Strategies to Improve the Energy Density of Non-Aqueous Organic Redox Flow Batteries
Guangtao Cong , Yi-Chun Lu
1 Institute of Low-dimensional Materials Genome Initiative, College of Chemistry and Environmental Engineering, Shenzhen University,Shenzhen 518060, Guangdong Province, China.
2 Electrochemical Energy and Interfaces Laboratory, Department of Mechanical and Automation Engineering, The Chinese University of Hong Kong, Shatin, N.T. 999077, Hong Kong SAR, China.
Abstract: Redox flow batteries (RFBs) have been widely recognized as the primary choice for large-scale energy storage due to their high energy efficiency, low cost, and versatile design of decoupled energy storage and power output. However, the broad deployment of RFBs in the power grid has long been plagued by the high cost and low energy density of existing inorganic metal-based electrodes. Redox-active organic molecules(ROMs), on the other hand, have recently been extensively explored as the potentials electrodes in RFBs for their potential low cost, abundant resources, and highly tunable structure. The energy density of RFBs is proportional to the number of electrons transferred per unit reaction, the concentration of active materials, and the cell voltage. Therefore,strategies to improve the energy density of RFBs could be categorized into three classes: (1) expanding the cell voltage;(2) maximizing the practical concentration of active materials; (3) realizing multi-redox process. Benefited by the highly tunable structure and properties of ROMs, the cell voltage of RFBs could be realized by lowering the redox potentials of anolytes or/and increasing the redox potentials of catholytes. To fully exploit the low-potential anolytes and high-potential catholytes, non-aqueous electrolytes with wider electrochemical potential windows (EPWs) are preferred over the aqueous systems. However, the solubility of most ROMs in commonly used non-aqueous electrolytes is very limited. Several effective strategies to improve the practical concentrations of ROMs have been proposed: (1) the solubility of ROMs could be easily tailored by adjusting the intermolecular interactions between ROMs and the interactions between ROMs and electrolytes via molecular engineering; (2) the redox-active eutectic systems remain liquid at or near room temperature,allowing us to reduce or completely remove the inactive solvent used in the conventional electrolyte of RFBs, which leads to an enhanced practical concentration of the redox-active components; (3) the semi-solid suspension achieves a high practical concentration of ROMs by combining the advantages of solid ROMs with high energy density and liquid electrolytes with flowability; (4) the redox-targeting approach breaks the solubility limitation by realizing remote charge exchange between the solid active materials deposited in the tanks and the current collectors of the electrochemical stacks via ROMs dissolved in electrolytes. The first three strategies employ a homogeneous flowing redox-active fluid which suffers from deteriorated physical and electrochemical properties as the practical concentration of ROMs increase, e.g.,high viscosity, phase separation, and salt precipitation. The redox-targeting approach uses a hybrid flowing liquid/static solid system, which avoids the aforementioned unfavorable changes in electrolyte properties, however, this design introduces additional chemical reactions between the ROMs and the solid active materials, which may reduce the power output. Another efficient method to improve the energy density of RFBs without affecting the properties of the electrolyte is achieved by realizing the multi-redox process of ROMs, however, the generated high valence state ROMs are highly reactive. Further optimization of the structure of these ROMs is required to improve their lifetime at high valence states. In this perspective, we summarize the general working principle of the RFBs, highlight the recent developments of the ROMs in non-aqueous redox flow batteries (NRFBs), with an emphasis on the strategies to improve the energy density of NRFBs,and discuss the remaining challenges and future directions of the non-aqueous organic redox flow batteries (NORFBs).
Key Words: Redox-active organic molecule; Flow battery; Energy density; Power output
1 Introduction
The increasing conflict between the climate crisis and energy security promotes the deep penetration of renewable energy in the global energy consumption market. However, the intermittent nature of the renewable energy resources and the demand for peak-valley regulation require the wide deployment of large-scale energy storage systems. RFBs distinguished themselves from other energy storage systems for potential low cost, independent control of power and energy, high scalability,and energy efficiency1-7. One of the main hurdles in the widespread application of RFBs is that the energy density of the liquid electrolytes is much lower than those of conventional solid electrodes. To increase the energy density of RFBs, we need to concentrate our efforts on expanding the cell voltage and/or improve the specific capacity.
