A Novel Salicylaldehyde Schiff-base Fluorescent Probe for Selective Detection of Cu2+ Ion①
LI Yuan-Da KUANG Jin-Hao QIN Hai-Bo LIU Ke-Xiang FAN Xu LI Zhong-Yan YUAN Lin
(Hunan University of Science and Engineering, Yongzhou 425199, China)
ABSTRACTA novel Schiff-base fluorescent probe 6,6′-((1E,1′E)-(ethane-1,2-diylbis(azaneylyliden e))bis-(methaneylylidene))bis(3-(diethylamino)phenol) (L) was derived from the 2:1 M condensation of 4-(diethylamino)-2-hydroxybenzaldehyde with ethylenediamine and characterized by 1H NMR, 13C NMR and FT-IR spectroscopies.The results of spectral analysis showed that the probe L is selective and sensitive to Cu2+. The detection limit of L is found to be 19 nmol·L-1. There is a good linear relationship between the fluorescence intensity of probe L and the concentration of Cu2+ in the range of 0 to 20 μmol⋅L-1. X-ray crystal structure of the L-Cu2+ complex and the Job plot revealed a 1:1 L-Cu2+ identification.
Keywords: Cu2+, salicylaldehyde, Schiff-base, fluorescent probe;
1 INTRODUCTION
Cu2+ion is a very important transition metal ion and has been widely used in the manufacturing industry. In animals and plants, Cu2+ion also plays a very important physiological role[1-3]. For example, in the human body, Cu2+ion is an essential trace metal ion, which is the second only to zinc ion and iron ion. Absence or excess of Cu2+ion can cause various diseases, such as osteoporosis, Parkinson’s disease, Alzheimer’s disease, et al.[4-7]. Therefore, quantitative detection of Cu2+ion is of great significance. Among many detection methods, the fluorescence sensor has attracted much attention due to its advantages such as good selectivity, high sensitivity, simple operation and real-time online detection. At present, the most researched fluorescent probes are usually divided into rhodamines, fused ring aromatic hydrocarbons, naphthimides,quinolines and borofluorodipyrrole[8-10]. Some of these probes are applied to the detection of Cu2+ion[11-14]. However, most of the fluorescent probes have the disadvantages of difficulty in synthesis or poor biocompatibility, which limits their further practical application.
Owing to the simple synthesis and good coordination ability,Schiff-base derivatives are often designed as fluorescent probes for detecting metal cations[15,16]. In this work, we have synthesized a novel Schiff-base fluorescent probe L from the 2:1 M condensation of 4-(diethylamino)-2-hydroxybenzaldehyde with ethylenediamine, as shown in Scheme 1. In addition, the possible fluorescent sensors and photochemical properties for certain metal ions have been explored for this Schiff-base L. The designed L exhibited strong yellow fluorescence (excitation: 393 nm, emission: 519 nm), and the fluorescence was quenched clearly by adding Cu2+ion. And L showed high selectivity and sensitivity for Cu2+without interference by many other common metal ions.

Scheme 1. Synthesis of Schiff-base L
2 EXPERIMENTAL
2. 1 Chemicals and instruments
All reagents and solvents for the synthesis and analysis were of analytical grade and used without further purification.Infrared (IR) spectra in the 4000~400 cm-1region were recorded on a Niconet AVATAR-360 spectrometer using KBr pellets.1H NMR and13C NMR were measured at 400 MHz using a Bruker AV400 spectrometer with CDCl3as the solvent and tetramethylsilane (TMS) as the internal standard.The crystallographic data were collected with a Bruker APEX II CCD area detector diffractometer using Mo-Kαradiation (λ= 0.71073 Å).
