低硅/铝比介孔USY分子筛的制备及其VGO加氢裂化性能
王晶晶, 王延飞, 赵梓贺, 付凯妹, 王嘉祎, 霍明辰, 徐 华, 王 燕, 喻 昊, 余颖龙
(中国石油 石油化工研究院,北京 102206)
Modified zeolite Y, such as USY, possesses high cracking activity and selectivity, which maks it a general and essential acidic component of hydrocracking catalysts. Recently, various strategies including bottom-up[1-3]and top-down[4-11]approaches have been developed to improve the turnover frequency, selectivity, catalytic activity, as well as lifetime of the catalysts. Post-modification method, which tailors the physical and chemical properties of catalysts via chemical strategies, has been receiving increasing attentions in current years. As traditional chemical strategies, hydrothermal treatment, which operates in water vapor atmosphere at elevated temperatures, as well as the selective acidic leaching or dealumination by acid solution, have been well developed[12-16]. Although hydrothermal treatment could not change the silica-alumina ratio of zeolite Y in bulk, the silica-alumina ratio of the framework could be increased as some of Al atoms are converted into non-framework species, which makes it possible to tailor total acidity and strength. The acid leaching on USY zeolite could remove non-framework aluminum species, as well as those in framework, producing USY with high silica-aluminum molar ratios[6,8]. Although these two approaches are beneficial to tailor the chemical properties such as acidity and hydrothermal stability, along with creation of mesopores, the generated mesopores are mainly dominated with cavity located in the internal zone and surrounded with micropores[17]. Consequently, these created mesopores are inaccessible to large molecules.
As proved, mesoporous structure could promote the diffusion of reactants and products, increase the apparent reaction activity, and improve the conversion capacity of zeolite Y for large molecules by optimizing the architecture[18]. Alkaline treatment of zeolites has been getting more attention and well developed for zeolites with different crystal structures in the past decade[4,6]. Detailed strategies and methods of zeolite Y modification have been summarized[19].
As a popular means to create mesoporosity, alkaline treatment was usually performed under various conditions according to the Si/Al ratio of starting Y zeolites. Taking NaY as an example, mild treatments (0.10 mol/L NaOH, treated at 80 ℃ for 2 h) were undesirable to create mesoporous structures, while harsh conditions (3.0 mol/L NaOH, treated temperature above 65 ℃) resulted in a relatively low modification efficiency and crystallinity, leading to a slight increase in mesoporous surface area of only 1 m2/g per 1% loss of zeolite by mass fraction[5-6]. Therefore, the conditions of alkaline treatment for NaY should be optimized to dissolve or disrupt the crystal structure to form mesoporous structures. Meanwhile, the desilication of USY with a high Si/Al ratio was also examined. Inappropriate alkaline treatment conditions can cause structural collapse or even amorphization in USY[4,6-8,20]. Hereby, optimal conditions are the keys to obtain mesoporous USY with a high crystallinity retention rate, a high efficiency, and an outstanding performance, which are crucial for industrial applications. As to USY with low silicon-aluminum ratio (n(Si)/n(Al)<10), ammonium fluoride (NH4F) and high alkaline solutions, which have a significant impact on the environment, are inevitable in its preparation process. Moreover, the activity of mesoporous USY for industrial feedstock has been rarely reported.
In this study, mesoporous USY (MUSY) is obtained via a novel method where parent USY with low Si/Al molar ratio (n(Si)/n(Al)=7.0) was treated using a wet impregnation with lower concentration alkaline solution (0.20 mol/L NaOH) and a subsequent heat treatment. As the water evaporates, the concentration of the alkaline solution in the pores gradually increases, thereby the desiliconization reaction was promoted. MUSY shows a mesopore volume of 0.31 cm3/g, which increases by 63% compared with that of USY. Similarly, it shows an external surface area of 150.1 m2/g, which increases by 50%. Meanwhile, as the bulk Si/Al molar ratio decreases to 5.2, the acid amount of MUSY increases approximately 40%. The zeolites were further prepared into hydrocracking catalysts. The specific area and mesoporous volume of catalyst contained MUSY were improved 46% and 27% in the range of 1.5 to 12 nm, which led to higher selectivity of middle distillate. Due to the increase in the acid amount of the MUSY, the acid amount of the corresponding catalyst also increases nearly 40%, resulting in higher hydrocracking activity.
1 Materials and Experiments
1.1 Raw Materials and Reagents
NaOH, (NH4)2SO4, nitric acid, Ni(NO3)2·6H2O and (NH4)6Mo7O24·4H2O are analytical reagents produced by Sinopharm.
USY zeolite (DAY-12,n(Si)/n(Al)=7.0) is a commercial product produced by Qilu Huaxin High-Tech. Co.Ltd. Pseudoboehmite and sesbania are industrial products produced by Shandong Xingdu Petrochemical Technology Co. Ltd.
The evaluation feedstock is laboratory mixed vacuum gas oil, and its properties are shown in Table 1.
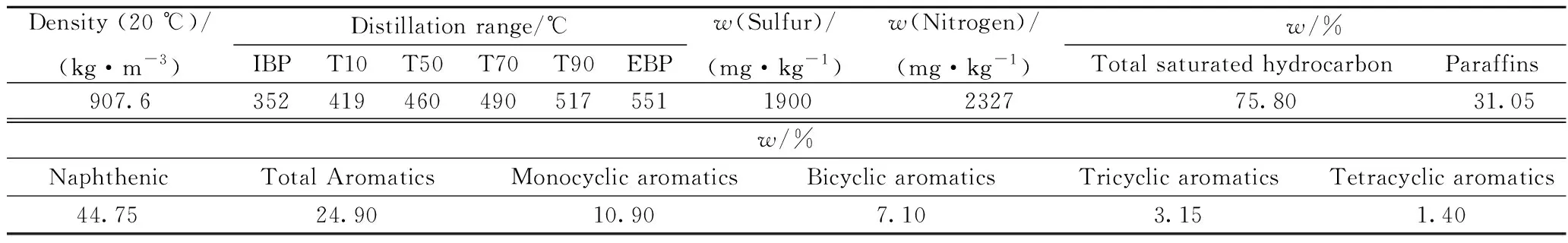
Table 1 Basic properties of feedstock
1.2 Preparation of MUSY
First, the parent USY was dispersed in an alkaline solution with a molar concentration of 0.2 mol/L, followed by vigorous stirring at room temperature for 1 h, the suspension was then subject to filtration without washing by deionized water, followed by heat treatment at 80 ℃ for 3 h. Secondly, the dried precipitate was then grounded into powder and then re-mixed with an alkaline solution of 0.2 mol/L for 0.5 h at room temperature, followed by filtration, washing by deionized water, drying and ion-exchange with ammonium solution. Finally, the product was obtained after calcination at 550 ℃ for 2 h, named as MUSY. The typical procedure is described as follows (Fig.1).

