洋金花叶化学成分及其抗炎活性研究
刘艳荣晓惠谭金燕潘娟管伟匡海学杨炳友*
(1.黑龙江中医药大学,教育部北药基础与应用研究重点实验室,黑龙江 哈尔滨150040; 2.山西中医药大学,山西 晋中030619)
洋金花Datura metelL.为茄科曼陀罗属植物,始载于《本草纲目》,其花入药,具有定喘,祛风,麻醉止痛的功效[1],药用历史悠久,临床应用广泛,但其生长周期长、产量低造成资源紧张。洋金花叶具有抗炎[2]、抗菌、抗氧化[3]、抑制免疫[4]等多种生物活性,并含可治疗银屑病的醉茄内酯类成分,其含量约为洋金花中的3.5倍[5]。为了充分利用洋金花植物资源,本实验对洋金花叶化学成分及抗炎活性进行研究,共分离鉴定出28 个化合物,其中化合物15~17、20、23、28 为首次从该茄科植物中分离得到,2、19、24、27 首次从曼陀罗属植物中分离得到,9、20 表现出抗炎活性。
1 材料
Waters 2695-2998-2414 分析高效液相色谱仪(美国Waters 公司);CBM-20A 半制备HPLC 色谱仪(日本岛津公司);Bruker-400/600 超导核磁共振光谱仪(德国Bruker 公司);UHPLC-Orbitrap-MS 质谱系统(美国Thermo Fisher Scientific 公司);旋转蒸发仪(日本东京理化器械株式会社);Sepacore 型中压液相色谱仪(瑞士Buchi 公司)。GF254 型薄层硅胶(烟台江友硅胶开发有限公司);柱层色谱用硅胶(80~100、200~300目,青岛海洋化工厂);柱用ODS(12 nm,s-50 μm,日本YMC 公司)。RAW264.7 细胞系(武汉大学细胞保藏中心);FBS 胎牛血清(以色列BI 公司);LPS(美国Sigma 公司)。洋金花叶于2017 年9 月采收于哈尔滨双城区,经黑龙江中医药大学药学院药用植物教研室樊锐锋教授鉴定为茄科曼陀罗属植物白花曼陀罗Datura metelL.的叶,标本(20170901)保存于黑龙江中医药大学中药化学教研室。色谱层析用分析纯试剂(天津试剂一厂);色谱纯甲醇(德国Merck 公司)。
2 提取与分离
将20 kg 干燥洋金花叶以8 倍量70% 乙醇回流提取3次,每次2 h,滤过,合并提取液,减压浓缩得70%乙醇总提物4.82 kg,经HP-20 大孔吸附树脂,分别用水、30%乙醇、95%乙醇洗脱后减压浓缩,分别得水洗脱组分930 g、30%乙醇洗脱组分1 195 g、95%乙醇洗脱组分1 894 g。
取30%乙醇洗脱组分400 g,经硅胶柱[二氯甲烷-甲醇(1∶0~0∶1)]梯度洗脱,洗脱液浓缩后经TLC 薄层检识,得到Fr.A~Fr.I 流分。其中,Fr.B 经硅胶柱、半制备型HPLC(甲醇-水,58∶42,3 mL/min)得化合物15(21.8 mg,tR=43.1 min);Fr.D 经硅胶柱、ODS 柱、半制备型HPLC(甲醇-水,60∶40,3 mL/min)得化合物16(3.1 mg,tR=18.8 min)、23(2.1 mg,tR=19.3 min);Fr.F 经硅胶柱、半制备型HPLC(甲醇-水,40∶60,3 mL/min)得化合物25(2.6 mg,tR=15.4 min)、27(3.3 mg,tR=14.8 min)、28(4.3 mg,tR=15.2 min);Fr.G 经硅胶柱、ODS 柱、半制备型HPLC(甲醇-水,35∶65,3 mL/min)得化合物24(2.4 mg,tR=16.4 min);Fr.H 经硅胶柱、半制备型HPLC(甲醇-水,50∶50,3 mL/min)得化合物22(1.9 mg,tR=15.6 min)。
取95%乙醇洗脱组分400 g,经硅胶柱[二氯甲烷-甲醇(100∶1~0∶1)]梯度洗脱,洗脱液浓缩后经TLC 薄层检识,得到Fr.A~Fr.F 流分。其中,Fr.B 经硅胶柱、半制备型HPLC(甲醇-水,60∶40,3 mL/min)得化合物1(20.2 mg,tR=45.2 min)、2(2.5 mg,tR=40.8 min);Fr.D 经硅胶柱、ODS 柱、半制备型HPLC(甲醇-水,65∶35,3 mL/min)得化合物3(30.1 mg,tR=45.9 min)、8(2.5 mg,tR=40.5 min)、10(12.9 mg,tR=47.2 min)、11(14.8 mg,tR=41.9 min)、12(22.6 mg,tR=43.6 min)、14(16.8 mg,tR=49.6 min)、18(2.8 mg,tR=38.3 min)、20(2.2 mg,tR=36.2 min)、21(15.0 mg,tR=34.6 min);Fr.E 经硅胶柱、ODS 柱、半制备型HPLC(甲醇-水,60∶40,3 mL/min)得化合物4(7.0 mg,tR=44.4 min)、6(4.1 mg,tR=45.9 min)、7(2.3 mg,tR=48.0 min)、13(7.8 mg,tR=49.9 min)、17(2.1 mg,tR=42.3 min)、19(1.0 mg,tR=38.1 min);Fr.F 经硅胶柱、半制备型HPLC(甲醇-水,65∶35,3 mL/min)得化合物5(4.1 mg,tR=44.3 min)、9(3.2 mg,tR=49.4 min)、26(0.7 mg,tR=50.4 min)。
3 结构鉴定
化合物1:白色无定形粉末(甲醇),分子式C28H38O5;HR-ESI-MSm/z477.260 6 [M +Na]+。1H-NMR(CD3OD,400 MHz)δ:6.92(1H,ddd,J=10.4,5.2,2.8 Hz,H-3),5.85(1H,m,H-2),5.79(1H,dd,J=6.0,2.0 Hz,H-6),4.47(1H,dt,J=13.2,3.2 Hz,H-22),4.38(1H,d,J=11.6 Hz,H-27a),4.31(1H,d,J=11.6 Hz,H-27b),3.79(1H,t,J=4.4 Hz,H-7),3.41(1H,br.d,J=21.2 Hz,H-4a),2.94(1H,dd,J=21.2,5.2 Hz,H-4b),2.56(1H,dd,J=18.0,13.2 Hz,H-23a),2.23(1H,m,H-11a),2.20(1H,dd,J=18.0,3.2 Hz,H-23b),2.10(3H,s,H-28),1.99(3H,m,H-9,12a,20),1.81(2H,m,H-15a,16a),1.60(1H,m,H-11b),1.45(1H,m,H-8),1.40(1H,m,H-16b),1.35(1H,m,H-12b),1.30(2H,m,H-14,17),1.25(3H,s,H-19),1.19(1H,m,H-15b),1.06(3H,d,J=6.8 Hz,H-21),0.79(3H,s,H-18);13C-NMR(CD3OD,100 MHz)δ:205.6(C-1),168.6(C-26),157.9(C-24),147.6(C-3),141.6(C-5),128.4(C-2),127.5(C-6),126.4(C-25),80.2(C-22),64.8(C-7),56.4(C-27),53.2(C-17),52.3(C-10),51.0(C-14),43.5(C-13),40.8(C-12),40.5(C-20),39.5(C-8),36.4(C-9),34.4(C-4),30.7(C-23),28.2(C-16),25.0(C-15),24.6(C-11),20.2(C-28),18.7(C-19),13.7(C-21),12.1(C-18)。与文献[6]报道一致,故鉴定为 7,27-dihydroxy-1-oxowitha-2,5,24-trienolide。
