morphology of the prepared membranes was analyzed by a scanning electron microscope(SEM)(Tescan Vega-II XMU SEM),and the morphology of coated PAN mats was evaluated by the transmission electron microscopy(TEM)(Zeiss-EM10C-100 kV).Fourier transform infrared spectroscopy(FTIR)spectra were recorded by a BRUKERVECTOR22 FTIR spectrophotometer.UV-vis spectra were analyzed by a UV-vis spectrometer(JASCO,V-350).pH measurements were performed by a pH meter(TWT,pH7110).The direct current(DC)was used to measure conductivity(σ)of the membranes with the fourprobe technique.To perform the measurement ofσ,a homemade four-probe device was used,which is composed of four parallel platinum wires of 0.4-mm diameter positioned 2 mm from one another.KEITHELY-220 and KEITHELY-617 were used as a programmable current source and programmable electrometer,respectively.An atomic absorption spectrophotometer(Shimadzu,AA-6800)was used to measure the absorbance of heavy metals on the prepared membranes.The DigiScope digital microscope program with a digital camera and a holder was used to measure the contact angle.σwas estimated by the Van der Pauw equation: where d is the distance between the electrodes,t is the thickness,w is the width of the sample,V is the electric voltage,and I is the electric current.Wettability tests were performed by a digital camera Promate to measure the contact angle. For the adsorption tests,10 mg of the samples were immersed in 10 mL of metal ion solution(with an adsorbent dosage of 1 g/L)for 24 h to make sure that the adsorption process reached the equilibrium(Aluigi et al.,2014).The adsorption capacity(q)and removal efficiency(R)were calculated using the following equations(Aluigi et al.,2014): where C0is the initial dye concentration,Ceis the equilibrium dye concentration,v is the volume of the dye solution,and m is the mass of the membranes.The specific surface area(S)was calculated according to the prepared membranes and the SEM images by measuring the density,average diameter,and the specific surface of the prepared membranes.The specific porosity and area were calculated for the prepared membranes on a square sample of 4 cm2.In practical terms,S of the membranes is expressed as a ratio between the total area and the mass of the nanofiber membrane,assuming that the nanocytes are similar to the cylinders of indefinite length,and the total area A can be calculated from each membrane using Eq.(4)(Garg et al.,2004):where D is the average fiber diameter and r is the fiber density calculated according to the Archimedes'procedure. Accordingly,the formula for calculating the specific surface area becomes the following: Also,the porosity(P)was calculated with the following equation(Aluigi et al.,2014;Selatile et al.,2019): where vais the apparent volume estimated by measuring the thickness and surface area of the membrane,and vtis the theoretical volume estimated by the membrane mass and bulk density. The PAN membranes were prepared using electrospinning techniques starting from homogenous solutions,which were prepared as the same way as in a previous study that involved testing the membranes to remove methylene blue from aqueous solutions(Mohammad and Atassi,2020). Generally,random PAN membranes have circular shapes with a diameter of 15 cm and a thickness of 100μm.The PAN/PANI nanocomposite membranes were prepared using the same procedure described in a previous study(Mohammad and Atassi,2020). The prepared membranes were characterized with an SEMto investigate their nanostructure and their effect on the adsorption performance.The SEM images(Fig.1)show that PAN had long,smooth,continuous nanofibers with a homogenous diameter of 330 nm.The morphology of PAN/PANI was similar to that of PAN.Moreover,it is covered by PANI homogeneously with no agglomerates and with an average diameter of 418 nm.This increase of PAN diameter confirms the successful precipitation of the conductive polymer on the PAN membrane.By zooming further into PAN/PANI,a rougher surface as compared to PAN can be observed. The membranes'specific surface area and porosity were calculated with Eqs.(5)and(6).The results are summarized in Table 1 along with the porosity values resulting from the SEM images using ImageJ software.Table 1 shows that the specific surface area of PAN is greater than the specific surface area of PAN/PANI,and the porosity of PAN is greater than the porosity of a PAN/PANI membrane.This is due to the increase in the diameter resulting from the deposition of the PANI on the PAN.It is worth noting that the porosity values calculated based on Eq.(6)and the porosity resulting from the SEM images are close to one another.On the other hand,we must not overlook the fact that there is an increase in surface groups resulting from the deposition of the PANI on the PAN. Table 1Specific surface area and porosity(P)calculated from Eq.