2. 2 Synthesis of compound L
A mixture of 4-(diethylamino)-2-hydroxybenzaldehyde (2 mmol), ethylenediamine (1 mmol) and anhydrous ethanol (20 mL) was heated at 80 ℃ and monitored by TLC. After the reaction is over, the reaction mixture was cooled to room temperature and a large amount of solid precipitates appeared.The crude product was filtered from the solvent, and then recrystallized from absolute ethanol to get light yellow crystal of L (0.35 g, yield: 85%).1H NMR(CDCl3, 400MHz)δ(ppm): 13.577 (br, 2H), 7.928 (s, 2H), 6.893 (d,J= 8.703 Hz, 2H), 6.053 (d,J= 8.785 Hz, 2H), 6.006 (s, 2H), 3.668 (s,4H), 3.276 (q,J= 21.163 Hz, 8H), 1.085 (t,J= 14.080 Hz,12H).13C NMR (CDCl3, 100MHz)δ(ppm): 165.034,163.259, 150.522, 132.026, 107.198, 102.007, 97.077, 58.971,43.432, 11.682. FT-IR: Selected IR data (KBr, cm-1): 3423.2,2971.9, 1614.2, 1452.7, 1284.5, 1036.8, 857.1, 703.3.
2. 3 UV-Vis and fluorescence spectra of L
The spectral analyses were done in ethanol solution at 25 ℃. The concentration of L was 16 μmol·L-1. Solutions of Ag+, K+, Na+, Ba2+, Ca2+, Co2+, Cu2+, Zn2+, Ni2+, Pb2+, Sn2+,Cr3+, Al3+and Fe3+ions were prepared with nitrate or hydrochloride salts in water (2 mmol·L-1). The excitation wavelength was 362 nm and the fluorescence emission spectra were recorded within the scope of 400~600 nm.
2. 4 Single-crystal diffraction studies
In order to study the binding mode of L and Cu2+ion, the crystal of complex L-Cu2+was prepared. The crystallographic data for L-Cu2+were collected with a Bruker APEX II CCD area detector diffractometer using Mo-Kαradiation (λ=0.71073 Å). Data collections, cell refinements, data reductions and absorption corrections were performed using multi-scan methods with Bruker software[17]. The structures were solved by direct methods using SIR2004[18]. The non-hydrogen atoms were refined anisotropically by full-matrix least-squares method onF2using SHELXL[19]. Molecular graphics were prepared with the Olex2 program[20].
3 RESULTS AND DISCUSSION
3. 1 Synthesis
As shown in Scheme 1, compound L was obtained by the condensation reaction between tris base with 4-(diethylamino)-2-hydroxybenzaldehyde in ethanol at 80 ℃ with a satisfactory yield. The Schiff-base L is light yellow powder,stable in air and soluble in the most common organic solvents such as MeOH, EtOH, CHCl3, DMSO and DMF. In the1H NMR spectra, the signal for the imine proton in L appears at 7.928 ppm, as a singlet peak. The1H NMR spectra of L exhibit -OHphenolicproton resonances at 13.577 ppm in turn,which suggests that here the -OHphenolictakes along some positive charges.

Fig. 1. UV-Vis spectra of L and L-Cu2+

Fig. 2. Fluorescence spectra of L (16 μmol/L) in the presence of different concentrations of Cu2+ (from 0 to 20 μmol·L-1)
3. 2 Spectral characterization
The UV-Vis spectra of L and L-Cu2+were measured. As shown in Fig. 1, the maximum absorption wavelength of L was red-shifted from 413 to 455 nm, with adding Cu2+ion.To get insight into fluorescence intensity changes with the increase of Cu2+concentration, the fluorescence spectra changes of L towards Cu2+were measured. As shown in Fig. 2,upon the addition of Cu2+to the solution of L, the fluorescence spectra at 519 nm clearly quenched under the excitation of 393 nm. Moreover, there was a good linear relationship between the fluorescence intensity of probe L and the concentration of Cu2+in the range of 0 to 20μmol·L-1.According to the reported definition (LOD = 3σ/k), the detection limit of L for Cu2+was found to be 19 nmol·L-1.The fluorescence spectra results proved that L has a high sensitivity toward Cu2+.