化合物2:白色无定形粉末(甲醇),分子式C28H40O6;HR-ESI-MSm/z495.271 5 [M+Na]+。1HNMR(CD3OD,400 MHz)δ:6.65(1H,ddd,J=10.4,5.2,2.4 Hz,H-3),5.77(1H,dd,J=10.4,2.4 Hz,H-2),4.46(1H,dt,J=13.2,3.2 Hz,H-22),4.38(1H,d,J=11.6 Hz,H-27a),4.31(1H,d,J=11.6 Hz,H-27b),3.52(1H,t,J=2.8 Hz,H-6),3.26(1H,dt,J=20.0,2.8 Hz,H-4a),2.55(1H,dd,J=17.6,13.6 Hz,H-23a),2.20(1H,dd,J=17.6,2.8 Hz,H-23b),2.20(1H,overlap,H-11a),2.10(3H,s,H-28),2.04(1H,dd,J=20.0,5.0 Hz,H-4b),1.97(2H,m,H-12a,20),1.78(3H,m,H-9,15a,16a),1.67(2H,m,H-7a,8),1.54(1H,m,H-7b),1.40(1H,m,H-15b),1.38(1H,m,H-16b),1.36(2H,m,H-11b,12b),1.30(3H,s,H-19),1.27(1H,m,H-17),1.20(1H,m,H-14),1.04(3H,d,J=6.8 Hz,H-21),0.80(3H,s,H-18);13CNMR(CD3OD,100 MHz)δ:207.6(C-1),168.6(C-26),157.9(C-24),143.8(C-3),129.0(C-2),126.4(C-25),80.2(C-22),78.3(C-5),75.3(C-6),57.1(C-14),56.4(C-27),53.4(C-17),53.0(C-10),44.2(C-13),42.6(C-9),41.5(C-12),40.5(C-20),36.6(C-4),34.1(C-7),31.4(C-8),30.7(C-23),28.2(C-16),25.4(C-15),24.5(C-11),20.2(C-28),16.2(C-19),13.7(C-21),12.7(C-18)。与文献[7]报道一致,故鉴定为acnistoferin。
化合物3:白色无定形粉末(甲醇),分子式C28H42O6;HR-ESI-MSm/z497.287 1 [M+Na]+。1HNMR(CD3OD,400 MHz)δ:5.71(1H,d,J=5.3 Hz,H-6),4.47(1H,dt,J=13.2,3.4 Hz,H-22),4.38(1H,d,J=11.7 Hz,H-27a),4.31(1H,d,J=11.7 Hz,H-27b),3.91(1H,m,H-3),3.82(1H,s,H-1),3.72(1H,t,J=3.8 Hz,H-7),2.54(1H,dd,J=17.9,13.4 Hz,H-23a),2.33(2H,m,H-4),2.20(1H,dd,J=17.9,3.4 Hz,H-23b),2.10(3H,s,H-28),2.00(3H,m,H-2a,12a,20),1.90(1H,m,H-8),1.82(2H,m,H-15a,16a),1.72(1H,m,H-2b),1.57(2H,m,H-11a,14),1.53(1H,m,H-11b),1.45(1H,m,H-9),1.41(1H,m,H-16b),1.25(2H,m,H-12b,15b),1.19(1H,m,H-17),1.04(3H,d,J=6.6 Hz,H-21),1.01(3H,s,H-19),0.78(3H,s,H-18);13C-NMR(CD3OD,100 MHz)δ:168.6(C-26),157.9(C-24),144.3(C-5),127.3(C-6),126.4(C-25),80.2(C-22),73.5(C-1),66.7(C-3),65.9(C-7),56.4(C-27),53.2(C-17),50.7(C-14),43.7(C-13),43.3(C-10),42.4(C-4),40.5(C-12),40.4(C-20),39.1(C-2),38.9(C-9),35.1(C-8),30.7(C-23),28.3(C-16),25.1(C-15),21.0(C-11),20.2(C-28),18.8(C-19),13.8(C-21),12.0(C-18)。与文献 [4 ]报道一致,故鉴定为baimantuoluoline K。
化合物4:白色无定形粉末(甲醇),Molish反应呈阳性,分子式C35H52O11;HR-ESI-MSm/z671.340 0 [M+Na]+。1H-NMR(CD3OD,600 MHz)δ:5.72(1H,d,J=4.3 Hz,H-6),4.62(1H,d,J=11.2 Hz,H-27a),4.49(1H,dt,J=13.4,3.4 Hz,H-22),4.46(1H,d,J=11.2 Hz,H-27b),4.32(1H,d,J=7.8 Hz,H-1′),3.85(2H,m,H-3,6′a),3.72(1H,m,H-7),3.67(1H,dd,J=11.9,5.5 Hz,H-6′b),3.32(1H,m,H-3′),3.27(1H,m,H-4′),3.28(3H,s,3-OCH3),3.24(1H,m,H-5′),3.16(1H,t,J=8.1 Hz,H-2′),2.99(1H,dd,J=14.0,3.5 Hz,H-2a),2.87(1H,brd,J=14.9 Hz,H-4a),2.57(1H,dd,J=18.0,13.6 Hz,H-23a),2.51(2H,m,H-2b,4b),2.22(1H,dd,J=18.0,3.2 Hz,H-23b),2.13(3H,s,H-28),2.00(1H,m,H-9),1.98(2H,m,H-12a,20),1.84(3H,m,H-11a,15a,16a),1.55(1H,m,H-14),1.45(1H,m,H-11b),1.41(1H,m,H-8),1.38(1H,m,H-16b),1.30(3H,s,H-19),1.28(2H,m,H-12b,17),1.19(1H,m,H-15b),1.04(3H,d,J=6.6 Hz,H-21),0.77(3H,s,H-18);13C-NMR(CD3OD,150 MHz)δ:212.9(C-1),168.6(C-26),160.4(C-24),142.9(C-5),128.4(C-6),123.6(C-25),104.0(C-1′),80.2(C-22),78.3(C-3),78.0(C-3′,5′),75.0(C-2′),71.6(C-4′),65.2(C-7),63.6(C-27),62.7(C-6′),56.2(3-OCH3),55.4(C-10),53.2(C-17),50.8(C-14),43.7(C-2,13),40.6(C-12),40.4(C-20),38.7(C-8),36.7(C-4),36.0(C-9),30.8(C-23),28.2(C-16),25.0(C-15),23.5(C-11),20.7(C-28),19.2(C-19),13.7(C-21),12.1(C-18)。与文献 [2]报道一致,故鉴定为daturafoliside G。