Fig.1 Preparation of MUSY
1.3 Catalyst Preparation for Hydrocracking
To prepare the nickel-molybdenum catalysts, the support was prepared as follows: certain amount of selected MUSY, pseudoboehmite, and sesbania were mechanically mixed for enough time, followed by the addition of nitric acid and deionized water, and stirring, the obtained paste was then extruded in a cylinder form, and the extrudes were then dried and calcined.
The catalyst was prepared via an incipient impregnation method, Ni(NO3)2·6H2O and (NH4)6Mo7O24·4H2O were selected as the precursors for metal oxides, and the concentration of metal solution was controlled to achieve a metal oxide loading of 5%NiO and 16%MoO3by mass fraction. The final catalyst was then obtained after drying and calcination. The obtained catalyst was named as NiMo/(Al2O3+MUSY), while NiMo/(Al2O3+USY) was also prepared for comparison using the same technique with USY as the acidic component instead of MUSY.
1.4 Characterization
N2sorption was performed on a Micromeritics ASAP 2460 analyzer at -196 ℃. Prior to the analysis, the samples had been degassed in vacuum at 350 ℃ for 8 h. The total specific surface area was calculated with BET equation, while the external surface area, microporous surface area, as well as the microporous pore volume were obtained with t-plot method. The pore size distribution was derived with desorption branch using BJH model, the total pore volume was measured at volume of N2adsorbed atp/p0=0.995.
X-ray diffraction (XRD) patterns of the samples were obtained on a PANalytial X′Pert Power X-ray diffract meter with monochromatized CuKαradiation (40 kV, 40 mA).
The acidic properties of the samples were measured by temperature-programmed desorption of ammonia (NH3-TPD) using a Micromeritics Autochem 2920 instrument. The samples were packed in the sample tube and pretreated in Helium atmosphere, then cooled for NH3adsorption to reach saturation. The desorption profile was programmed with heating ramp of 8 ℃/min in Helium atmosphere.
The surface morphology was captured by FEI NanoSEM450 Scanning Electron Microscope.
The transmission electron microscopy was captured by JEOL JEM 2100 Plus, 200 kV.
The elemental composition of zeolites and catalysts were measured by a RIGAKU PRIMUS II X-ray fluorescence spectrometer. The zeolites and catalysts were grinded into fine powder and sampled by a polyvinyl chloride sample ring.
Acid type distributions and acid sites of the catalysts were measured by a thermo Fisher Nicolet IS10 FT-IR spectrometer using pyridine as the probe. The Nicolet 6700 infrared spectrometer quartz infrared double-in-situ cell vacuum device is also used. Firstly, 20 mg of the sample was pressed into a film, and then placed in an in-situ cell, followed by evacuation to 1×10-4Pa, degassing at 400 ℃ for 2 h, afterwards, the cell was then cooled down to room temperature. The pyridine is adsorbed for 30 min, and then the corresponding absorption spectrum in the range of 1000—2000 cm-1is captured.
1.5 Hydrocracking Performance Evaluation
To investigate the cracking performance of prepared catalysts in this study, hydrocracking reaction with vacuum gas oil as the feedstock was employed on a fixed bed reactor system which is self-built device in laboratory. The basic properties of the feedstock are listed in Table 1. The whole system for evaluation consists of three sections: Feeding, reacting, and separation. The reacting system includes two reactors in a tandem form, where the hydrocracking section follows the hydrotreating section. The catalysts are charged in the isothermal zone of the reactor, (internal diameter 15 mm, isothermal length 500 mm), and the reaction heating are controlled with four furnaces and three thermocouples loaded in the top, middle and bottom of the catalyst bed.
For the study here, one commercial hydro-treating catalyst is placed in the first reactor, and the prepared hydrocracking catalysts (NiMo/(Al2O3+USY) or NiMo/(Al2O3+MUSY)) are loaded in the second reactor. The evaluation was performed under the following conditions: Catalysts loadings for hydro-treating and hydrocracking were 30 g and 20 g, and liquid hourly space velocity (LHSV) for hydrotreating and hydrocracking reaction were 1.2 and 1.5 h-1, respectively. Hydrogen pressure is 15 MPa, and H2/Oil volume ratio is 1500 and the reaction temperature for hydrotreating was controlled at 380 ℃ to make sure the nitrogen concentration is below 15 mg/kg, while the hydrocracking temperature is adjusted to control the conversion for the feedstock (>360 ℃ fraction) at an equivalent level for comparison. The degree of cracking in the hydrocracking step could be represented by conversion. Conversion here is defined as the proportion of the cracked fraction heavier than 360 of the crackeds. The middle distillation selectivity represents the yield of middle distillate fraction (180—360 ℃) in hydrocracking products.
The catalysts employed in this evaluation system are sulfided using dimethyl disulfide (DMDS) in a jet fuel fraction with a concentration of 2% by mass fraction. The sulfidation is performed at 15 MPa,with a H2/ Oil volume ratio of 1500 and LHSV for hydrocracking of 1.5 h-1. The sulfidation follows the program below: Drying in H2up to 150 ℃ for 5 h, maintenance at 150 ℃ with DMDS in jet fuel for 2 h, heating up to 220 ℃ with a ramp of 20 ℃/h, and holding at 220 ℃ for at least 8 h; heating up to 330 ℃ with a rate of 10 ℃/h, and maintenance at 330 ℃ for 8 h. Afterwards, the vacuum gasoil was fed into the reactors with a fixed amount along with the reactor temperature increasing to the target temperature for achieving required conversion.