(4)and porosity(P SEM)estimated from SEM micrographs of prepared membranes. Fig.1.SEM micrographs of prepared membranes. Fig.2 shows a TEMmicrograph of the prepared PAN/PANI membrane.In this figure,long and two-layer fibers can be observed.The inner part is transparent and bonded to PAN fibers,while the outer part is opaque and linked to PANI deposition.This result indicates successful deposition on the PAN fibers and formation of a homogeneous layer of PANI on the PAN fibers.The diameters of the PAN and PAN/PANI fibers are(325±13)nm and(410±15)nm,respectively.These results correlate closely with those obtained using SEM scanning electron microscopy images. FTIR spectra of PAN and PAN/PANI membranes are exhibited in Fig.3.The PAN spectrum shows distinct peaks corresponding to the stretching of the triple bond between carbon and nitrogen,C-H scissoring,and C-H stretching at 2 252,1 470,2 950 cm-1,respectively.When PANI is deposited on PAN,new peaks appear such as the C=C aromatic stretching at 1 483 cm-1and 1 640 cm-1,as well as the C-N stretching at 1 312 cm-1,the N-H stretching at 3 510 cm-1,and=C-H stretching with sp2 hybridization within the aromatic ring.The triple bond between the carbon and nitrogen has a red shift and its intensity has markedly decreased,confirming the interaction between PAN as the membrane and PANI,and suggesting the successful deposition of PANI on PAN membrane. The contact angle method has been used to investigate the wettability performance of the prepared membranes toward water,especially since the membranes are prepared to work in aqueous solutions.According to digital photos(Fig.4),the PAN/PANI membrane exhibits more hydrophobicity compared with the PAN membrane.This can be explained by the presence of amine groups in the semi-doped PANI cover and suggests the beneficial role of coating PAN membranes with PANI in promoting the wettability of the membrane and enhancing the interactions between the adsorbate and the adsorbent.From another perspective,the high hydrophobicity of the PAN membrane has two main origins:the first is the chemical group presented in PAN,and the second is the roughness on its surface,which increases its hydrophobicity,as suggested by the Cassie Baxter model.The PAN membrane wetting angle is about 51°,which is close to the value of the contact angle mentioned by Pan et al.(2018),whereas it is 34°in the case of the PAN/PANI membrane. The DC conductivity was measured using a four-probe technique with a KEITHELY-220 programmable current source and a KEITHELY-617 programmable electrometer. Fig.2.TEM micrograph of PAN/PANI membrane. Fig.3.FTIR spectra of PAN nanofiber membrane and PAN/PANI composite nanofiber membrane. The value of the conductivity was investigated before and after immersion of the prepared membrane with a solution of lead and chromate ions at different concentrations.PANI is considered a p-type semiconductor,where conductivity is caused by the movement of conjugated electrons along the polymeric chain(Li and Bai,2006;Al-Jallad and Atassi,2018)and by movement of chlorine dopant along and in between chains.Before adsorption,the PAN/PANI membrane conductivities were 8 S/cm.As the concentration of ions increased,the conductivity of the membrane decreased(Fig.5).The decrease in electrical conductivity with the increase of ion concentration could be linked to the fact that the adsorbate forms a kind of insulating layer on top of the surface of the membrane that decreases its conductivity.It is worth noting that the correlation between the conductivity of the membrane and ion concentration allows the monitoring of pollutant concentration with simple conductivity measurements,especially in a region where the correlation is linear. Pb2+ions adsorbed on the PANI layer can replace amine hydrogen atoms in PANI,and this can be considered somehow a crosslinking of different polymer chains,which causes a reduction in the ability of the double bonds to move along polymer chains and in turn reduces the conductivity.In the case of chromate,a replacement of the dopant occurs. The feasibility of the prepared membranes to be used for water treatment was evaluated by studying their adsorption capacity toward two species of ions:Pb2+and.A series of Pb2+solutions with concentration ranges from 5 to 350 mg/L was prepared for this purpose and a series of Cr2O2-7solution with concentration in between 5 and 500 mg/L was prepared as well.All the adsorption experiments were carried out at a pH value of 7,a membrane mass to solution volume ratio of 1/1,and room temperature.Fig.6 shows the adsorption capacityies and removal efficiencies of PAN and PAN/PANI membranes in both Pb2+and Cr2O2-7cases and the maximum corresponding values are listed in Table 2.The adsorption capacity increases with concentration until it reaches a maximum value(Table 2),where the free adsorption sites on the substrate become saturated or hindered.In both cases,the PAN/PANI membrane exhibits better adsorption properties.This can be attributed to the fiber roughness,which provides a freer adsorption site from one side,and the higher affinity of PANI to metallic ions as compared to PAN,due to the presence of amine groups along