化合物5:白色无定形粉末(甲醇),Molish反应呈阳性,分子式C38H58O11;HR-ESI-MSm/z713.387 1 [M+Na]+。1H-NMR(CD3OD,600 MHz)δ:5.73(1H,d,J=5.6 Hz,H-6),4.63(1H,d,J=11.2 Hz,H-27a),4.52(1H,m,H-22),4.47(1H,d,J=11.2 Hz,H-27b),4.33(1H,d,J=7.8 Hz,H-1′),3.94(1H,t,J=2.9 Hz,H-3),3.86(1H,dd,J=11.9,1.7 Hz,H-6′a),3.74(1H,t,J=3.8 Hz,H-7),3.68(1H,dd,J=11.9,5.4 Hz,H-6′b),3.43(1H,m,H-1″a),3.40(1H,m,H-3′),3.34(1H,m,H-1″b),3.28(2H,m,H-4′,5′),3.17(1H,t,J=7.8 Hz,H-2′),2.98(1H,dd,J=13.9,3.7 Hz,H-2a),2.87(1H,br.d,J=14.8 Hz,H-4a),2.58(1H,dd,J=17.8,13.8 Hz,H-23a),2.48(2H,m,H-2b,4b),2.23(1H,dd,J=17.8,2.9 Hz,H-23b),2.14(3H,s,H-28),2.03(1H,m,H-9),2.00(1H,m,H-20),1.98(1H,m,H-12a),1.83(3H,m,H-11a,15a,16a),1.56(1H,m,H-14),1.49(1H,m,H-2″a),1.46(1H,m,H-11b),1.45(2H,m,H-8,2″b),1.40(1H,m,H-16b),1.36(1H,m,H-3″a),1.32(2H,m,H-3″b,12b),1.31(3H,s,H-19),1.25(1H,m,H-17),1.20(1H,m,H-15b),1.05(3H,d,J=6.6 Hz,H-21),0.92(3H,t,J=7.4 Hz,H-4″),0.78(3H,s,H-18);13C-NMR(CD3OD,150 MHz)δ:213.2(C-1),168.6(C-26),160.4(C-24),143.0(C-5),128.3(C-6),123.6(C-25),104.0(C-1′),80.2(C-22),78.0(C-3′,5′),76.8(C-3),75.0(C-2′),71.6(C-4′),69.5(C-1″),65.3(C-7),63.6(C-27),62.7(C-6′),55.4(C-10),53.1(C-17),50.8(C-14),44.2(C-2),43.7(C-13),40.5(C-12),40.4(C-20),38.6(C-8),37.1(C-4),36.1(C-9),32.8(C-2″),30.8(C-23),28.2(C-16),25.0(C-15),23.4(C-11),20.7(C-28),20.3(C-3″),19.1(C-19),14.2(C-4″),13.7(C-21),12.1(C-18)。与文献[2]报道一致,故鉴定为daturafoliside H。
化合物6:白色无定形粉末(甲醇),Molish反应呈阳性,分子式C34H48O10;HR-ESI-MSm/z639.313 9 [M+Na]+。1H-NMR(CD3OD,600 MHz)δ:6.08(1H,dd,J=9.7,1.9 Hz,H-4),5.78(1H,m,H-3),5.52(1H,d,J=2.2 Hz,H-6),4.62(1H,d,J=11.2 Hz,H-27a),4.50(1H,dt,J=13.3,3.4 Hz,H-22),4.46(1H,d,J=11.2 Hz,H-27b),4.32(1H,d,J=8.0 Hz,H-1′),3.85(1H,dd,J=11.9,2.0 Hz,H-6′a),3.79(1H,d,J=8.3 Hz,H-7),3.67(1H,dd,J=11.9,5.4 Hz,H-6′b),3.41(1H,m,H-3′),3.26(2H,m,H-4′,5′),3.16(1H,t,J=8.0 Hz,H-2′),2.68(1H,dd,J=20.0,4.7 Hz,H-2a),2.57(1H,dd,J=18.0,13.3 Hz,H-23a),2.21(1H,dd,J=18.0,3.4 Hz,H-23b),2.13(3H,s,H-28),1.98(2H,m,H-12a,20),1.96(1H,m,H-2b),1.88(3H,m,H-11,15a),1.81(2H,m,H-9,16a),1.52(2H,m,H-8,11b),1.42(3H,s,H-19),1.40(2H,m,H-15b,16b),1.34(1H,m,H-12b),1.29(1H,m,H-14),1.24(1H,m,H-17),1.04(3H,d,J=6.6 Hz,H-21),0.80(3H,s,H-18);13C-NMR(CD3OD,150 MHz)δ:212.1(C-1),168.6(C-26),160.3(C-24),143.4(C-5),131.7(C-6),129.5(C-4),125.6(C-3),123.7(C-25),104.0(C-1′),80.2(C-22),78.0(C-3′,5′),75.0(C-2′),72.4(C-7),71.6(C-4′),63.6(C-27),62.7(C-6′),57.1(C-14),53.2(C-10),52.7(C-17),44.7(C-13),41.4(C-8),41.1(C-9),40.8(C-2),40.7(C-12),40.4(C-20),30.8(C-23),28.5(C-16),27.4(C-15),23.8(C-11),20.8(C-28),20.5(C-19),13.8(C-21),12.4(C-18)。与文献[2]报道一致,故鉴定为daturafoliside I。
化合物7:白色无定形粉末(甲醇),Molish反应呈阳性,分子式C35H50O10;HR-ESI-MSm/z653.332 7 [M+Na]+。1H-NMR(CD3OD,600 MHz)δ:6.08(1H,dd,J=9.9,2.5 Hz,H-7),5.71(1H,br.s,H-6),5.69(1H,br.s,H-4),4.63(1H,d,J=11.3 Hz,H-27a),4.49(1H,m,H-22),4.46(1H,d,J=11.3 Hz,H-27b),4.32(1H,d,J=7.8 Hz,H-1′),4.31(1H,m,H-3),3.86(1H,dd,J=12.0,2.1 Hz,H-6′a),3.67(1H,dd,J=12.0,5.2 Hz,H-6′b),3.35(3H,s,3-OCH3),3.34(1H,m,H-3′),3.28(1H,m,H-4′),3.24(1H,m,H-5′),3.15(1H,t,J=7.8 Hz,H-2′),3.01(1H,dd,J=13.4,5.8 Hz,H-2a),2.56(1H,m,H-23a),2.55(1H,m,H-2b),2.23(1H,m,H-23b),2.15(1H,m,H-8),2.12(3H,s,H-28),2.01(1H,m,H-12a),2.00(1H,m,H-20),1.83(2H,m,H-15a,16a),1.67(1H,m,H-11a),1.52(1H,m,H-9),1.44(1H,m,H-16b),1.43(1H,m,H-11b),1.33(1H,m,H-15b),1.29(1H,m,H-14),1.28(1H,m,H-17),1.26(1H,m,H-12b),1.19(3H,s,H-19),1.02(3H,d,J=6.6 Hz,H-21),0.81(3H,s,H-18);13C-NMR(CD3OD,150 MHz)δ:214.1(C-1),168.6(C-26),160.3(C-24),144.9(C-5),132.7(C-6),128.3(C-7),125.8(C-4),123.6(C-25),104.0(C-1′),80.0(C-22),78.0(C-3′,5′),77.1(C-3),75.0(C-2′),71.6(C-4′),63.6(C-27),62.7(C-6′),56.2(3-OMe),55.1(C-14),53.0(C-17),50.4(C-10),47.0(C-9),44.9(C-13),44.7(C-2),40.8(C-12),40.4(C-20),38.4(C-8),30.8(C-23),28.2(C-16),25.1(C-15),23.8(C-11),20.7(C-28),18.1(C-19),13.6(C-21),12.3(C-18)。与文献[5]报道一致,故鉴定为daturafoliside R。