Limited by the electrolysis limit of the water molecule,aqueous electrolyte allows a relatively narrow voltage range of less than 2.0 V. To widen the operation voltage to 3.0 V and above, non-aqueous electrolytes must be adopted. Normally the solubility of inorganic compounds exploited in aqueous RFBs is relatively low in non-aqueous electrolytes and the cost of the metal-based inorganic redox compounds is too high for largescale application. Redox-active organic molecules (ROMs) have been increasingly exploited in both aqueous redox-flow batteries and NRFBs for their potential low cost, material abundance, high tunability in structures and associated physical,chemical and electrochemical properties. Although aqueous electrolytes support narrower EPWs than their non-aqueous counterparts, aqueous organic redox flow batteries (AORFBs)exhibit superior safety, lower capital cost, and higher electrochemical performances. Much progress has been achieved regarding the development of ROMs for AORFBs8-10and the results have been comprehensively reviewed and summarized elsewhere5,6,11-13. In this work, we focus our attention on the development of NORFBs. The solubility of most reported ROMs in non-aqueous solvents is less than 1.0 mol·L-1, corresponding to a volumetric capacity of less than 26.8 Ah·L-1. Many methods had been proposed to increase the practical concentration of active molecules of RFBs. Next, we will briefly introduce the working principles of RFBs, review and categorize the recent innovative advances on strategies to improve the energy density of non-aqueous organic redox flow batteries (NORFBs). Finally,the remaining challenges and future outlooks of the NORFBs are discussed.
2 Working principles of RFBs
As illustrated in Fig. 1, the core components of a typical RFB include electrochemical cell stacks where the redox reactions happen, external tanks which store the redox-active anolyte and catholyte, and pumps which are used to circulate the redoxactive electrolytes between the cell stacks and the external tanks.The power output of the RFBs is specifically determined by the reaction areas in the cell stacks while the energy storage capacity is proportional to the size of the external tanks, therefore the power and capacity could be scaled independently.Mathematically the energy density of the RFBs could be evaluated according to Eq. (1):

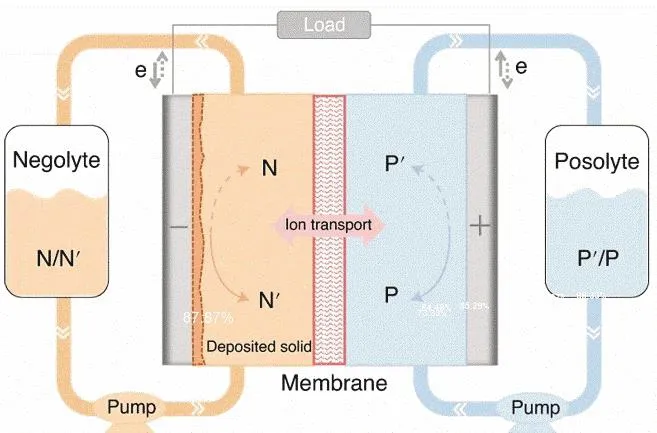
Fig. 1 Schematic illustrations of the structure of typical RFBs.
where F is a constant (26.8 Ah·L-1), E is the cell voltage, Vcand Vaare the volumes of the catholyte and anolyte respectively (L),n, C, and V are parameters associated with the capacity-limiting redox-active electrolyte (could be either anolyte or catholyte). C is the concentration of the redox-active molecules of the capacity-limiting electrolyte (mol·L-1), n is the number of electrons transferred per molecule dissolved in the capacitylimiting electrolyte, V is the volume of the capacity-limiting electrolyte (L). Consequently, the energy density of RFBs is linearly dictated by the number of electrons transferred per molecule, the potential difference between the catholyte and anolyte, and the concentration of redox-active species. Based on the above analysis, the strategies to improve the energy density of RFBs generally fall into the following three categories: (1)expanding the cell voltage by lowering the potential of anolyte and/or increasing the potential of catholyte; (2) maximizing the concentration of redox-active molecules; and (3) developing molecules which support multi-redox process. In this perspective, we review the recent advances of non-aqueous organic RFBs with a special emphasis on strategies to improve the energy densities and discuss the challenges and future directions of NORFBs.