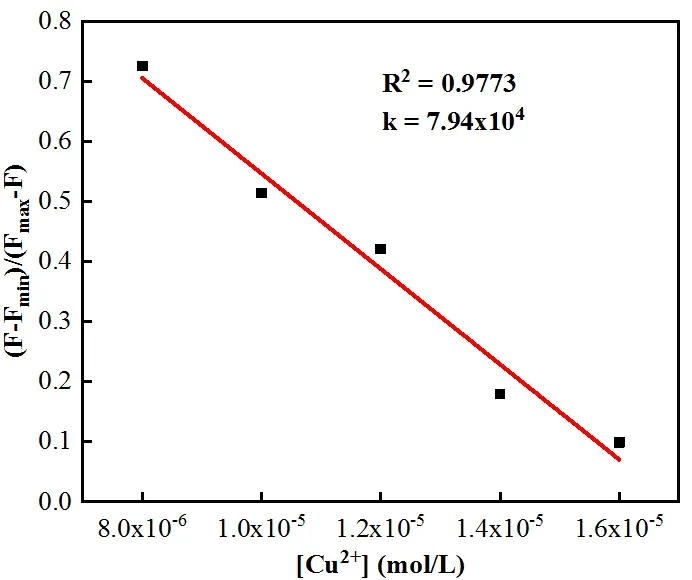
Fig. 3. Benesi-Hildebrand plot from fluorescence titration data of L with Cu2+
The binding constantKof L-Cu2+was calculated according to the Benesi-Hildebrand equation. Depending on the slope, the results obtained wereKL= 7.94 × 104L·mol-1,indicating that L had a great binding affinity to Cu2+(Fig. 3).In order to study the binding of L with Cu2+, the Job plot was measured by using a total concentration of 16 μmol·L-1L and Cu2+, and the result indicated that the combination of L and Cu2+is 1:1 stoichiometry (Fig. 4).OH group in L disappeared from 3423 cm-1, with adding the Cu2+ion, which indicated that OH was deprotonated. By combining the above information, the binding mode of L and Cu2+was speculated, as shown in Fig. 5b.
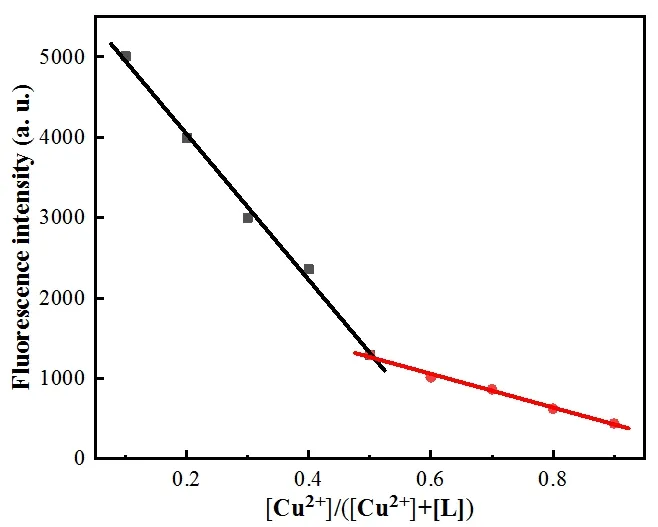
Fig. 4. Job-plot for Cu2+ and L showing 1:1 stoichiometry

Fig. 5. (a) Infrared spectra of L and L-Cu2+, (b) Possible binding mode of L-Cu2+
To further understand the fluorescent property of L with Cu2+, the fluorescence response behaviors of L to various metal ions were investigated (Fig. 6). The results showed no obvious changes in the fluorescence intensity after adding other metal ions to L solution, including Ag+, K+, Na+, Ba2+,Ca2+, Zn2+, Ni2+, Pb2+, Sn2+, Cr3+, Al3+and Fe3+. However,fluorescence intensities declined with adding Co2+possibly because of the heavy metal effect. Moreover, the competitive experiments proved that the presence of metal ions, such as Ag+, K+, Na+, Ba2+, Ca2+, Co2+, Zn2+, Ni2+, Pb2+, Sn2+, Cr3+,Al3+and Fe3+ions, did not interfere with the quenched fluorescence. The results indicated that L has good sensibility and selectivity toward Cu2+.