2 Results and Discussion
2.1 Characterization of USY Zeolites
2.1.1 XRD of the zeolites
Fig.2 shows XRD patterns of USY and prepared MUSY samples between 5° and 35°. MUSY showed all the characteristic peaks of zeolite Y, such as crystal planes of 111, 220, 311, 533 and 555. Compared to USY, the diffraction angles of each crystal planes in MUSY moved forward slightly, because the bulk Si/Al ratio decreases. The intensity of these peaks in MUSY also decreased about 30%, indicating the good preservation of crystal framework structure according to the intensity after alkaline treatment. It is speculated that the crystal framework and microporous structure are partially destroyed due to the alkaline treatment. The baseline and peak width of MUSY remaining the same as USY suggested that the amorphous components were eliminated effectively.

Fig.2 XRD patterns of USY zeolite and the prepared MUSY zeolite
2.1.2 Textural properties
The alkaline treatment developed in this study could generate mesoporous structure in the parent matrix. To investigate the mesoporosity evolution, N2sorption is applied for characterization. As shown in Fig. 3, both zeolites display a typical IV adsorption-desorption isotherms with a H3 hysteresis loop in the range of 0.4—0.9 for relative pressure indicating the presence of mesopores. Additionally, the sharp increase at the relative pressure (p/p0) below 0.01 shows the presence of microporosity. This confirms the combination of microprosity and mesoporosity in the parent (USY) and prepared zeolites (MUSY). Regarding the isotherms, compared with USY, the prepared 2MUSY features a lower adsorption at lower relative pressure, while has higher adsorption capacity at higherp/p0, which is consistent with surface area, microporous surface area, and mesoporous surface area before and after alkaline treatment. Table 2 showed that the microporous surface area decreased by 34%, while the mesoporous surface area increases by 50%, and the mesoporous volume increases by 63% from 0.19 to 0.31 cm3/g. After treatment, MUSY displays a bimodal mesopore size distribution in the range centered at 6 nm and 29 nm, which is consistent with earlier research[1-2]. It is suggested that the mesopores are produced by the collapse of zeolite′s intrinsic framework. Furthermore, the bulk Si/Al ratio reduction from 7.0 to 5.2, along with the results of XRD and pore structure, reveals that the formation of mesopores is initialized by acid-base neutralization between silicon species and alkaline.
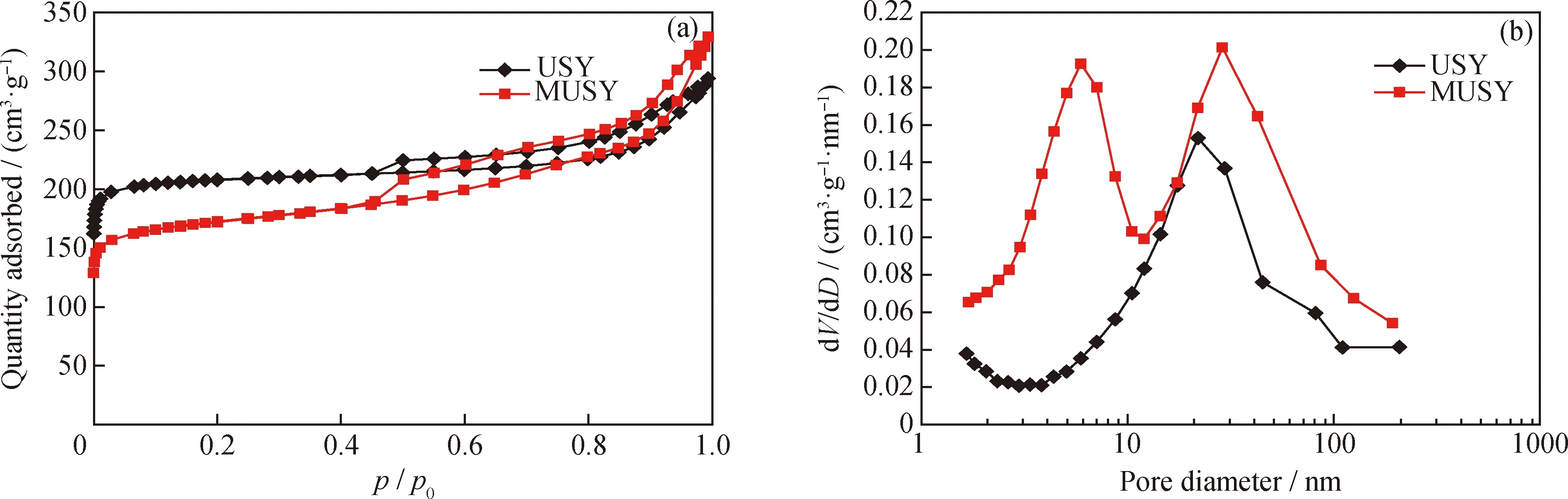
Fig.3 N2 adsorption-desorption of USY and MUSY zeolites(a) Isotherms; (b) BJH adsorption pore size distributions
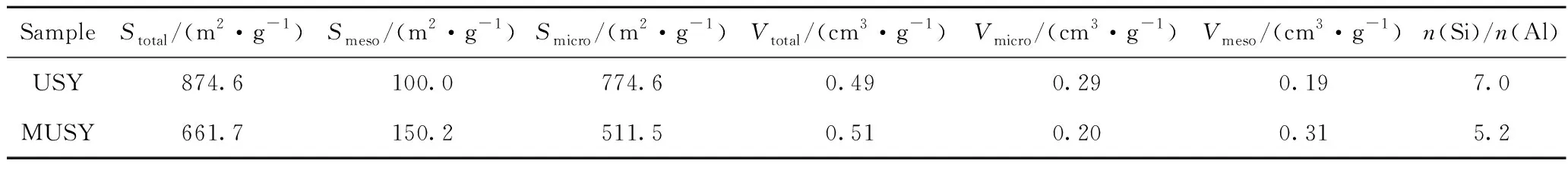
Table 2 Textural properties and bulk composition of USY zeolite and the prepared MUSY zeolite
Stotal—Total specific surface area, derived by BET method;Smeso—The surface area for mesoporosity, which is derived by subtracting microporous surface area from total surface area;Smicro—Microporous surface area from t-plot method;Vtotal—Total pore volume measured atp/p0=0.995;Vmicro—Microporous volume from t-plot method;Vmeso—Mesoporous volume, which obtained by subtracting microporous volume from total pore volume;n(Si)/n(Al)—Molar ratio measured by X-ray fluorescence (XRF)
2.1.3 Acidity properties
The acidity of zeolites was studied by NH3-TPD and shown in Fig. 4. Both zeolites possessed acidity from 200 to 500 ℃. MUSY showed a higher total acidity than that of USY, mainly due to the increase of medium strong acid around 200—350 ℃ and strong acid above 450 ℃. It could be inferred that dissolution of silicon species led to a lower bulk Si/Al ratio, which accordingly increased the acidity of MUSY.