3 Expanding the potential difference between anolyte and catholyte
One of the most compelling features of the NRFBs over the aqueous ones is their much wider EPWs, which is defined as the potential difference between the cathodic and anodic limits of the electrolyte (including solvents and supporting electrolytes).The expanded EPWs of non-aqueous electrolytes enables the application of high-potential catholytes and low-potential anolytes, leading to an improved cell voltage. The EPWs of the non-aqueous electrolytes are highly determined by the test conditions, including temperature, supporting electrolytes, and the materials of the current collector. Fig. 2 summarizes the EPWs of some representative aqueous, non-aqueous, and deep eutectic solvents with corresponding test conditions14.
As the cell voltage linearly relates to the energy density of the RFBs (Eq. (1)), it is favorable to achieve high operation voltage by increasing the potential of the catholyte and meanwhile lowering the potential of the anolyte. One of the most compelling features of the ROMs over the inorganic ones is that their structure and associated physical, chemical, and electrochemical properties could be tailored according to practical needs.Specifically, the redox potentials of the ROMs could be positively shifted by adding electron-withdrawing functional groups (EWGs) or removing the electron-donating functional groups (EDGs). On the other hand, the addition of EDGs or removal of EWGs would lower the redox potentials of the ROMs. The commonly used EDGs and EWGs are listed in Fig.3 in sequence from EDGs (activators) to EWGs (deactivators).For a more detailed study of substituent effects of different functional groups, one could refer to the computational study by Remya et al.15, in which the influence of around 381 functional groups on the molecular electrostatic potential is discussed.Next, we discuss and summarize the recent developments in high-potential organic catholytes (> 3.8 V vs. Li/Li+) and lowpotential organic anolytes (< -1.0 V vs. Ag/Ag+) in the following section.
