High-humidity Sensor of a New Trinuclear Ti3-Oxo Cluster①
SUN Shi-Hao ZHANG Qian-Chong YE Xiao-Liang
KASHI Chivanje evulub LI Wen-Huab WANG Guan-Eb② XU Gangb
a (College of Chemistry and Materials Science, Fujian Normal University, Fuzhou 350007, China)
b (State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China)
ABSTRACT Crystalline polyoxo-titanium clusters (PTCs), as a molecular model of TiO2 nanomaterials, have attracted unprecedented attention due to their designable structure, tunable band gap, catalysis, and photochromic properties. A new trinuclear Ti3-oxo cluster, [Ti3(μ2-O)(μ3-O)(abz)6(OiPr)2]·CH3CN·H2O (Ti3), was synthesized by solvothermal method with a yield of 60% by using 4-aminobenzoic acid as ligand. Single-crystal X-ray diffraction shows that it has a [Ti3(μ2-O)(μ3-O)(abz)6(OiPr)2] trinuclear cluster structure. Ti3 crystallizes in monoclinic space group P21/c with a = 11.091(1), b = 22.837(2), c = 22.754(1) Å, β = 90.580(6)°, V = 5763.0(6) Å3, Z = 4, Dc =1.345 g·cm-3, F(000) = 2412, μ = 2.743 mm-1, R = 0.0796, and wR = 0.2260 (I > 2σ(I)). Ti3 shows typical semiconductive behavior determined by temperature-dependent conductivity test. The chemiresistive humidity sensor fabricated by Ti3 showed good performance, including high response (four orders of magnitude current change from 0 to 100% RH) and fast response time (160 s) and recovery time (26 s).
Keywords: polyoxo-titanium clusters, semiconductor, chemiresistive sensor, humidity sensor;
1 INTRODUCTION
Crystalline polyoxo-titanium clusters (PTCs) are still in their infancy stage and have a large room for development compared with other well-developed polyoxometalates[1].Owing to their precise structure, PTCs are a good bridge between TiO2nanoparticles and a precise molecular model. A 3.6 nm Ti52-oxo nanocluster was found byWei-Hui Fanget al[2]. A fullerene-like polyoxotitanium cage [Ti42(μ3-O)60(OiPr)42(OH)12]6-was also synthesized by Mei-Yan Gaoet al[3]. Bandgap engineering regulated based on Ti6core has been systematically studied by Jin Xiu Liuet al[4]. Xi Fanet al.reported the first pair of isomeric titanium-oxo clusters[Ti20(μ2-O)8(μ3-O)20(PA)14(8-OQ)10] and [Ti20(μ2-O)10(μ3-O)16(μ4-O)2(PA)14(8-OQ)10] with anatase model and explored their photocatalytic activity[5]. The photochromic behavior of[Ti6(μ3-O)2(PZ)4(TAZ)2(OiPr)14] and [Ti10(μ2-O)4(μ3-O)8-(PZ)12(OiPr)8] was also studied by Xi Fanet al[6]. Up to now,studies on PTCs are mainly focused on catalysis, photochromic, structure design, and band gap control[1,2,4,6-11]. A great challenge still remains for PTCs to be a gas sensor material with fast responsibility, high sensitivity, and good stability.
Humidity is one of the most commonly measured physical quantities. Humidity sensor has been used in hospitals, food processing, and other industries and fields[12-15]. At present,many types of humidity sensors have been developed, such as capacitance, impedance, mass-sensitive, and optical sensors.Resistance-type sensors are portable, cheap, and easy to design. Thus, this type of humidity sensor is the most popular one[16]. TiO2-based nanotubes[17], TiO2nanotubes[18], TiO2nanofibers[19], and TiO2slanted nanorod arrays[20]have been explored as humidity sense materials. Although many TiO2materials with different morphologies have been used for sensor materials, cluster-based sensors are rarely reported.Very rare titanium-oxo cluster-based sensors have been reported. Furthermore, owing to the lack of precise information of the adsorption model, exploring the sensing mechanism is still a great challenge. PTCs with a clear structure may become a good structural platform for mechanism investigation.