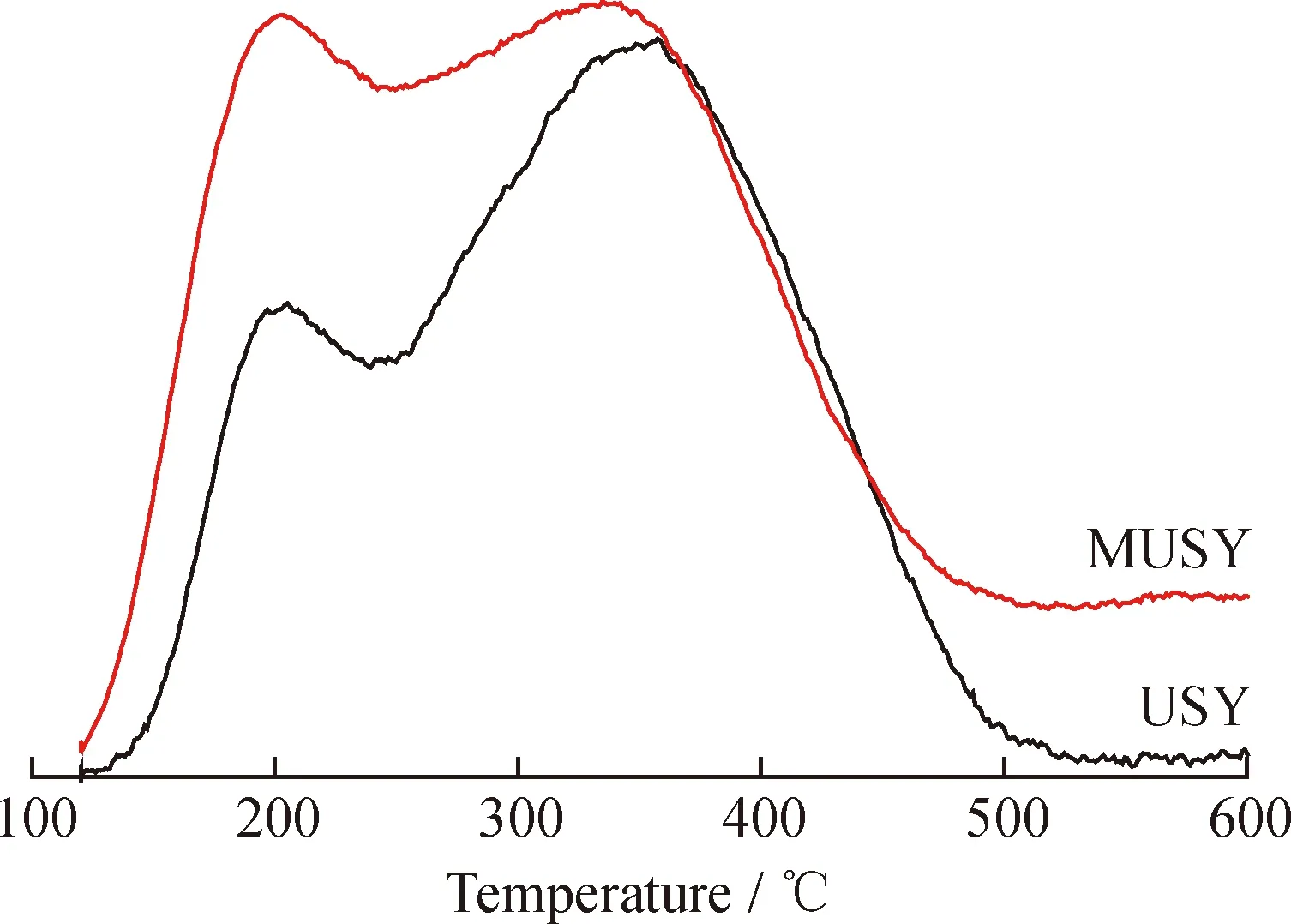
Fig.4 NH3-TPD profiles of USY zeolite andthe prepared MUSY zeolite
2.1.4 Morphology of the zeolites
To further study the effect of alkaline treatment on the surface morphology, SEM technology was involved (Fig.5). The particle shape is well preserved and the occurrence of mesopores is obvious. SEM images showed many holes and slits are formed on the crystal surface and extend into internal zone. Crystal breakage is also clearly observed on MUSY, and the increase in small fragments may be responsible for the significant increase in the first X-ray diffraction peak at 6.4°.

Fig.5 SEM images with various magnifications of zeolites(a), (b) USY; (c), (d) MUSY
Transmission electron microscopy was used to explore internal changes (Fig.6). The TEM images of MUSY showed that the created mesopores possessed random conformation. Compared to USY, the alkaline treated samples are rich in mesopores which extend from the crystal surface to the inside. It can also be seen that the mesoporous channels are clear, indicating that there is no amorphous component inside.

Fig.6 TEM images of zeolites(a) USY; (b) MUSY
2.2 Characterization of the Oxide Catalysts
2.2.1 XRD of the catalysts
XRD was employed to identify the dispersion of metal species of catalysts prepared with USY and MUSY. According to the XRD patterns (Fig.7), characteristic peak attributed to Al2O3(doubled*), and zeolite Y (singled*) are found, while the peaks related to MoO3and NiO metal oxides are absent indicating that molybdenum and nickel oxide species dispersed well on the support.