化合物8:白色无定形粉末(甲醇),分子式C28H38O6;HR-ESI-MSm/z493.255 8 [M+Na]+。1HNMR(CD3OD,600 MHz)δ:7.07(1H,dd,J=9.6,6.0 Hz,H-3),6.23(1H,J=6.0 Hz,H-4),5.96(1H,d,J=9.6 Hz,H-2),4.45(1H,dt,J=13.4,3.4 Hz,H-22),4.37(1H,d,J=11.8 Hz,H-27a),4.30(1H,d,J=11.8 Hz,H-27b),4.22(1H,d,J=3.2 Hz,H-6),3.73(1H,t,J=2.8 Hz,H-7),2.52(1H,dd,J=18.0,13.4 Hz,H-23a),2.16(1H,dd,J=18.0,3.4 Hz,H-23b),2.08(3H,s,H-28),2.06(1H,m,H-8),2.00(1H,m,H-12a),1.96(2H,m,H-11a,20),1.79(2H,m,H-15a,16a),1.57(3H,m,H-9,11b,14),1.43(3H,s,H-19),1.43(1H,overlap,H-16b),1.28(1H,m,H-15b),1.26(1H,m,H-17),1.14(1H,m,H-12b),1.03(3H,d,J=6.7 Hz,H-21),0.84(3H,s,H-18);13C-NMR(CD3OD,150 MHz)δ:208.7(C-1),168.5(C-26),157.8(C-24),157.4(C-5),142.4(C-3),126.7(C-2),126.4(C-25),122.1(C-4),80.1(C-22),79.2(C-6),73.7(C-7),56.4(C-27),55.2(C-10),53.1(C-17),51.3(C-14),43.8(C-13),42.6(C-9),40.6(C-12),40.4(C-20),36.4(C-8),30.7(C-23),28.2(C-16),24.8(C-15),22.5(C-11),20.2(C-28),19.9(C-19),13.6(C-21),12.1(C-18)。与文献[5]报道一致,故鉴定为daturafoliside S。
化合物9:白色无定形粉末(甲醇),Molish反应呈阳性,分子式C34H48O9;HR-ESI-MSm/z623.319 2 [M+Na]+。1H-NMR(CD3OD,600 MHz)δ:6.91(1H,ddd,J=10.0,4.9,2.4 Hz,H-3),5.83(1H,dd,J=10.0,2.4 Hz,H-2),5.62(1H,d,J=5.8 Hz,H-6),4.63(1H,d,J=11.2 Hz,H-27a),4.49(1H,m,H-22),4.47(1H,d,J=11.2 Hz,H-27b),4.33(1H,d,J=7.8 Hz,H-1′),3.86(1H,dd,J=12.0,2.0 Hz,H-6′a),3.68(1H,dd,J=12.0,5.4 Hz,H-6′b),3.35(2H,m,H-4a,3′),3.27(2H,m,H-4′,5′),3.17(1H,t,J=8.1 Hz,H-2′),2.88(1H,dd,J=21.4,4.9 Hz,H-4b),2.58(1H,dd,J=18.1,13.6 Hz,H-23a),2.23(1H,dd,J=18.1,3.1 Hz,H-23b),2.18(1H,m,H-11a),2.14(3H,s,H-28),2.03(1H,m,H-12a),2.02(1H,m,H-7a),1.97(1H,m,H-20),1.80(2H,m,H-9,16a),1.68(1H,m,H-15a),1.59(1H,m,H-11b),1.58(1H,m,H-7b),1.47(1H,m,H-8),1.38(2H,m,H-12b,16b),1.28(1H,m,H-14),1.26(3H,s,H-19),1.17(2H,m,H-15b,17),1.05(3H,d,J=6.6 Hz,H-21),0.80(3H,s,H-18);13C-NMR(CD3OD,150 MHz)δ:206.7(C-1),168.6(C-26),160.3(C-24),148.1(C-3),137.4(C-5),128.3(C-2),125.7(C-6),123.6(C-25),104.0(C-1′),80.1(C-22),78.0(C-3′,5′),75.0(C-2′),71.6(C-4′),63.6(C-27),62.8(C-6′),57.6(C-14),53.3(C-17),51.8(C-10),44.5(C-9),43.8(C-13),41.0(C-12),40.4(C-20),34.5(C-4),34.4(C-8),31.9(C-7),30.8(C-23),28.2(C-16),25.4(C-15),24.9(C-11),20.8(C-28),19.5(C-19),13.8(C-21),12.4(C-18)。与文献[8]报道一致,故鉴定为daturametelin A。
化合物10:白色无定形粉末(甲醇),Molish反应呈阳性,分子式C34H48O10;HR-ESI-MSm/z639.314 1 [M+Na]+。1H-NMR(CD3OD,400 MHz)δ:6.14(1H,dd,J=10.0,1.9 Hz,H-4),5.83(1H,m,H-3),5.79(1H,d,J=5.5 Hz,H-6),4.63(1H,d,J=11.2 Hz,H-27a),4.50(1H,m,H-22),4.47(1H,d,J=11.2 Hz,H-27b),4.32(1H,d,J=7.8 Hz,H-1′),3.90(1H,m,H-7),3.85(1H,dd,J=11.9,2.0 Hz,H-6′a),3.67(1H,dd,J=11.9,5.2 Hz,H-6′b),3.40(1H,m,H-5′),3.28(2H,m,H-3′,4′),3.16(1H,t,J=7.8 Hz,H-2′),2.71(1H,dd,J=20.4,4.3 Hz,H-2),2.58(1H,dd,J=18.0,13.5 Hz,H-23a),2.23(1H,dd,J=18.0,3.2 Hz,H-23b),2.14(3H,s,H-28),2.12(1H,m,H-9),1.99(1H,m,H-20),1.98(1H,m,H-8),1.95(1H,m,H-12a),1.85(2H,m,H-15a,16a),1.81(1H,m,H-11a),1.60(1H,m,H-16b),1.57(1H,m,H-11b),1.40(1H,m,H-14),1.37(3H,s,H-19),1.33(1H,m,H-15b),1.31(1H,m,H-12b),1.22(1H,m,H-17),1.05(3H,d,J=6.6 Hz,H-21),0.80(3H,s,H-18);13C-NMR(CD3OD,100 MHz)δ:211.6(C-1),168.6(C-26),160.3(C-24),145.2(C-5),130.2(C-4),128.7(C-6),126.2(C-3),123.7(C-25),104.0(C-1′),80.2(C-22),78.0(C-3′,5′),75.0(C-2′),71.6(C-4′),65.0(C-7),63.6(C-27),62.8(C-6′),54.0(C-10),53.2(C-17),50.6(C-14),43.8(C-13),40.6(C-12),40.5(C-2,20),38.4(C-8),35.0(C-9),30.8(C-23),28.2(C-16),25.0(C-15),23.3(C-11),20.8(C-28),20.0(C-19),13.8(C-21),12.2(C-18)。与文献[6]报道一致,故鉴定为daturametelin I。