In this work, the synthesis and crystal structure of a new PTC, [Ti3(μ2-O)(μ3-O)(abz)6(OiPr)2]·CH3CN·H2O (Ti3), and its application in chemiresistive humidity sensor (Habz: 4-aminobenzoic acid;iPrOH: isopropanol) were explored. This work was the first to report a chemiresistive humidity sensor of titanium-oxo clusters. The direct current (DC) chemiresistive sensor of these PTCs showed excellent humidity sensing performance, with a high response of four orders of magnitude enhanced conductivity under 100% RH and fast response time (160 s) and recovery time (26 s).
2 EXPERIMENTAL
2. 1 Synthesis of the materials
All the reagents and solvents employed were purchased commercially and used as received without further treatment.Titanium isopropoxide was purchased from Adamas-beta,and 4-aminobenzoic acid was purchased from Aladdin.Acetonitrile was acquired from Sinopharm Chemical Reagent Beijing. 4-Aminobenzoic acid (0.634 g, 4.693 mmol) was dissolved in 8 mL acetonitrile, and titanium isopropoxide(312.5 µL, 1.057 mmol) was added quickly to obtain a red solution. Then, the red solution was sealed in a 25 mL glass bottle and heated at 85 °C for 3 days. After cooling to room temperature, red-rodlike crystals of Ti3 are obtained (Fig. 1a),washed with excess amount of acetonitrile, and dried under vacuum. The purity of the compounds was proven by powder X-ray diffraction (PXRD) (Fig. 1b). EA, Calcd.: H, 4.74; N,8.38; C, 51.34%. Found: H, 4.43; N, 7.75; C, 48.72%.

Fig. 1. (a) Crystal photo of Ti3; (b) Experiment PXRD of Ti3 and simulation
2. 2 Crystal structure determination
The structure data of Ti3 were collected on a Rigaku Hyoix (293 K) by using graphite-monochromated GaKαradiation (λ= 1.3405 Å). A total of 31351 reflections were collected for Ti3 red crystals, of which 9873 (Rint= 0.0362)were independent in the range of 2.38°≤θ≤53.22° by using anωscan mode. The structure was solved by direct methods and refined by full-matrix least-squares onF2by using SHELX2018 package. All non-hydrogen atoms were refined anisotropically except the O from water molecule. Hydrogen atoms were geometrically generated. The finalR= 0.0796 andwR= 0.2260 (w= 1/[σ2(Fo2) + (0.1138P)2+ 4.9551P],whereP= (Fo2+ 2Fc2)/3), (Δ/σ)max= 0.008,S= 1.025, (Δρ)max= 0.695 and (Δρ)min= -0.530 e/Å3. Selected bond lengths and bond angles are shown in Tables 1 and 2. The Ti-O bond lengths are from 1.821(3) to 2.046(3) Å and the bond angles fall in the 78.00(1)~127.88(8)° range.
2. 3 Characterization
The data of PXRD were acquired from a MiniFlex II diffractometer using CuKαradiation (λ= 1.540598 Å) at 30 kV and 15 mA. The simulated PXRD patterns of Ti3 were derived from the Mercury Version 3.9 software. UV-vis spectrum was collected on a PerkinElmer Lambda-950 UV/Vis/NIR spectrophotometer. Spectrally pure BaSO4was used as a background. The temperature-dependent I-V curves were measured by KEITH-LEY4200-SCS semiconductor characterization system. The electrode was made using silver paste and 50 μm-diameter gold wires by placing the pressed pellets of the samples between two electrodes.
Acetonitrile suspension liquid (40 mg/mL) was made using Ti3 powders. Then, 10 µL of the abovementioned liquid was dropped onto an Al2O3-based silver platinum interdigital electrode and dried at 60 °C for 24 h in air. The devices were used for humidity sensing performance test after drying at 60 °C for 48 h. Humidity sensing characterization was conducted using a home-made system previously reported[21,22]at room temperature. Different humidity levels were controlled by mixing dry air with 100% RH moisture in a closed quartz chamber. Dry air with a flow rate of 600 mL·min-1was purged for 5 min, followed by 5 min of different humidity gases for response. A bias voltage of 5 V was applied, and the current was recorded by a Keithley 2602B source meter.