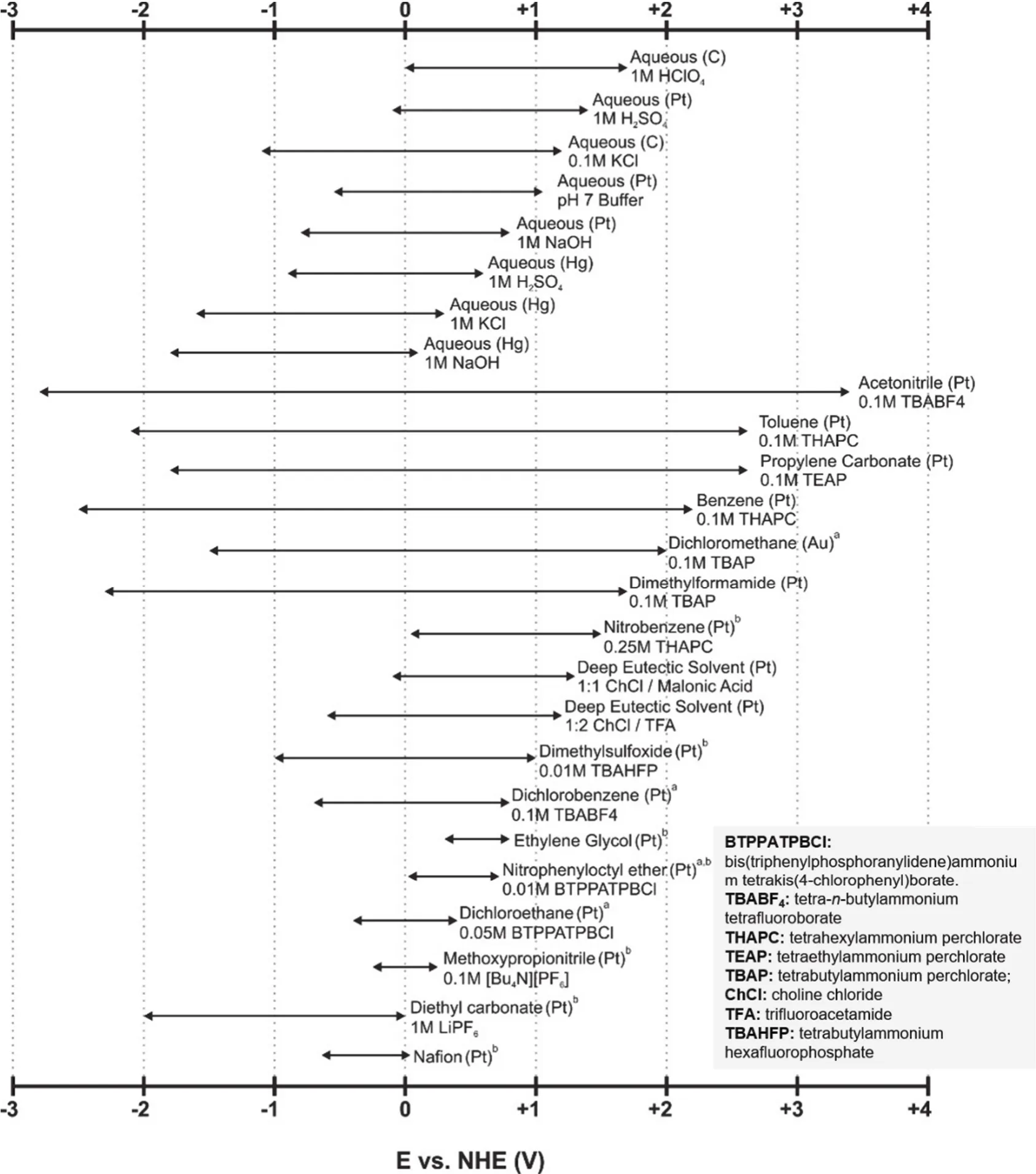
Fig. 2 The electrochemical windows of some representative electrolytes including non-aqueous, aqueous, and deep eutectic solvent systems.

Fig. 3 Representative electron-donating (activating) and electron-withdrawing (deactivating) groups.
3.1 Developing low-potential anolytes
Compared with the aqueous systems, the non-aqueous electrolytes exhibit significantly expanded cathodic limits lower than -3.0 V vs. saturated calomel electrode (SCE). To make full use of the low cathodic limit of the non-aqueous electrolytes, it is highly desired to improve the output cell voltage by employing anolytes with extremely low potentials. Among all the reported organic anodes, carbonyl compounds and polycyclic aromatic hydrocarbons exhibit redox potentials lower than -1.0 V vs.Ag/Ag+with superior reversibility and high solubility in aprotic solvents such as Acetonitrile (MeCN) and 1,2-dimethoxyethane(DME), making them promising anolytes for NORFBs.Carbonyl compounds have been used as organic electrodes since 1969 when Williams et al.17used the liquid dichloroisocyanuric acid cathode in a primary battery system. The carbonyl compounds are a family of organic molecules containing at least one carbon-oxygen double bond. In this polar bond, the electron density is shifted toward the electronegative oxygen atom, which makes the oxygen atom partially negatively charged and susceptible to the electrophilic attack of cations. Stabilized by the adjacent segments, the carbonyl group undergoes reversible one-electron transfer forming radical anion (Scheme 1). The most frequently studied carbonyl compounds are quinones,however, quinones usually exhibit redox potentials higher than-1.0 V vs. Ag/Ag+, which is frequently exploited as anolytes in aqueous RFBs. In this perspective, we focus on the representative carbonyl compounds with redox potential lower than -1.0 V vs. Ag/Ag+.