化合物11:白色无定形粉末(甲醇),Molish反应呈阳性。分子式C34H48O11;HR-ESI-MSm/z655.308 8 [M+Na]+。1H-NMR(CD3OD,400 MHz)δ:7.08(1H,dd,J=9.7,5.8 Hz,H-3),6.23(1H,d,J=5.8 Hz,H-4),5.97(1H,d,J=9.7 Hz,H-2),4.62(1H,d,J=11.2 Hz,H-27a),4.48(1H,m,H-22),4.46(1H,d,J=11.2 Hz,H-27b),4.32(1H,d,J=7.8 Hz,H-1′),4.22(1H,d,J=3.3 Hz,H-6),3.85(1H,dd,J=12.0,2.0 Hz,H-6′a),3.74(1H,t,J=2.8 Hz,H-7),3.67(1H,m,H-6′b),3.34(1H,overlap,H-5′),3.28(1H,m,H-4′),3.26(1H,m,H-3′),3.15(1H,m,H-2′),2.56(1H,dd,J=18.0,13.4 Hz,H-23a),2.18(1H,dd,J=18.0,3.2 Hz,H-23b),2.12(3H,s,H-28),2.10(1H,m,H-8),1.98(3H,m,H-11a,12a,20),1.80(2H,m,H-15a,16a),1.58(2H,m,H-9,11b),1.46(1H,m,H-16b),1.43(3H,s,H-19),1.42(1H,m,H-14),1.28(2H,m,H-15b,17),1.15(1H,m,H-12b),1.03(3H,d,J=6.6 Hz,H-21),0.84(3H,s,H-18);13C-NMR(CD3OD,100 MHz)δ:208.7(C-1),168.6(C-26),160.2(C-24),157.4(C-5),142.4(C-3),126.7(C-2),123.7(C-25),122.0(C-4),104.0(C-1′),80.1(C-22),79.2(C-6),78.0(C-3′,5′),75.0(C-2′),73.7(C-7),71.6(C-4′),63.6(C-27),62.8(C-6′),55.2(C-10),53.1(C-17),51.3(C-14),43.8(C-13),42.6(C-9),40.6(C-12),40.5(C-20),36.4(C-8),30.8(C-23),28.2(C-16),24.9(C-15),22.5(C-11),20.7(C-28),19.9(C-19),13.6(C-21),12.2(C-18)。与文献[6]报道一致,故鉴定为daturametelin J。
化合物12:白色无定形粉末(甲醇),Molish反应呈阳性,分子式C34H48O10;HR-ESI-MSm/z639.313 8 [M+Na]+。1H-NMR(CD3OD,400 MHz)δ:6.92(1H,ddd,J=10.0,4.9,2.4 Hz,H-3),5.85(1H,dd,J=10.0,2.4 Hz,H-2),5.79(1H,dd,J=5.9,1.7 Hz,H-6),4.63(1H,d,J=11.2 Hz,H-27a),4.50(1H,m,H-22),4.47(1H,d,J=11.2 Hz,H-27b),4.32(1H,d,J=7.8 Hz,H-1′),3.85(1H,dd,J=12.0,2.2 Hz,H-6′a),3.79(1H,t,J=4.2 Hz,H-7),3.67(1H,dd,J=12.0,5.2 Hz,H-6′b),3.41(1H,m,H-4a),3.34(1H,m,H-3′),3.28(2H,m,H-4′,5′),3.16(1H,t,J=8.1 Hz,H-2′),2.94(1H,dd,J=21.4,4.9 Hz,H-4b),2.58(1H,dd,J=17.9,13.5 Hz,H-23a),2.22(1H,m,23b),2.14(3H,s,H-28),1.99(4H,m,H-8,9,12a,20),1.82(3H,m,H-11,16a),1.60(1H,m,H-15a),1.44(1H,m,H-16b),1.40(1H,m,H-14),1.35(1H,m,H-15b),1.30(1H,m,H-12b),1.25(3H,s,H-19),1.20(1H,m,H-17),1.06(1H,d,J=6.6 Hz,H-21),0.79(3H,s,H-18);13CNMR(CD3OD,100 MHz)δ:205.6(C-1),168.6(C-26),160.3(C-24),147.6(C-3),141.6(C-5),128.4(C-6),127.5(C-2),123.6(C-25),104.0(C-1′),80.2(C-22),78.0(C-3′,5′),75.0(C-2′),71.6(C-4′),64.8(C-7),63.6(C-27),62.7(C-6′),53.2(C-14),52.2(C-17),51.0(C-10),43.5(C-13),40.8(C-12),40.5(C-9),39.5(C-20),36.4(C-8),34.4(C-4),30.8(C-23),28.2(C-16),25.0(C-15),24.6(C-11),20.7(C-28),18.7(C-19),13.7(C-21),12.1(C-18)。与文献[8]报道一致,故鉴定为daturataturin A。