Table 1. Selected Bond Lengths (Å) for Ti3
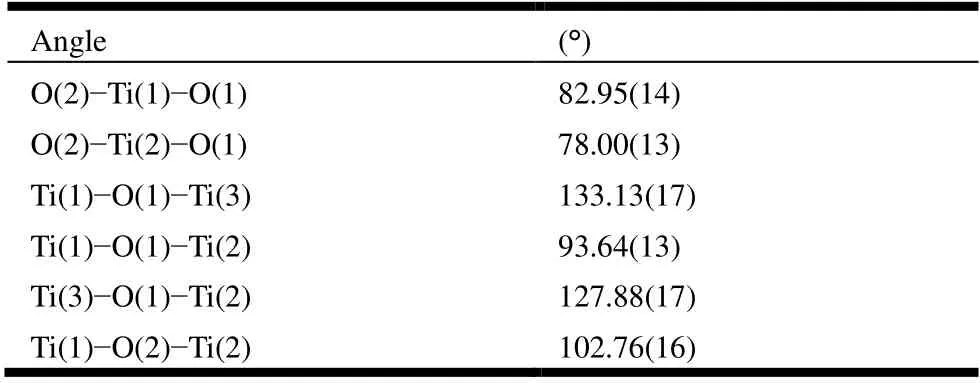
Table 2. Selected Bond Angles (°) for Ti3
3 RESULTS AND DISCUSSION
3. 1 Structure description
Single-crystal X-ray diffraction analysis revealed that Ti3 crystallizes in monoclinic groupP21/c. As shown in Fig. 1a,each Ti atom is coordinated by six O atoms to form an isolated octahedron. These octahedra are connected by bridged O atoms to form a Ti3-oxo cluster core. The O atoms on the terminal Ti3-oxo cluster core build five chelating 4-aminobenzoic acids, one 4-aminobenzoic acid, and two isopropanols(Fig. 2a). The Ti3-oxo cluster interacts with two neighboring ones through Van der Waals interactions to form a 1Dsupramolecular chain extending along theaaxis (Fig. 2b), and such 1Dchains are stacked in thebcplane to form a 3Dstructure (Fig. 2c).

Fig. 2. (a) Structure of the single cluster; (b) Extending along the a axis; (c) Packing model
3. 2 Spectrum analyses
Compared with the 4-aminobenzoic acid, the peak intensity at 1720~1706 cm-1(-C=O) was reduced for the coordination of Ti and carboxylic acid in the benzene ring.The peak at 3500~3100 cm-1for -NH remains, indicating that the amino group has not been coordinated to Ti (Fig. 3a).These results are consistent with the single-crystal structure through single-crystal X-ray diffraction. Band gaps (Fig. 3b)of Ti3 were 1.97 eV, smaller than that of pure TiO2.

Fig. 3. (a) FT-IR spectra of Ti3 and 4-aminobenzoic acid; (b) Solid-state UV-Vis diffuse spectrum of Ti3
3. 3 Thermal stability and semiconductive property
Ti3 exhibited a weight loss of approximately 5.68%(theoretical value: 5.05%) from 25 to 200 °C, corresponding to the occupancy of solvent and water molecules.The residual concentration was 21.50%, which may be TiO2(theoretical value: 20.05%, Fig. 4a). The I-V curves of Ti3 were tested in the range of 30~120 ℃. As shown in Fig. 4b, the conductivity at 30 ℃ was 2.79 × 10-11S/cm,which increased to 3.78 × 10-9S/cm at 120 ℃. The values and the trend of the conductivity increasing upon raising the temperature revealed its typical semiconductive property (Fig. 4c).