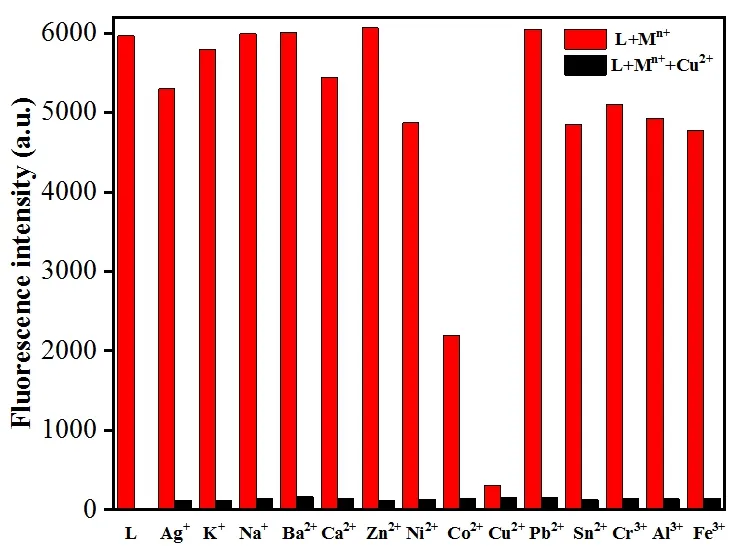
Fig. 6. Relative emission of L (16 μmol/L) and its complexation with Cu2+ (32 μmol/L) in the presence of different metal ions (32 μmol/L). The response of L on its own is used as a control
3. 3 Crystal structure
The crystal of Schiff-base L-Cu2+was cultivated to further confirm the real binding mode of L and Cu2+ion. The molecular structure of L-Cu2+is shown in Fig. 7. It is obvious that L and Cu2+ion are combined according to the ratio of 1:1. Cu and N(1), N(2), O(1), O(2) form four coordinate bonds, with the H atom of the phenol group disappearing.

Fig. 7. ORTEP representation of L-Cu2+
4 CONCLUSION
In summary, one novel Schiff-base probe L was developed as efficient chemical sensor for the highly selective detection of Cu2+. The maximum absorption wave length of L was redshifted from 413 to 455 nm, with adding Cu2+ion. After the addition of Cu2+ion, the fluorescence quenching of L was observed at 519 nm under the excitation of 393 nm, which did not take effect for other metal ions. Probe L is highly selective for Cu2+detection, which may be attributed to the fact that the Jahn-Teller deformation of Cu2+complexes provides excellent thermodynamic stability among all metal cations.The job plot revealed that the binding ratio of probe L to Cu2+ion is 1:1, which could be further confirmed by the crystal structure of the complex L-Cu2+. Cu2+ion detection limit of probe L was measured to be 19 nmol·L-1. Our group job herein could provide certain new insights for exploring highly selective and sensitive Schiff-base fluorescent sensors.
- 结构化学的其它文章
- Structural and Electronic Properties of Lutetium Doped Germanium Clusters LuGen(+/0/-) (n = 6~19):A Density Functional Theory Investigation①
- Discovery of Benzimidazole Derivatives as Novel Aldosterone Synthase Inhibitors: QSAR, Docking Studies, and Molecular Dynamics Simulation①
- QSAR Models for Predicting Additive and Synergistic Toxicities of Binary Pesticide Mixtures on Scenedesmus Obliquus①
- Preparation, Crystal Structure and Fungicidal Activity of N-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)amide Compounds①
- Antibiotic Silver Particles Coated Graphene Oxide/polyurethane Nanocomposites Foams and Its Mechanical Properties①
- Planar Tetracoordinate Carbon in 6σ + 2π Double Aromatic CBe42- Derivatives①