Ketones and imides represent another two classes of promising ROMs. Wei et al.18built an all organic RFB with 9-fluorenone (FL) as anolyte and 2,5-di-tert-butyl-1-methoxy-4-(2’-methoxyethoxy) benzene (DBMMB) as catholyte. The FL anolyte showed a redox potential as low as -1.64 V vs. Ag/Ag+and high solubility of 2.0 mol·L-1in MeCN. In addition, they found that the stability of the FL·-radical anion is highly associated with the electrolytes. Nucleophilic attack or H extraction of the MeCN molecules by FL·-may occur, leading to an 80% capacity decay after 100 cycles. By employing more stable DME and bulky TEA+salt, the cycling stability was greatly improved (90% capacity retention over 100 cycles)18. As another member of the ketone family, benzophenone shows even lower redox potential (-2.16 V vs. Ag/Ag+). Xing et al.19introduced benzophenone as the anolyte and 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) as catholyte. The proposed all organic RFB has a voltage of 2.41 V and a high theoretical energy density of 139 Wh·L-1, however, the cycling stability is still unsatisfactory which may result from the parasitic cross-talk between the anolyte and catholyte, since the anion exchange membrane could not prevent the crossover of benzophenone anion radical to the TEMPO catholyte. Imides have also been extensively studied as electrode materials. By employing N-methylphthalimide as anolyte coupled with the TEMPO catholyte, Li et al.20designed an all organic RFB with a theoretical cell voltage of 1.6 V and a high coulombic efficiency (CE) of 90%. The N-methylphthalimide anolyte demonstrated a low redox potential of -1.79 V vs. Ag/Ag+and more interestingly, the imide structure has two carbonyl groups,thus allowing a second-electron transfer process21,22. Other carbonyl compounds with N or S heterocycles also show promising features as potential anolytes22-25. The key performance parameters of representative carbonyl anolytes are summarized in Table 1.

Scheme 1 Reaction mechanism of carbonyl compounds.
Polycyclic aromatic hydrocarbons (PAH) could reversibly uptake/release one electron to/from their lowest unoccupied molecular orbital (LUMO) at extremely low potentials26,27. The unpaired electron is delocalized through the conjugation of the π orbitals of the aromatic ring, which empowers the reduced PAH anion radical with a strong reducing power comparable to those of alkali metals. Generally, the redox potentials of PAHs increase with the number of aromatic rings. Composed of two benzene rings linked by a single C―C bond, biphenyl shows the lowest redox potential (0.09 V vs. Na/Na+) among almost all the PAHs.The conflict between the conjugation effect of the two benzene rings and the repulsion of the overlapping ortho-hydrogen results in its nonplanar structure with a torsional angle of ~40°in its neutral state in solution and decreases to ~9° after the addition of an electron to its π orbitals. Electron uptake and release in a biphenyl molecule are accompanied by the twisting of the two benzene rings. The concept of employing biphenyl as the anolyte was first proposed by Yan and coworkers28. Later,Yu et al.29demonstrated the application of sodium biphenyl complex as a class of liquid anode in a sulfur battery to eliminate the notorious sodium dendrite formation in Na/S battery. Wang et al.30systematically studied seven representative PAHs including biphenyl, naphthalene, phenanthrene, anthracene,tetracene, pyrene, and perylene. All the tested PAHs exhibits redox potentials lower than -1.5 V vs. SHE and a correlation between the PAH structures and redox potentials was established(Fig. 4)30,31. Besides, most PAHs show high solubility in ether electrolytes. All these characteristics of PAHs making them promising anolytes for constructing high voltage RFBs.