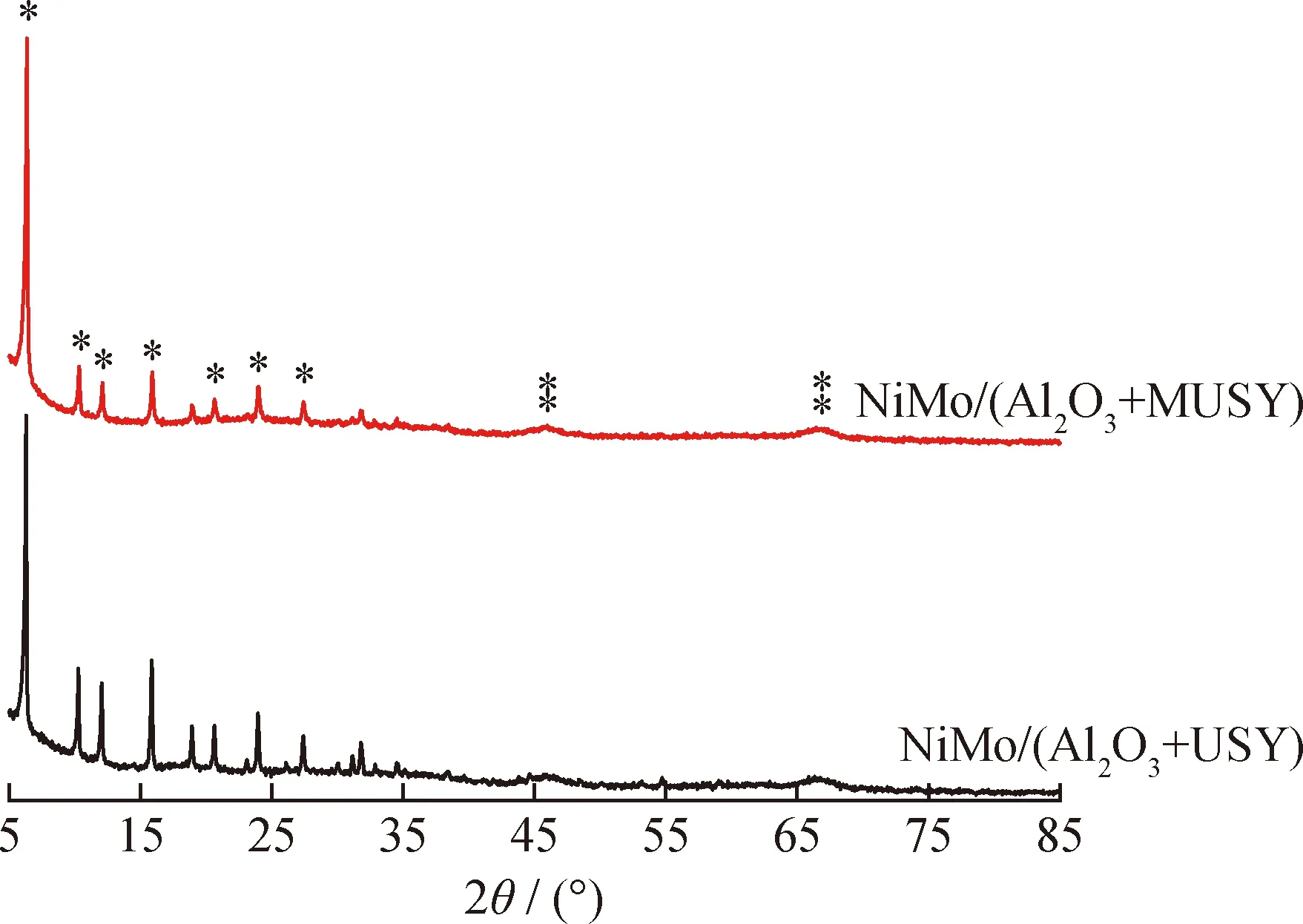
—Al2O3; *—Zeolite YFig.7 XRD patterns of prepared catalysts
2.2.2 Textural properties
The textures of the catalysts are listed in Table 3. Since MUSY was used, not only the total surface area and total pore volume of NiMo/(Al2O3+MUSY) were higher than those of NiMo/(Al2O3+USY), but the small mesopores (1.5—12 nm) specific surface area and pore volume of NiMo/(Al2O3+MUSY) were also significantly promoted by 45.8% and 26.7%.

Table 3 Textural properties of catalysts
2.2.3 Acidity of the catalysts
The NH3-TPD profiles of the catalysts are shown in Fig.8. The acid distribution of both catalysts ranged from 200 to 600 ℃. The total acid amount of NiMo/(Al2O3+MUSY) is 41% higher than NiMo/(Al2O3+USY), which is consistent with the distinct change of two zeolites by 39% (Table 4). The consistency of the changes in the acidity of the prepared zeolites and catalysts indicates that the acid sites of these two prepared hydrocracking catalysts are mainly provided by zeolites.