Fig. 4. (a) Thermogravimetric (TG) analysis curve;(b) I-V curves at different temperature; (c) Arrhenius plot at different temperature
3. 4 Humidity sensing
Humidity is the most common physical quantity used to express the content of water vapor in air. Preparing a highperformance humidity sensor remains a challenge. Several sensing devices were produced by dropping a suspension liquid of Ti3 to the interdigital electrode. As shown in Fig. 5a,the Ti3 showed humidity response in the broad RH range from 10% to 100%. The baseline current was 1 × 10-12A under dry air flow. The electrical current rapidly increased when they were exposed to humidity atmosphere, and then gradually reached a relatively stable value. The current dropped quickly back to the baseline current when the dry air was purged in. The value of response was calculated, and the sensing properties of the compound under different water concentrations were revealed. The sensor’s response value in detecting humidity is defined as the resistance ratio between dry air and humidity gas as follows[22]:

The response of Ti3 was 1166.11 at RH 100%, which was comparable to that in other metal oxides and metalorganic framework-based humidity sensors[23-26]. The repeating dynamic response of Ti3-based sensor to rapid variations in dry air and 60% RH is shown in Fig. 5b. The result indicated that the humidity-sensing process is extremely reversible. Response time is a very important parameter of gas sensor. The response of a single cycle(RH = 60%) was normalized to evaluate the response recovery level of the sensor. The response time (tresponse)was set to 90% of the maximum current value, and the recovery time (trecovery) was set to 10% of the maximum current value (Fig. 5c). The response and recovery time for Ti3 were 160 and 26 s, respectively.
H2O molecules are easy to adsorb/desorb on the surface of materials, especially with hydrophilic group, indicating that water is mainly gathered on the surface of sensing materials. This situation is beneficial for rapid response and recovery[27]. The mechanism of humidity sensing is mainly surface transmission mechanism. The resistance of sensing material is changed by H2O molecules, which gather on the surface of sense materials through chemical and physical adsorption. The resistance between electrode and materials was reduced by the introduction of surface H+, OH-, H3O+, and