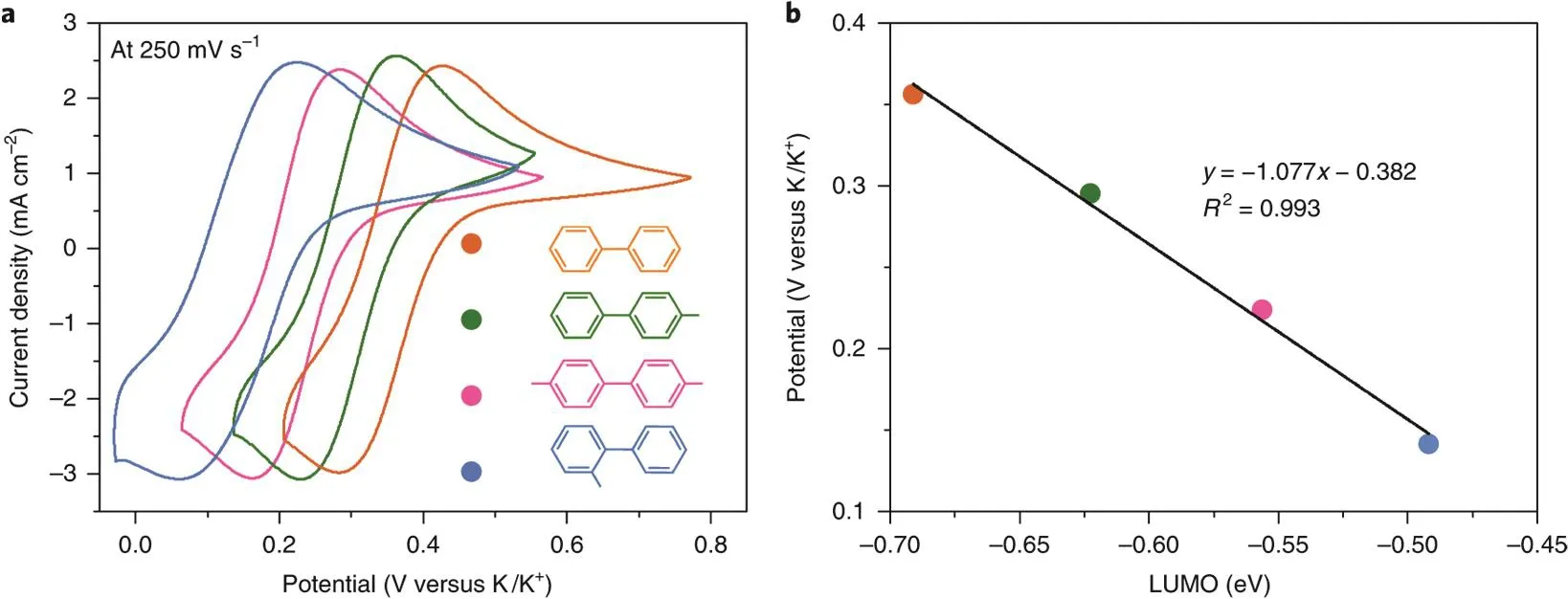
Fig. 4 (a) Cyclic voltammetry (CV) curves of the PAH anolytes; (b) the correlation between the redox potential and LUMO energies of PAHs.
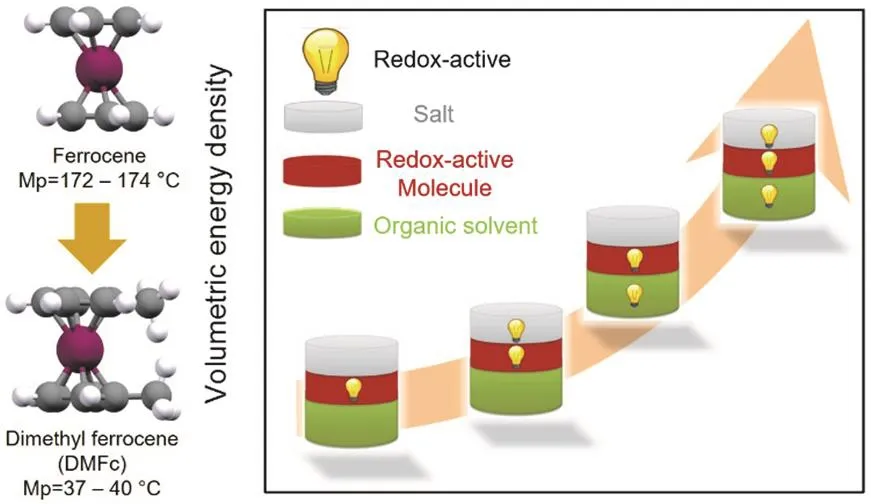
Fig. 5 Molecular engineering approach to increase the solubility of Fc and schematic illustration to boost the energy density of RFBs by activating the inactive solvent.