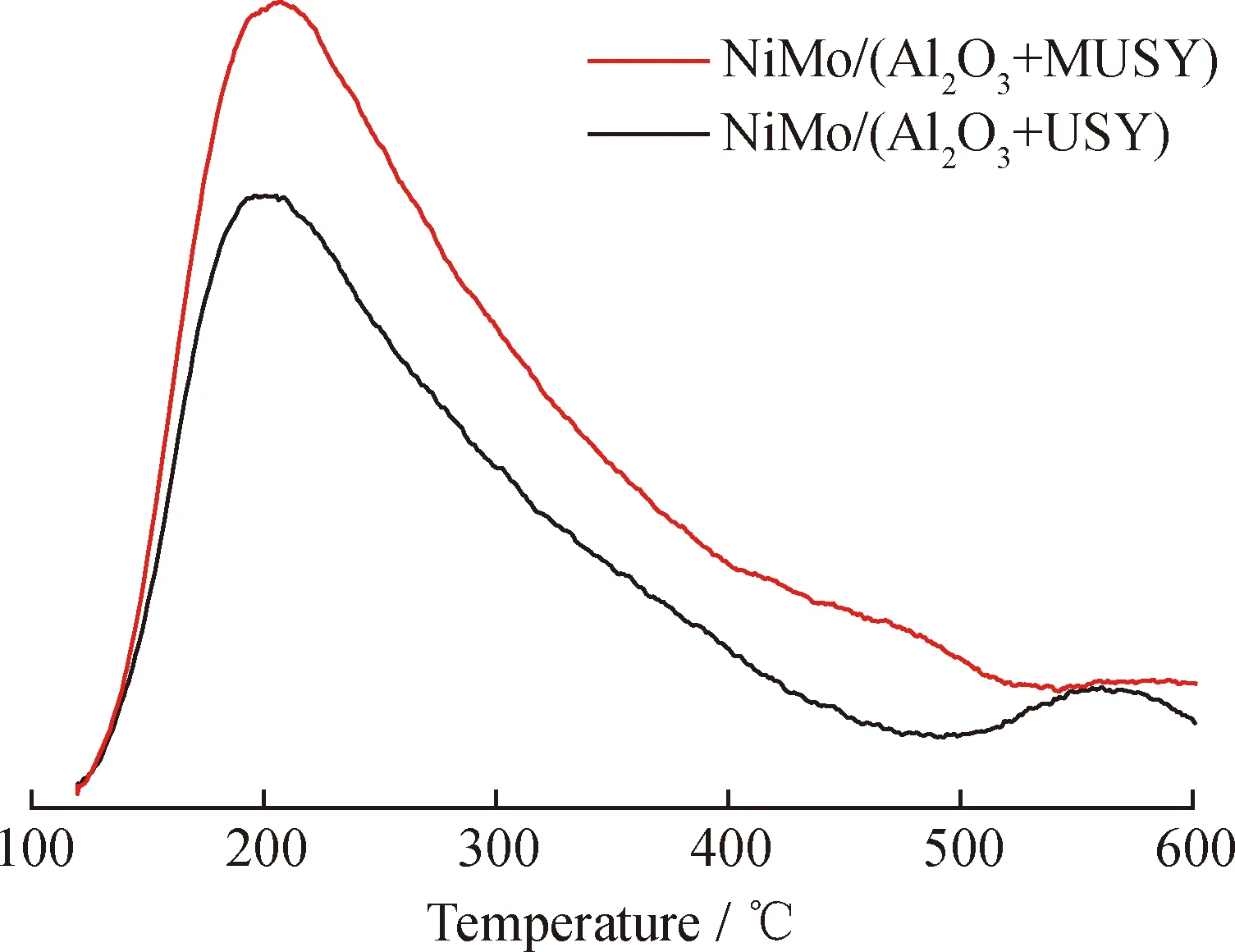
Fig.8 NH3-TPD profiles of catalysts
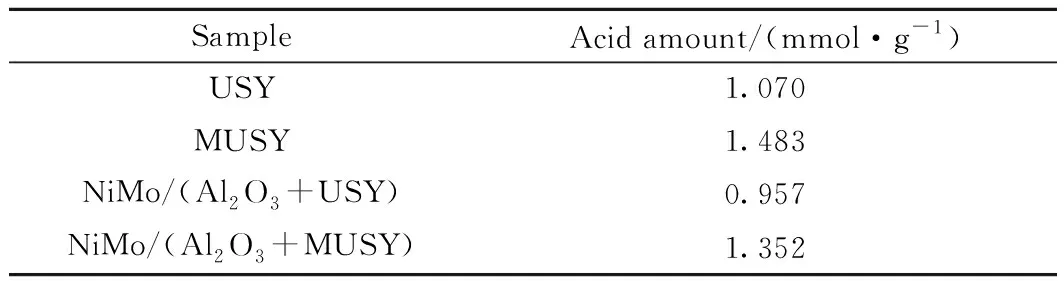
Table 4 Acid amount of zeolites and catalystsderived from NH3-TPD
Table 5 shows the acidic strength and relative abundance of Brönsted and Lewis acid sites. Compared with NiMo/(Al2O3+USY), the total acid amount of catalysts probed with pyridine after desorption (200 ℃) concentration of NiMo/(Al2O3+MUSY) increased by 39%, which is consistent with the NH3-TPD results. Moreover, Compared with NiMo/(Al2O3+USY), the overall acid content of the catalyst has increased, the total amount of L acid (1450 cm-1) of NiMo/(Al2O3+MUSY) increased by about 39%, the total amount of B acid (1540 cm-1) increased by about 65%, and the total B/L acid amount ratio increased from 0.217 to 0.257. Medium-strong L acid (>350 ℃) decreased by 20%, medium-strong B acid (>350 ℃) increased by about 24%, and medium-strong B/L increased from 0.372 to 0.576 (Table 5 and Fig.9). Since the content of each component in the two catalysts is the same, these changes are mainly due to the acid changes of the MUSY. The higher B acid content and B/L acid amount ratio are beneficial to improve the stability and cracking ability of the catalyst[21].