the PANI chain,and chlorine dopant,which can be replaced by pollutant ions.This explanation can be confirmed with conductivity measurements.The membranes exhibit better Pb2+adsorption efficiency due to smaller volume as compared to,which permits better penetration and with less hindrance. Fig.4.Wettability test for prepared membranes. Fig.5.PAN/PANI membrane conductivity after immersion in Pb2+and Cr2O2-7 solution with different concentrations. Table 3 shows a comparison with permissible concentrations of Pb2+andin water for drinking,agriculture,wastewater,and sea water.The results obtained in practical experiments were compared with previously established standards(the Environmental Protection Agency(EPA);Food and Agriculture Organization(FAO)).It was found that the water resulting from purification at low concentrations of lead ions is suitable for drinking and agriculture and it is within acceptable limits for sea water,agricultural drainage channels,industrial wastewater,rivers,and land surface in case of using PAN/PANI membranes.PAN membranes are suitable for water purification within acceptable limits of agricultural drainage channels and seas.Table 3 shows the maximum permissible limits for Pb2+and Cr2O2-7in different water sources,as well as the values obtained in our experiments. Table 2 Comparison of removal efficiencies of membranes of lead and chromate droplets at different concentrations. Table 3 Permissible limits for Pb2+and Cr2 O2-7 in different water types. Two adsorption models were selected and used to study the adsorption:the Freundlich and Langmuir models.They werestudied by varying ion concentrations at a constant adsorbent dosage.For the Freundlich model,Eq.(7)describes the linear isotherm: where qeis the amount of sorbent adsorbed per weight(mg/g),and kFand 1/n are Freundlich constants. The Langmuir model expresses the adsorption isotherm using the following equation: where C1is the equilibrium concentration of heavy metal ions,q1is the equilibrium adsorption capacity at certain concentration of heavy metal ions,qmaxis the maximum adsorption capacity,and kLis the effective dissociation constant(Deng and Chen,2003). Fig.6.Adsorption capacities and removal efficiencies of PAN and PAN/PANI membranes with regard to lead and chromate ions. The Langmuir and Freundlich isotherm curves forand Pb2+ions are depicted in Figs.7 and 8,respectively.In addition,the models'constants are listed in Tables 4 and 5,in the cases of Pb2+and Cr2O2-7,respectively. It is clear from the adsorption models that the Freundlich model provides the best characterization,which indicates that adsorption occurs mostly between the pores of the membranes and not only on the surface.This in turn confirms the effectiveness of nanopores in the retention of heavy metal ions,providing greater purity of water,which is confirmed by the results obtained,i.e.,removal of 99%of ions at minimum concentrations. Table 6 shows the adsorption capacities of Pb2+and Cr2O2-7on the prepared membrane in single and multielement aqueous solutions.The adsorption capacities of the prepared membranes are 290.12 and 1 202.53 mg/g for Pb2+and,respectively.Since wastewater often contains more than one heavy metal species,the behavior of particular metal specie is usually affected by the presence of other metals.Therefore,a competitive adsorption study of heavy metal ions from their mixtures was also performed.The adsorption capacities decreased,as shown in Table 6.This decrease is expected,due to the increased ionic strength of the aqueous solution. Table 7 and Table 8 compare lead and chromate ion removal capacity found in this study with the relevant scientific literature.The stronger performance of the prepared PAN/PANI membrane compared with other membranes suggested in the literature can be observed. The pH of contaminated aqueous solutions is an important factor in use of nanomembranes.The filtration process relates to two main factors:the size of the pollutants and the interaction between heavy metal ions and the surface of charged membranes.The pH value affects the charge of the surface of the membrane as well as the polluting ions.Lead nitrate salt is stable at a pH value lower than 6.Table 9 shows the effect of pH on the removal efficiency of membranes for Pb2+and Cr2O27-.The initial concentration of Pb2+and Cr2O27-solution was 100 mg/L,prepared with three different pH values(3,7,and 13).The prepared membranes(PAN/PANI)were immersed in these solutions at room temperature,and the ratio of volume to mass was equal to one.The adsorption time was set at 3 h.Table 9 clearly shows that the removal efficiency varies with the change of pH according to the ion,since the greatest removal of the positive lead ion is at a pH value of 13 due to increased interaction between the surface of the neutral membrane and the cationic lead ion charge.In contrast,at a low pH value of 3,the removal rate is minimized due to the dissonance between the surface of the positive charge membrane at this pH value and the positive