化合物13:白色无定形粉末(甲醇),Molish反应呈阳性,分子式C34H46O9;HR-ESI-MSm/z621.303 3 [M+Na]+。1H-NMR(CD3OD,400 MHz)δ:7.11(1H,dd,J=10.0,6.2 Hz,H-3),6.20(1H,dd,J=10.0,2.6 Hz,H-6),6.03(1H,d,J=6.2 Hz,H-4),5.88(2H,d,J=10.0 Hz,H-2,7),4.62(1H,d,J=11.2 Hz,H-27a),4.48(1H,m,H-22),4.46(1H,overlap,H-27b),4.32(1H,d,J=7.8 Hz,H-1′),3.85(1H,dd,J=12.0,2.1 Hz,H-6′a),3.67(1H,m,H-6′b),3.33(1H,m,H-3′),3.26(2H,m,H-4′,5′),3.15(1H,t,J=8.4 Hz,H-2′),2.55(1H,dd,J=17.9,13.2 Hz,H-23a),2.37(1H,t,J=10.0 Hz,H-8),2.18(2H,m,H-15a,23b),2.12(3H,s,H-28),2.06(1H,m,H-12a),1.97(1H,m,H-20),1.83(2H,m,H-11a,16a),1.69(1H,m,H-15b),1.56(1H,m,H-9),1.45(1H,m,H-16b),1.35(1H,m,H-11b),1.28(2H,m,H-14,17),1.24(3H,s,H-19),1.19(1H,m,H-12b),1.03(3H,d,J=6.6 Hz,H-21),0.86(3H,s,H-18);13C-NMR(CD3OD,100 MHz)δ:207.2(C-1),168.6(C-26),160.3(C-24),157.5(C-5),143.2(C-3),136.2(C-7),128.5(C-6),125.8(C-2),123.6(C-25),118.4(C-4),104.0(C-1′),80.0(C-22),78.0(C-3′,5′),75.0(C-2′),71.5(C-4′),63.6(C-27),62.7(C-6′),55.1(C-14),53.0(C-17),52.3(C-10),48.8(C-9),45.0(C-13),41.0(C-12),40.4(C-20),39.3(C-8),30.7(C-23),28.1(C-16),25.0(C-11),23.7(C-15),20.8(C-28),20.5(C-19),13.6(C-21),12.2(C-18)。与文献[8]报道一致,故鉴定为baimantuoluoside H。
化合物14:白色无定形粉末(甲醇),Molish反应呈阳性,分子式C35H50O10;HR-ESI-MSm/z653.329 4 [M+Na]+。1H-NMR(CD3OD,400 MHz)δ:6.16(1H,dd,J=10.0,1.6 Hz,H-4),6.02(1H,d,J=5.2 Hz,H-6),5.84(1H,m,H-3),4.62(1H,d,J=11.2 Hz,H-27a),4.49(1H,m,H-22),4.46(1H,overlap,H-27b),4.32(1H,d,J=7.8 Hz,H-1′),3.85(1H,dd,J=12.0,2.0 Hz,H-6′a),3.67(1H,dd,J=12.0,5.2 Hz,H-6′b),3.48(1H,m,H-7),3.44(1H,m,H-2b),3.35(1H,m,H-3′),3.30(3H,overlap,7-OCH3),3.26(2H,m,H-4′,5′),3.16(1H,t,J=8.4 Hz,H-2′),2.72(1H,dd,J=20.3,5.0 Hz,H-2a),2.57(1H,m,H-23a),2.22(1H,dd,J=18.1,3.2 Hz,H-23b),2.13(3H,s,H-28),2.10(1H,m,H-9),1.97(2H,m,H-12a,20),1.81(2H,m,H-11a,16a),1.74(2H,m,H-11b,15a),1.61(2H,m,H-8,14),1.42(1H,m,H-16b),1.36(3H,s,H-19),1.28(3H,m,H-12b,15b,17),1.04(3H,d,J=6.6 Hz,H-21),0.78(3H,s,H-18);13C-NMR(CD3OD,100 MHz)δ:211.5(C-1),168.6(C-26),160.4(C-24),146.6(C-5),130.2(C-4),126.4(C-3),125.6(C-6),123.6(C-25),104.0(C-1′),80.1(C-22),78.0(C-3′,5′),75.0(C-2′),74.0(C-7),71.6(C-4′),63.6(C-27),62.7(C-6′),56.8(7-OCH3),54.1(C-10),53.2(C-17),50.4(C-14),43.8(C-13),40.5(C-2,20),40.4(C-12),38.0(C-8),35.7(C-9),30.7(C-23),28.2(C-16),25.1(C-15),23.4(C-11),20.7(C-28),20.1(C-19),13.7(C-21),11.9(C-18)。与文献[4]报道一致,故鉴定为(22R)-27-hydroxy-7α-methoxy-1-oxowitha-3,5,24-trienolide。
化合物15:白色无定形粉末(甲醇),Molish反应呈阳性,分子式C27H22O18;HR-ESI-MSm/z657.068 5 [M+Na]+。1H-NMR(CD3OD,600 MHz)δ:7.06(2H,s,H-23,27),6.69(1H,s,H-3),6.66(1H,s,H-12),6.37(1H,d,J=1.6 Hz,H-20),4.96(2H,m,H-16,18),4.52(1H,m,H-15a),4.47(1H,m,H-17),4.16(1H,dd,J=11.0,8.4 Hz,H-15b),3.99(1H,d,J=1.0 Hz,H-19);13C-NMR(CD3OD,150 MHz)δ:170.1(C-14),168.5(C-1),166.7(C-21),146.4(C-24,26),146.0(C-11),145.6(C-9),145.3(C-6),145.2(C-4),140.4(C-25),138.2(C-5),137.7(C-10),125.5(C-13),125.4(C-2),120.6(C-22),117.2(C-7),116.7(C-8),110.2(C-3),111.0(C-23,27),108.3(C-12),95.0(C-20),76.2(C-18),71.6(C-16),69.5(C-19),65.0(C-15),62.5(C-17)。与文献[9]报道一致,故鉴定为柯里拉京。
化合物16:黄色无定形粉末(甲醇),Molish反应呈阳性,分子式C23H26O10;HR-ESI-MSm/z485.123 5 [M+Na]+。1H-NMR(CD3OD,400 MHz)δ:7.04(2H,d,J=8.4 Hz,H-2′,6′),6.70(2H,d,J=8.4 Hz,H-3′,5′),6.52(1H,d,J=2.2 Hz,H-7),6.50(1H,br.s,H-5),4.98(1H,d,J=7.3 Hz,H-1‴),4.50(1H,m,H-3),3.89(1H,dd,J=12.2,2.2 Hz,H-6‴a),3.68(1H,dd,J=12.2,5.7 Hz,H-6‴b),3.31~3.50(4H,m,H-2‴,3‴,4‴,5‴),2.95(1H,m,H-4a),2.89(1H,m,H-4b),2.78(1H,m,H-2″a),2.69(1H,m,H-2″b),2.07(1H,m,H-1″a),1.96(1H,m,H-1″b);13C-NMR(CD3OD,100 MHz)δ:171.4(C-1),165.1(C-6,8),156.7(C-4′),143.4(C-10),133.1(C-1′),130.4(C-2′,6′),116.3(C-3′,5′),108.2(C-5),104.0(C-9),103.5(C-7),101.3(C-1‴),80.1(C-3),78.3(C-5‴),77.8(C-3‴),74.7(C-2‴),71.2(C-4‴),62.4(C-6‴),37.9(C-1″),33.9(C-4),31.1(C-2″)。与文 献 [10 ]报道一致,故鉴定为desmethylagrimonolide 6-O-β-D-glucopyranoside。