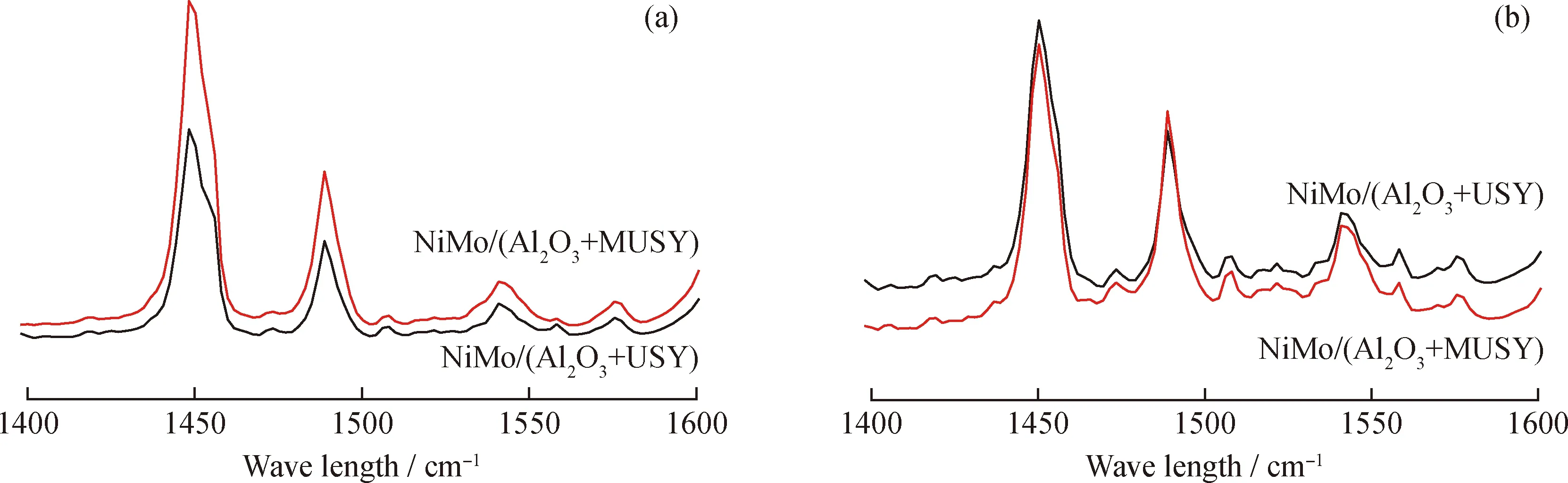
Fig.9 Py-IR spectra of catalysts(a) 200 ℃; (b) 350 ℃
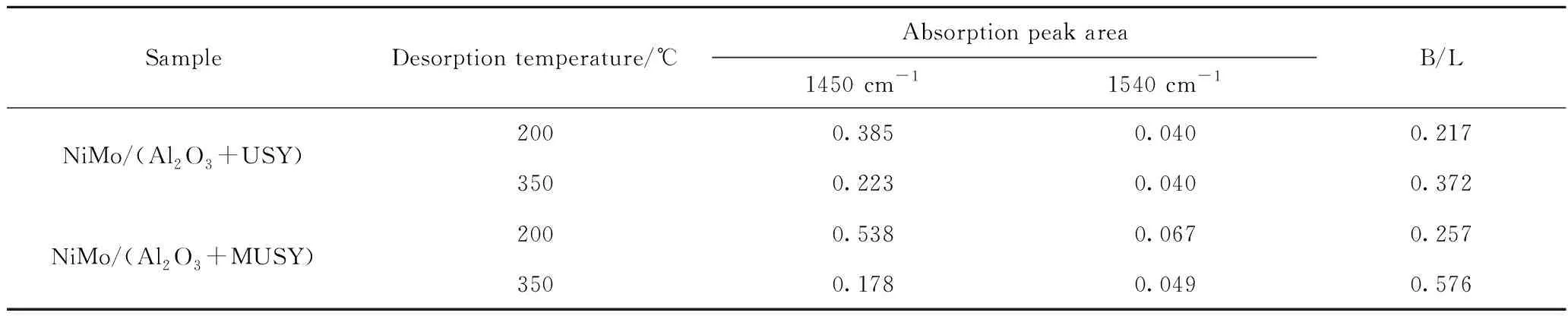
Table 5 FT-IR peak area of catalysts
2.3 Catalytic Performance
The conditions of hydro-treating reactor were adjusted to control the nitrogen mass fraction less than 15 mg/kg, and the conditions for hydrocracking reaction were adjusted by controlling the same conversion for fraction of higher than 360 ℃. Both catalysts yield similar products distributions, while NiMo/(Al2O3+MUSY) presents a slightly decreased selectivity for heavy naphtha (65—180 ℃) and an increased selectivity for middle distillate (180—360 ℃) (Table 6). It can be inferred that the generated mesopores can facilitate the diffusion, decreasing the possibility of secondary cracking, and therefore increasing the yield and selectivity of middle distillate. Of note is the fact that the catalytic activity regarding the conversion is increased by 8 ℃ for the MUSY based catalyst compared with USY based catalyst. This is probably due to the enhanced acidity, as well as the increased accessibility to acid sites.
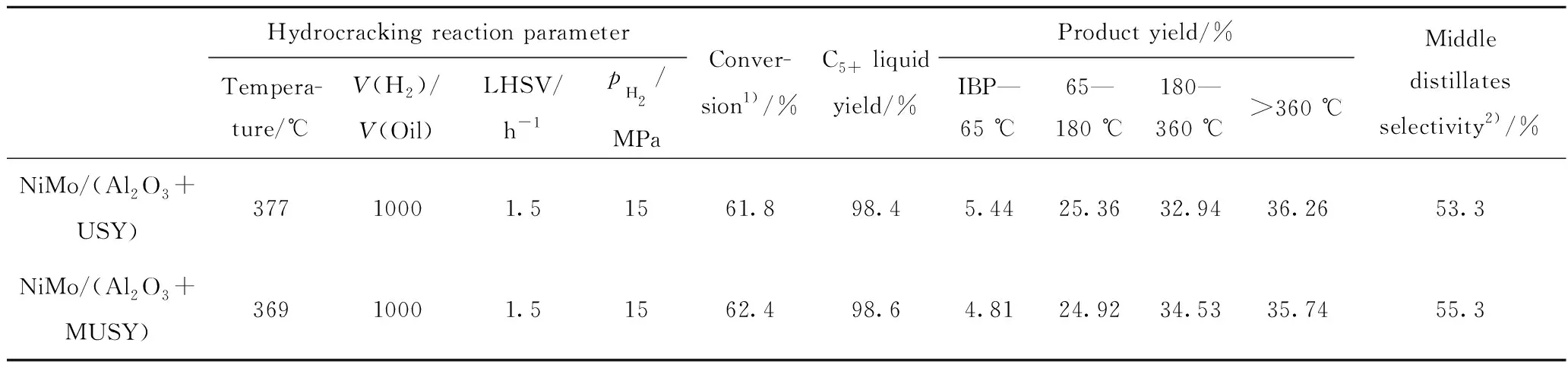
Table 6 Hydrocracking reaction parameters and product distribution under the action of two catalysts
1) The proportion of the cracked fraction heavier than 360 ℃ in fresh food; 2) The yield of middle distillate fraction (180—360 ℃) in hydrocracking products; IBP—Initial boiling point
The hydrocarbon composition of various products was listed in Table 7. Compared with NiMo/(Al2O3+USY), NiMo/(Al2O3+MUSY) possessed more naphthenes and aromatics and less isomeric hydrocarbons in the naphtha fraction (65—180 ℃). The composition of middle distillate (180—360 ℃) fraction shows that NiMo/(Al2O3+MUSY) can produce more paraffins, cycloalkanes and saturated hydrocarbons and less aromatics compared with NiMo/(Al2O3+USY), and indicates that NiMo/(Al2O3+MUSY) has a better hydrogenation ability. As for the unconverted oil (>360 ℃), there was less paraffines and more cycloalkanes with NiMo/(Al2O3+MUSY). It also showed that the more rings in the cycloalkanes, the faster the contents increase. These results may infer that the mesoporous structure, especially the higher external specific surface area, not only facilitates the efficient dispersion of the hydrogenation metal, which thereby improves the hydrogenation ability of the catalyst, but also promotes the diffusion of the macromolecules and produces more cycloalkanes.