lead ion.The negatively charged chromate Cr2O2-7moiety has the highest clearance at a low pH value and the lowest clearance at a high pH value for the same reason.At a moderate pH value of 7,the removal rate is intermediate due to the neutral membrane surface.This result agrees with the observed effect of environmental pH on water absorbency. Fig.7.Plots of fitted experimental data with Langmuir and Freundlich isotherm models for PAN and PAN/PANI membranes including Cr2O2-7 ions. Fig.8.Plots of fitted experimental data with Langmuir and Freundlich isotherm models for PAN and PAN/PANI membranes including Pb2+ions. The cycling stability of the PAN/PANI was investigated by performing four cycles ofadsorption and desorption.The adsorbed Cr2O2-7on PAN/PANI can be efficiently removed in acidic(5%HCl)conditions by reinforcing the electrostatic effect between PAN/PANI and Cr2O2-7.On the other hand,the adsorbed Pb2+on PAN/PANI can be efficiently removed in alkaline(5%NaOH)conditions by reinforcing the electrostatic effect between PAN/PANI and Pb2+.The reuse/reactivation experiment was conducted for both lead and chromate ions,as shown in Table 10.The PAN/PANI retains almost 58%and 60%of its initial adsorption capacity after four cycles for Cr2O2-7and Pb2+,respectively,indicating its superior recyclability for the removal of Cr2O2-7and Pb2+from wastewater. Table 4 Constants of adsorption isotherm models of PAN and PAN/PANI membranes in presence of Pb2+. Table 5 Constants of adsorption isotherm models of PAN and PAN/PANI membranes in presence of Cr2O2-7. Table 6 Adsorption capacities of Pb2+and Cr2O2-7 on PAN/PANI membrane in single and multi-element aqueous solutions. Table 7 Comparison of adsorption capacities of reported materials for Pb2+removal. Table 8 Comparison of adsorption capacities of reported materials for Cr2O2-7 removal. Table 9 Adsorption of Pb2+and Cr2O2-7 at different pH values on PAN/PANI membrane. Table 10 Removal efficiencies of PAN/PANI for Cr2O2-7 and Pb2+at different adsorption-desorption cycles(with initial Cr2O2-7 concentration of 250 mg/L at pHof 3 and initial Pb2+concentration of 250 mg/L at pHof 13). This study focused on the development of a composite material of polyacrylonitrile and polyaniline to form ananofiber membrane to remove heavy metal ions.Polyaniline played an important role in removing lead ions and chromate ions from aqueous solutions.We succeeded in this through deposition of polyaniline on an electrospinning membrane of polyacrylonitrile in the presence of an APS initiator.The preparation method is quick,easy,and low-cost.Infrared analysis showed an association between the polyaniline and the prepared membrane.The prepared membrane showed a high ability to remove heavy metal ions,especially in terms of the lower concentrations,where the lead ion removal rate reached 99%at 5 mg/L and chromate ions reached 90%removal at 5 mg/L,demonstrating the ability of this type of membrane to have a high removal capacity.The adsorption mechanism was characterized by the Langmuir and Freundlich models.The removal rate was tested according to the initial ion concentration and the pH values.The change of membrane surface conductivity was also studied by changing the ion concentration used.The result is that the membrane used is effective in removing contaminants from heavy metals from wastewater.Membrane reusability for lead ions and chromate adsorption was investigated and the membrane removal capacity was evident even after four cycles. Declaration of competing interest The authors declare no conflicts of interest.Acknowledgements Authors would like to thank the Higher Institute for Applied Sciences and Technology for providing the facilities to perform current research.Authors are grateful to Hohai University for supporting the publishing of the current work.2.Materials and methods
2.1.Materials
2.2.Instruments





2.3.Preparation of PAN and PAN/PANI membranes
3.Results and discussion
3.1.Morphology characterization

3.2.TEM micrograph
3.3.FTIR spectra
3.4.Membrane wettability
3.5.Conductivity measurement


3.6.Adsorption of metal ions by prepared membranes



3.7.Adsorption isotherm



3.8.Effect of initial pH


3.9.Desorption of Cr2O2-7 and Pb2+






4.Conclusions
 Water Science and Engineering2021年2期
Water Science and Engineering2021年2期
- Water Science and Engineering的其它文章
- Flood disaster monitoring based on Sentinel-1 data:A case study of Sihu Basin and Huaibei Plain,China
- Influence of cascade reservoirs on spatiotemporal variations of hydrogeochemistry in Jinsha River
- Preparation and characterization of Fe2O3/Bi2WO6 composite and photocatalytic degradation mechanism of microcystin-LR
- Photodegradation of reactive blue 19 dye using magnetic nanophotocatalyst α-Fe2O3/WO3:A comparison study ofα-Fe2O3/WO3 and WO3/NaOH
- Assessment of physicochemical properties of water and their seasonal variation in an urban river in Bangladesh
- Estimation of unloading relaxation depth of Baihetan Arch Dam foundation using long-short term memory network