3.2 Developing high-potential catholyte
Another direction to maximize the cell voltage is realized by applying high-potential catholytes. A library of ROMs with potentials higher than 3.5 V vs. Li/Li+has been developed for over-charge protection in lithium-ion batteries32. Researchers frequently scrutinized this library of ROMs for promising highpotential organic cathodes. Among the reported ROMs,dialkoxybenzenes and cyclopropenium stand out as the most appealing catholytes with redox potentials around 4.0 V vs.Li/Li+. With two alkoxy groups attached to a benzene ring,dialkoxybenzenes could be oxidized to corresponding cation radicals at about 4.0 V vs. Li/Li+. A pair of tert-butyl groups are necessary to stabilize the highly unstable cation radicals32.Researchers at the Argonne National Laboratory pioneered the research of dialkoxybenzene compounds as catholytes for NORFBs18,23,32,33. 2,5-di-tert-butyl-1,4-bis(2-methoxyethoxy)benzene (DBBB) was found to have compelling features of high redox potential and stability, however, its solubility in most non-aqueous solvents is too low for the application in RFBs. With combined computational and experimental efforts, Zhang and coworkers33synthesized and examined a series of dimethoxydi-tert-butyl-benzene analogues for high-potential catholytes.By introducing asymmetrical polyethylene oxide (PEO) groups with different lengths to the benzene ring, they successfully improved the solubility of the dimethoxy-di-tert-butyl-benzene analogs. Remarkably two of the newly synthesized derivatives(Entry 2-3 in Table 2) remain liquid at room temperature,showing great potential for the application in high-voltage NORFBs18.
Cyclopropeniums (CPs) represent another family of promising catholytes for NORFBs for their facile synthetic process, high solubility, superior ionic conductivity, and high redox potential(> 0.8 Vvs.Fc/Fc+)34-37. Sanford and coworkers34,36screened and synthesized a series of tri(disubstituted-amino)cyclopropeniums (TACPs). The alkyl-substituted TACP demonstrated high stability due to the steric hindrance effect of the bulky alkyl group. By replacing one of the N substituents with S (bis(disubstituted-amino)-substituted-sulfurcyclopropeniums, entry 6 in Table 2), they further improved the redox potential of the TACPs to 1.33 Vvs.Fc/Fc+, whichrepresents one of the highest redox potentials among reported organic cathodes35.

Table 2 Key performance parameters of representative high-potential catholytes.
4 Maximizing the concentration of redoxactive molecules
Another parameter that dictates the energy density of RFBs is the effective concentration of the ROMs. For the conventional liquid anolytes and catholytes, the concentration of dissolved ROMs is limited by their solubility in the corresponding electrolytes. The solubility of organic molecules is mainly determined by the intermolecular interaction between the organic solute and their interactions with the solvent molecules and supporting salts. Although many strategies have been proposed to improve the solubility of the ROMs, we have to point out that the practical concentration of ROMs (Cpractical)can’t exceed the value calculated based on pure ROMs (Eq. (2)).

whereCmax,ρROMs, andMROMsare the concentration, density, and molecular weight of the pure ROMs, respectively.
4.1 Molecular engineering of the organic molecule
A spontaneous dissolution process requires that the solvation energy exceeds the lattice energy. Generally, organic molecules with asymmetric structures exhibit lower lattice energy and have high solubility in most organic solvents, thus we could rationally tailor the structure of the organic molecules to improve their solubility, e.g., by introducing one methyl group to each of the cyclopentadienyl rings of the ferrocene (Fc) molecule, Conget al.38greatly reduced the melting point of Fc from over 170 °C to 37-40 °C for the newly synthesized dimethyl ferrocene(DMFc), which allows us to apply the redox-active DMFc liquid at near room temperature to replace the conventionally inactive solvent to improve the volumetric capacity of the catholyte (Fig.5). The sharp depression in melting point suggests the reduction of lattice energy, which eventually leads to the increased solubility. The solubility of Fc derivatives in the carbonate electrolytes was greatly improved from less than 1.0 mol·L-1for Fc to over 3.0 mol·L-1for DMFc38. However, the tailoring of organic molecules inevitably introduces redundant inactive motifs, which increases the molecular weight, leading to a reduced gravimetric energy density and sometimes shifts the redox potential to the undesired position, e.g., lowering the potential of catholytes or increasing the potential of anolytes. A collective strategy combining the influence of molecular engineering on solubility, redox potentials, stability,reversibility, and other critical parameters should be performed when optimizing the ROMs. In addition to molecular engineering, other effective strategies to increase the effective concentration of the ROMs are realized by circumventing the solubility limits, e.g., eutectic electrolyte, semi-solid suspension,and redox-targeting approach.