化合物17:黄色无定形粉末(甲醇),Molish反应呈阳性。分子式C24H28O10;HR-ESI-MSm/z499.157 0 [M+Na]+。1H-NMR(CD3OD,600 MHz)δ:7.14(2H,d,J=8.6 Hz,H-2′,6′),6.83(2H,d,J=8.6 Hz,H-3′,5′),6.52(1H,d,J=2.1 Hz,H-7),6.50(1H,br.s,H-5),4.97(1H,d,J=7.3 Hz,H-1‴),4.50(1H,m,H-3),3.89(1H,dd,J=12.1,2.0 Hz,H-6‴a),3.74(3H,s,4′-OCH3),3.68(1H,dd,J=12.1,5.8 Hz,H-6‴b),3.34~3.49(4H,m,H-2‴,3‴,4‴,5‴),2.96(1H,m,H-4a),2.92(1H,m,H-4b),2.82(1H,m,H-2″a),2.72(1H,m,H-2″b),2.09(1H,m,H-1″a),1.98(1H,m,H-1″b);13C-NMR(CD3OD,150 MHz)δ:171.3(C-1),165.2(C-6),165.1(C-8),159.6(C-4′),143.4(C-10),134.3(C-1′),130.4(C-2′,6′),115.0(C-3′,5′),108.3(C-5),104.0(C-9),103.5(C-7),101.4(C-1‴),80.1(C-3),78.4(C-5‴),77.9(C-3‴),74.7(C-2‴),71.2(C-4‴),62.4(C-6‴),55.7(4′-OCH3),37.8(C-1″),33.9(C-4),31.1(C-2″)。与文献[11]报道一致,故鉴定为仙鹤草内酯-6-O-β-D-葡萄吡喃糖苷。
化合物18:无色方晶(甲醇),分子式为C22H26O8;HR-ESI-MSm/z441.151 7 [M+Na]+。1HNMR(CD3OD,400 MHz)δ:6.65(4H,s,H-2,2′,6,6′),4.71(2H,d,J=4.3 Hz,H-7,7′),4.26(2H,dd,J=9.0,6.9 Hz,H-9a,9′a),3.88(2H,dd,J=9.0,3.6 Hz,H-9b,9′b),3.84(12H,s,3,3′,5,5′-OCH3),3.13(2H,m,H-8,8′);13C-NMR(CD3OD,100 MHz)δ:149.4(C-3,3′,5,5′),136.2(C-4,4′),133.2(C-1,1′),104.6(C-2,2′,6,6′),87.6(C-7,7′),72.8(C-9,9′),56.8(3,3′,5,5′-OCH3),55.5(C-8,8′)。与文献[12]报道一致,故鉴定为丁香脂素。
化合物19:白色无定形粉末(甲醇),Molish反应呈阳性,分子式C28H36O13;HR-ESI-MSm/z603.204 7 [M+Na]+。1H-NMR(CD3OD,400 MHz)δ:6.71(2H,s,H-2,6),6.65(2H,s,H-2′,6′),4.86(1H,d,J=7.5 Hz,H-1″),4.76(1H,d,J=4.0 Hz,H-7),4.71(1H,d,J=4.3 Hz,H-7′),4.28(2H,m,H-9a,9′a),3.91(2H,dd,J=9.2,3.0 Hz,H-9b,9′b),3.85(6H,s,3,5-OCH3),3.84(6H,s,3′,5′-OCH3),3.77(1H,dd,J=12.0,2.3 Hz,H-6″a),3.65(1H,dd,J=12.0,5.1 Hz,H-6″b),3.47(1H,m,H-2″),3.41(2H,m,H-3″,4″),3.18(1H,m,H-5″),3.13(2H,m,H-8,8′);13C-NMR(CD3OD,100 MHz)δ:154.4(C-3,5),149.4(C-3′,5′),139.6(C-1),136.2(C-4′),135.6(C-4),133.1(C-1′),105.3(C-1″),104.8(C-2,6),104.5(C-2′,6′),87.6(C-7′),87.2(C-7),78.4(C-5″),77.8(C-3″),75.7(C-2″),72.9(C-9,9′),71.3(C-4″),62.6(C-6″),57.1(3′,5′-OCH3),56.8(3,5-OCH3),55.7(C-8′),55.5(C-8)。与文献[12]报道一致,故鉴定为丁香脂素-4-O-β-D-吡喃葡萄糖苷。
化合物20:白色无定形粉末(甲醇),Molish反应呈阳性,分子式C24H24O10;HR-ESI-MSm/z471.128 6 [M-H]-。1H-NMR(CD3OD,400 MHz)δ:7.71(1H,d,J=15.9 Hz,H-7′),7.61(1H,d,J=15.9 Hz,H-7″),7.44(4H,J=8.6,4.4 Hz,H-2′,2″,6′,6″),6.79(4H,dd,J=8.7,2.2 Hz,H-3′,3″,5′,5″),6.37(1H,d,J=15.9 Hz,H-8′),6.33(1H,d,J=15.9 Hz,H-8″),5.60(1H,d,J=7.6 Hz,H-1),4.50(1H,dd,J=12.1,2.0 Hz,H-6a),4.32(1H,dd,J=12.1,5.6 Hz,H-6b),3.42~3.70(4H,m,H-2,3,4,5);13C-NMR(CD3OD,100 MHz)δ:169.2(C-9″),167.6(C-9′),161.6(C-4′),161.3(C-4″),148.1(C-7′),146.9(C-7″),131.4(C-2′,6′),131.3(C-2″,6″),127.2(C-1′),127.1(C-1″),116.9(C-3′,5′),116.8(C-3″,5″),114.9(C-8″),114.4(C-8′),95.8(C-1),77.9(C-3),76.3(C-5),74.0(C-2),71.4(C-4),64.4(C-6)。与文献[13]报道一致,故鉴定为 1,6-di-O-coumaroyl glucopyranoside。
化合物21:白色无定形粉末(甲醇),分子式C11H10O4;HR-ESI-MSm/z229.047 1 [M +Na]+。1H-NMR(CD3OD,600 MHz)δ:7.88(1H,d,J=9.5 Hz,H-4),7.13(1H,s,H-5),6.97(1H,s,H-8),6.26(1H,d,J=9.4 Hz,H-3),3.92(3H,s,6-OCH3),3.88(3H,s,7-OCH3);13C-NMR(CD3OD,150 MHz)δ:163.8(C-2),154.8(C-7),151.3(C-9),148.1(C-6),145.9(C-4),113.5(C-3),113.0(C-10),110.0(C-5),101.0(C-8),56.8(6,7-OCH3)。与文献[14]报道一致,故鉴定为滨蒿内酯。
化合物22:白色无定形粉末(甲醇),分子式C10H8O4;HR-ESI-MSm/z215.031 1 [M+Na]+。1HNMR(CD3OD,600 MHz)δ:7.87(1H,d,J=9.4 Hz,H-4),7.12(1H,s,H-5),6.78(1H,s,H-8),6.21(1H,d,J=9.4 Hz,H-3),3.92(3H,s,6-OCH3);13C-NMR(CD3OD,150 MHz)δ:164.1(C-2),153.0(C-7),151.5(C-9),147.1(C-4),146.2(C-6),112.7(C-5,10),110.0(C-3),104.0(C-8),56.9(6-OCH3)。与文献[15]报道一致,故鉴定为东莨菪内酯。