water. The grain boundary resistance and potential barrier may also be responsible. Instantaneous polarity reversal was applied on a DC circuit with an operating voltage of 1 V[28]to probe electronic and ionic contributions to the moisture-induced increase in electrical conduction (Fig. 5d). Therefore, the electronic mechanism may be the possible sensing mechanism in this work. When the DC voltage was applied onto the electrodes, the current decayed exponentially. Then, the currents finally stabilized at 0~3 orders of magnitude larger than the baseline value in accordance with different RH%values (Table 3).
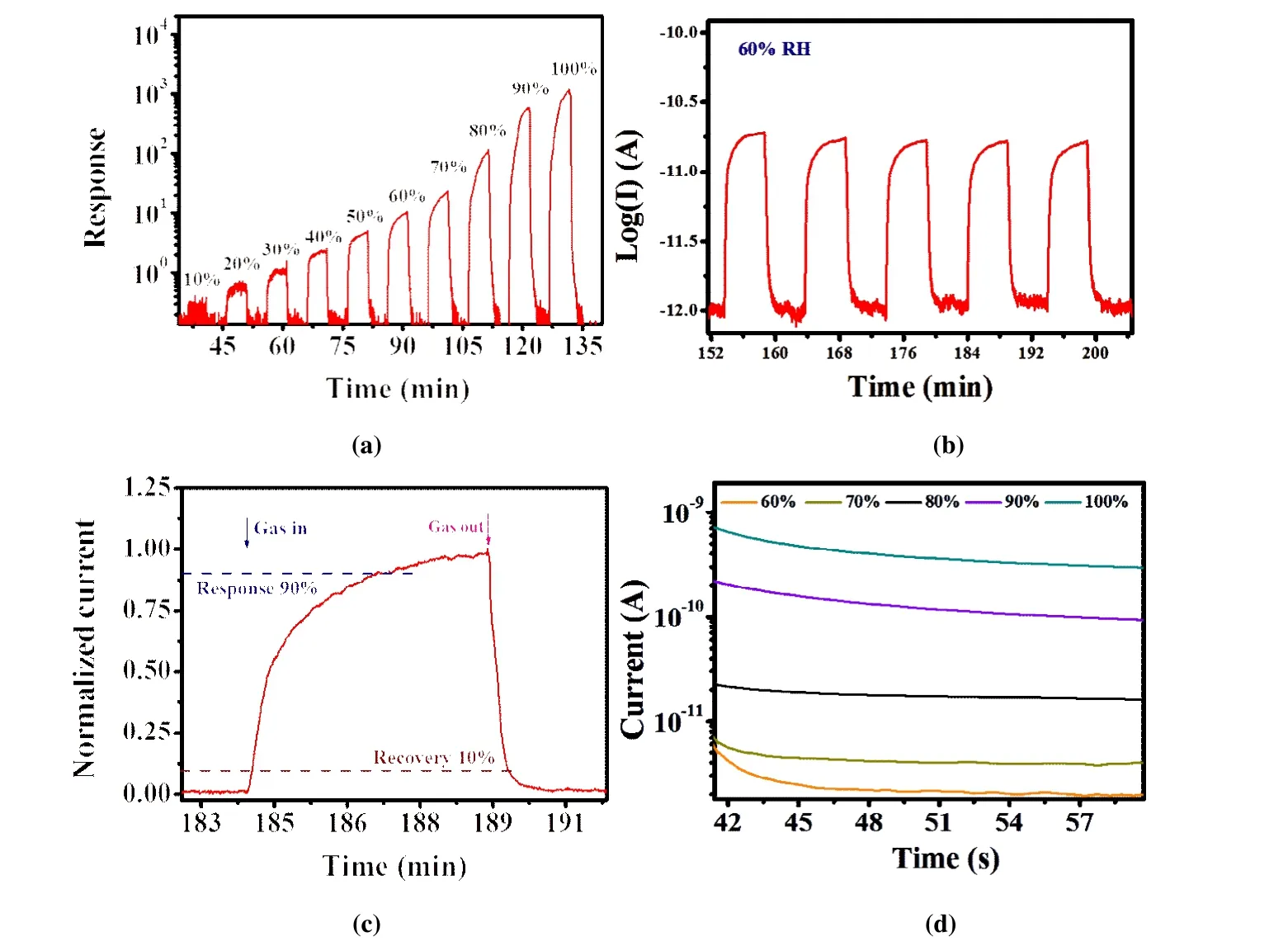
Fig. 5. (a) Response of Ti3 to different RH (10%~100%) at room temperature; (b) Response and recovery curve under 60% RH for 5 cycles;(c) Response and recovery time curve at 60% RH; (d) Curves of current vs. time of Ti3 based sensor at various RH obtained by the DC reverse polarity method

Table 3. Value of Response under Different RH% for Ti3
4 CONCLUSION
In summary, a titanium-oxo cluster semiconductive [Ti3(µ2-O)(µ3-O)(abz)6(OiPr)2]·CH3CN·H2O was prepared and characterized. The compound showed typical semiconductive behavior. The corresponding DC humidity sensor based on this compound exhibited fast response and recovery, together with a high response of four orders of magnitude at 100% RH,
5 AUTHOR CONTRIBUTIONS
All authors listed have made a substantial, direct, and which demonstrated its great potential for quantitatively detecting humidity.intellectual contribution to the work. The authors declare that they have no conflict of interest, and they approved this manuscript for publication.
- 结构化学的其它文章
- Structural and Electronic Properties of Lutetium Doped Germanium Clusters LuGen(+/0/-) (n = 6~19):A Density Functional Theory Investigation①
- Discovery of Benzimidazole Derivatives as Novel Aldosterone Synthase Inhibitors: QSAR, Docking Studies, and Molecular Dynamics Simulation①
- QSAR Models for Predicting Additive and Synergistic Toxicities of Binary Pesticide Mixtures on Scenedesmus Obliquus①
- Preparation, Crystal Structure and Fungicidal Activity of N-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)amide Compounds①
- Antibiotic Silver Particles Coated Graphene Oxide/polyurethane Nanocomposites Foams and Its Mechanical Properties①
- Planar Tetracoordinate Carbon in 6σ + 2π Double Aromatic CBe42- Derivatives①