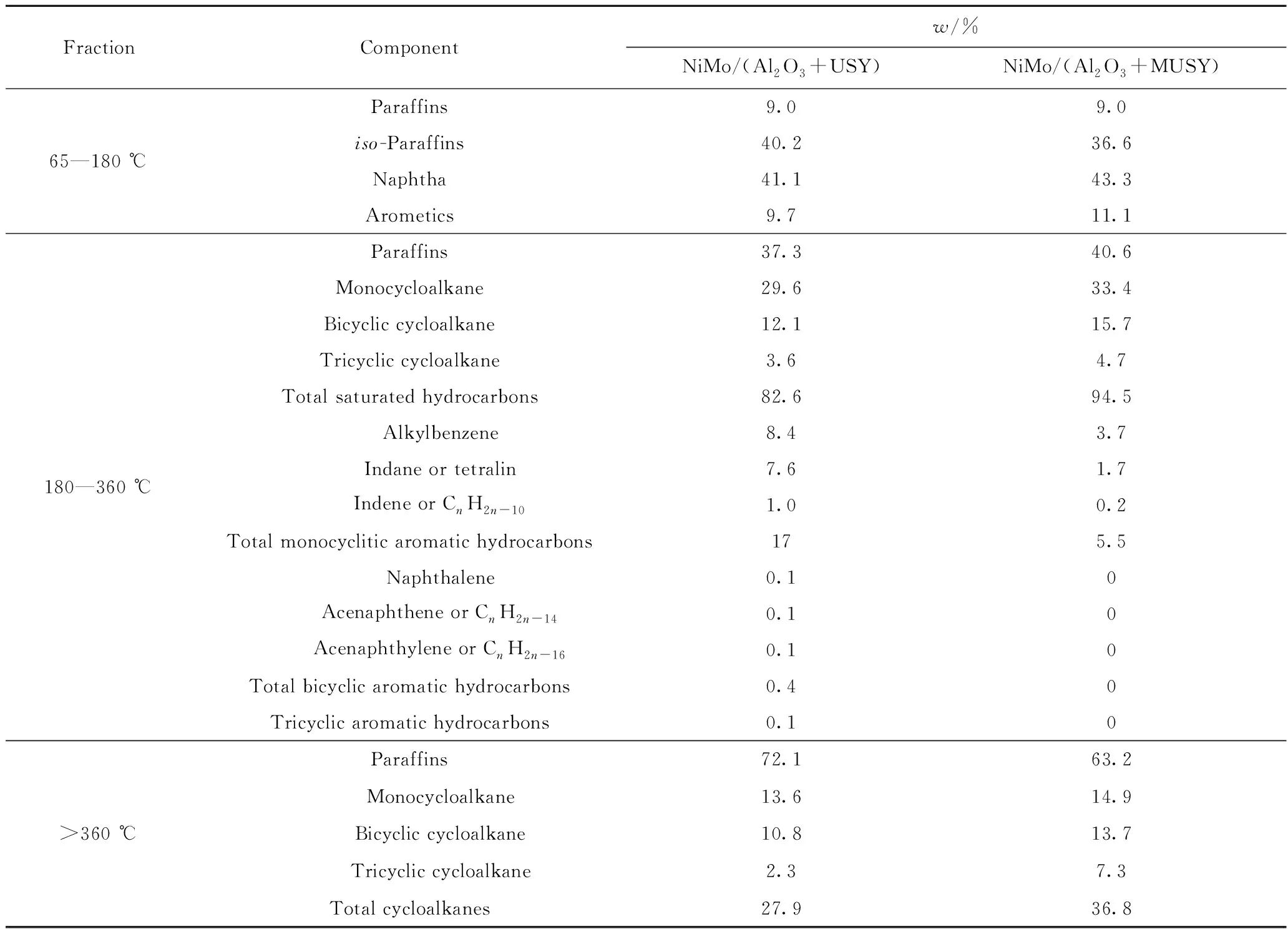
Table 7 Composition of various distillates under the action of two catalysts
3 Conclusions
(1) We reported a method to prepare MUSY with enhanced mesopores and acidity by low concentration alkali combined with low temperature thermal treatment of USY with lown(Si)/n(Al). This method can realize the effect of high concentration alkali solution in conventional alkali treatment methods.
(2) The MUSY (n(Si)/n(Al)=5.2) was derived by alkaline-thermal treating a parent USY (n(Si)/n(Al)=7). Compared with USY, the micropore volume and micro specific surface area were 0.20 cm3/g and 511.5 m2/g, which decreased by 31% and 34% respectively. The mesoporous volume and mesoporous specific surface area were 0.31 cm3/g and 150.5 m2/g, which increased by 63% and 50% respectively. A mesoporous structure with a pore diameter of 6 nm was created. Alkali treatment mainly removes silicon species from the framework, resulting in the loss of microporous structure and the increase of mesoporous structure. Then(Si)/n(Al) of MUSY was reduced to 5.2 and the acid amount determined by NH3-TPD was increased by 39%.
(3) The hydrocracking evaluation results of VGO show that NiMo/(Al2O3+MUSY) possessed higher activity, middle distillate selectivity and hydrogenation performance due to the enhanced mesoporosity and acidity of MUSY. The reaction temperature of catalyst containing MUSY was 8 ℃ lower than that of catalyst containing USY. The selectivity of middle distillate of catalyst containing MUSY was increased by about 2 percentages.
(4) Fluid catalytic cracking (FCC) and hydrocracking industrial units usually use cracking catalysts containing USY with lown(Si)/n(Al) to produce clean gasoline or naphtha. The lack of mesopores in USY leads to excessive cracking and carbon deposition. MUSY with enhanced mesoporosity may replace USY for preparing hydrocracking or FCC catalysts in the future. We anticipate that the method developed in this work will be applied to the synthetic or post-synthetic preparation of other hierarchically structured materials and to prepare functional materials with hybrid frameworks.