化合物23:白色无定形粉末(甲醇),Molish反应呈阳性,分子式C36H58O10;HR-ESI-MSm/z673.392 0 [M+Na]+。1H-NMR(CD3OD,600 MHz)δ:5.32(1H,d,J=8.1 Hz,H-1′),5.31(1H,t,J=2.5 Hz,H-12),3.80(1H,dd,J=12.0,2.0 Hz,H-6′a)3.69(1H,dd,J=12.0,4.7 Hz,H-6′b),3.63(1H,m,H-2),3.39(1H,d,J=8.8 Hz,H-3),1.33(3H,s,H-27),1.20(3H,s,H-29),1.01(6H,s,H-23,26),0.94(3H,d,J=6.7 Hz,H-30),0.80(3H,s,H-24),0.78(3H,s,H-25);13C-NMR(CD3OD,150 MHz)δ:178.5(C-28),139.7(C-13),129.5(C-12),95.8(C-1′),84.5(C-3),78.6(C-5′),78.3(C-3′),73.9(C-2′),73.6(C-19),71.1(C-4′),69.5(C-2),62.4(C-6′),56.7(C-5),55.0(C-18),48.7(C-17),48.5(C-9),48.2(C-1),42.9(C-14),42.7(C-20),41.3(C-8),40.5(C-10),39.2(C-4),38.3(C-22),34.1(C-7),29.6(C-15),29.3(C-23),27.2(C-21),27.0(C-29),26.5(C-16),24.8(C-11),24.6(C-27),19.7(C-6),17.6(C-26),17.4(C-24),17.1(C-30),16.6(C-25)。与文献[16]报道一致,故鉴定为野蔷薇亭。
化合物24:白色无定形粉末(甲醇),分子式C11H16O3;HR-ESI-MSm/z219.099 7 [M +Na]+。1H-NMR(CD3OD,600 MHz)δ:5.75(1H,s,H-7),4.22(1H,m,H-3),2.42(1H,dt,J=13.9,2.8 Hz,H-4b),1.99(1H,dt,J=14.4,2.8 Hz,H-2b),1.76(3H,s,H-11),1.74(1H,d,J=4.0 Hz,H-4a),1.53(1H,dd,J=14.4,3.7 Hz,H-2a),1.47(3H,s,H-9),1.28(3H,s,H-10);13C-NMR(CD3OD,150 MHz)δ:185.7(C-6),174.4(C-8),113.3(C-7),88.9(C-5),67.2(C-3),48.0(C-2),46.4(C-4),37.2(C-1),31.0(C-10),27.4(C-11),27.0(C-9)。与文献[17]报道一致,故鉴定为(-)-loliolide。
化合物25:白色无定形粉末(甲醇),分子式C11H16O3;HR-ESI-MSm/z219.099 7 [M+Na]+。1HNMR(CD3OD,600 MHz)δ:5.78(1H,s,H-7),4.10(1H,m,H-3),2.47(1H,ddd,J=11.7,4.0,2.2 Hz,H-4b),2.00(1H,ddd,J=12.7,4.0,2.2 Hz,H-2b),1.59(3H,s,H-11),1.42(1H,m,H-4a),1.32(3H,s,H-10),1.29(3H,s,H-9),1.29(1H,overlap,H-2a);13C-NMR(CD3OD,150 MHz)δ:183.9(C-6),174.0(C-8),113.7(C-7),88.5(C-5),65.2(C-3),50.7(C-2),48.6(C-4),36.1(C-1),30.3(C-10),25.8(C-11),25.3(C-9)。与文献[17]报道一致,故鉴定为(+)-isololiolide。
化合物26:黄色油状物(甲醇),分子式C16H22O4;HR-ESI-MSm/z301.140 7 [M+Na]+。1HNMR(CD3OD,600 MHz)7.72δ:(2H,dd,J=5.7,3.3 Hz,H-2,5),7.62(2H,dd,J=5.7,3.3 Hz,H-3,4),4.30(4H,t,J=6.6 Hz,H-1′,1″),1.73(4H,m,H-2′,2″),1.47(4H,m,H-3′,3″),1.00(6H,t,J=7.4 Hz,H-4′,4″);13C-NMR(CD3OD,150 MHz)δ:169.3(C-7,7′),133.6(C-1,6),132.3(C-3,4),129.9(C-2,5),66.6(C-1′,1″),31.7(C-2′,2″),20.2(C-3′,3″),14.0(C-4′,4″)。与文献[18]报道一致,故鉴定为邻苯二甲酸二丁酯。
化合物27:白色无定形粉末(甲醇),分子式C7H6O3;HR-ESI-MSm/z145.026 1 [M+Na]+。1HNMR(CD3OD,400 MHz)δ:9.75(1H,s,-CHO),7.76(2H,d,J=8.6 Hz,H-2,6),6.90(2H,d,J=8.6 Hz,H-3,5);13C-NMR(CD3OD,100 MHz)δ:192.8(-CHO),165.4(C-4),133.4(C-2,6),130.2(C-1),116.9(C-3,5)。与文献[17]报道一致,故鉴定为对羟基苯甲醛。
化合物28:白色无定形粉末(甲醇),分子式C9H10O5;HR-ESI-MSm/z221.041 8 [M+Na]+。1HNMR(CD3OD,600 MHz)δ:7.05(2H,s,H-2,6),4.27(2H,q,J=7.1 Hz,H-8),1.35(3H,t,J=7.1 Hz,H-9);13C-NMR(CD3OD,150 MHz)δ:168.6(C-7),146.5(C-3,5),139.7(C-4),121.8(C-1),110.0(C-2,6),61.6(C-8),14.6(C-9)。与文献[19]报道一致,故鉴定为没食子酸乙酯。
4 抗炎活性筛选
参照文献 [2],利用脂多糖(LPS)刺激RAW264.7 巨噬细胞炎症模型,对各化合物抑制NO 生成作用进行测定,结果见表1。各化合物均能对NO 释放呈现不同程度的抑制作用,其中化合物9、20 作用较强。
表1 各化合物对LPS 刺激RAW264.7 细胞中NO 生成的影响(μmol/L,, n=3)Tab.1 Effects of various compounds on NO production in LPS-stimulated RAW264.7 cells(μmol/L,, n=3)
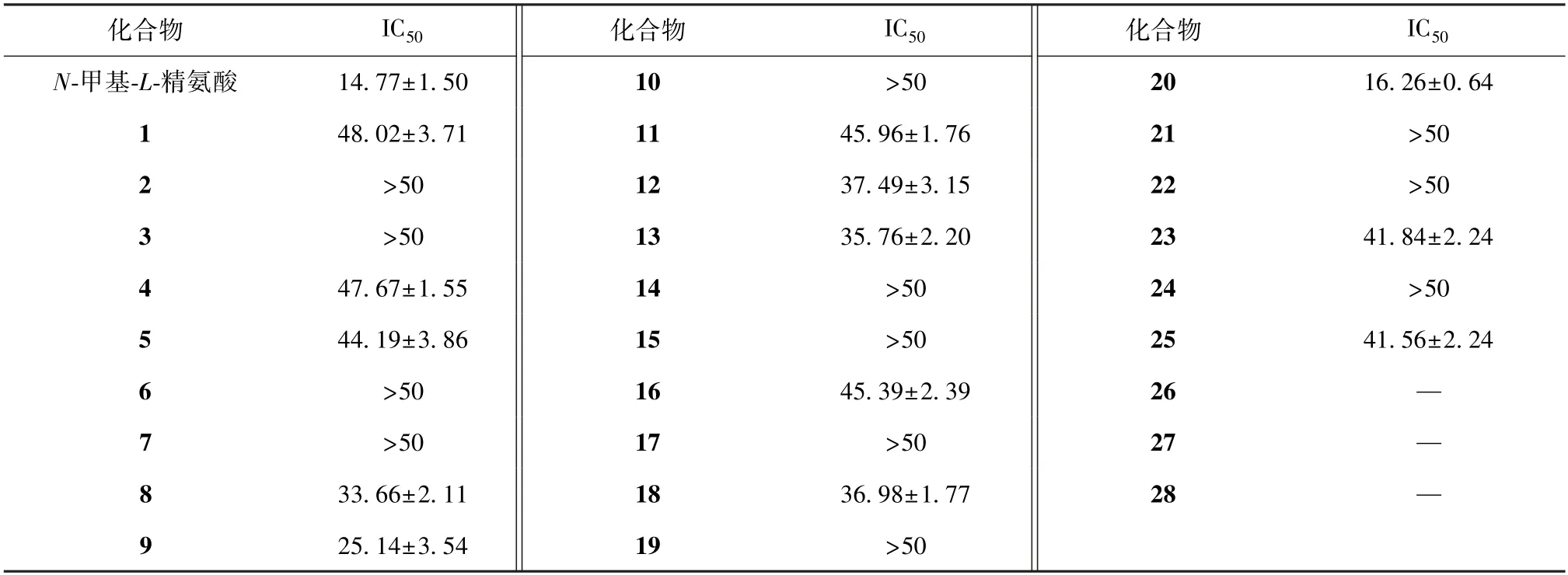
表1 各化合物对LPS 刺激RAW264.7 细胞中NO 生成的影响(μmol/L,, n=3)Tab.1 Effects of various compounds on NO production in LPS-stimulated RAW264.7 cells(μmol/L,, n=3)
5 讨论
本研究采用各种分离鉴定手段,最终从洋金花叶70%乙醇提取物中分离出28 个化合物,包括14个醉茄内酯类、1 个糖苷类、7 个苯丙素类、6 个其他类,并且各化合物均能不同程度地抑制NO 的释放,其中化合物9、20 作用更明显,3、13、14被发现有潜在的抗增殖、抗免疫作用[4],可为该药材开发利用、化学成分研究及生物活性筛选提供理论依据和实验基础。