4.2 Eutectic electrolyte
The concept of eutectic electrolyte has attracted growing attention as potential high-energy electrolytes in RFBs39,40. A eutectic solution is formed by mixing the redox-active molecules with salts and other inorganic or organic components. The eutectic mixture has a melting point lower than any component due to the reduced lattice energy by the interaction between the components. According to the redox-active components, the eutectic electrolyte could be categorized into metal-based and organic-based eutectic electrolytes. Here we focus on the recent developments on organic eutectic electrolyte (OEE)40.
The diversity in molecular structure and elemental abundance of organic molecules provide great opportunities for designing high-performance OEEs40-47. By mixing EG, malonic acid, and viologen-based ammonium salt, Goeltz et al.42obtained a metalfree low melting point OEE with viologen concentration as high as 4.2 mol·L-1. Although this viologen-based OEE exhibits high reversibility, a temperature higher than 30 °C is needed to maintain this OEE liquid. OEEs could also be prepared by mixing alkali salt with the ROMs. Yu and coworkers45recently reported an OEE anolyte by mixing phthalimide derivatives,urea, and LiTFSI. A nearly six-fold increase in solubility was achieved by optimizing the ratio of components. Takechi et al.46demonstrated the formation of a room temperature OEE by mixing 4-methoxy-2,2,6,6-tetra-methypiperidine 1-oxyl (MT)with LiTFSI (LT). The MT/LT OEE with a molar ratio of 1 : 1 has an effective MT concentration above 2.0 mol·L-1, which is much higher than the solubility of MT in an aqueous solution (<0.5 mol·L-1). However, the viscosity of the proposed MT/LT OEE is too high for practical operation and 17% (w) of water is needed to reduce the viscosity and improve the ionic conductivity and fluidity. Recently Yu and coworkers44systematically studied the formation of OEEs between organic molecules with carbonyl/ nitroxyl radical/methoxyl groups and alkali metal fluorinated sulfonylimide salts and proposed a general design methodology to prepare redox-active OEEs for RFBs (Fig. 6). Following this methodology, they found that methoxy-substituted Fc derivative remains liquid at both reduced and oxidized states. Coupled with the lithium metal anode and a 2.8 mol·L-1Fc derivative OEE, the Li hybrid cell achieved a high discharge energy density of 188 Wh·L-1.

Fig. 6 (a) Photo image of BQ-LiTFSI OEE with a molar ratio of 1 : 1; (b) snapshots of MD simulations of BQ-LiTFSI OEE. C: Brown, O: red,H: pink, Li: purple, N: blue, S: yellow and F: light gray; (c, d) molecular interactions of BQ-LiTFSI OEE; (e) photo image of Li/Na/K-TEMPO OEEs with molar ratios of 1 : 5, 1 : 2, 1 : 5, respectively; (f-g) MD simulations of the molecular interactions of Li-TEMPO OEE.(h) photo images of Fc-1-Li, Fc-2-Li, and Fc-2-Na OEEs at the molar ratio of 2 : 1; (i, j) the molecular interactions of Fc-1-Li and Fc-2-Li OEEs.
4.3 Semi-solid suspension
By mixing the redox-active materials, conducting carbon matrix and electrolytes, the semi-solid suspension approach represents another effective strategy to break the solubility limit of organic molecules24,48-50. Duduta et al.41first studied the electrochemical performance of a semi-solid slurry cathode containing LiCoO2/C composite and demonstrated a high energy capacity of 615 Wh·L-1in a hybrid flow cell. Later this concept was adopted in organic RFBs by Lu and coworkers51to improve the practical concentration of 10-methylphenothiazine (MPT).The proposed hybrid Li organic semi-solid RFB delivered a high volumetric capacity of 55 Ah·L-1with a high CE of > 98% over 100 cycles. By applying the semi-solid approach to both anolyte and catholyte, Xing et al.24reported an all-organic semi-solid flow battery with 10-methylphenothiazine@Ketjen black and thioxanthone@Ketjen black as the active materials for catholyte and anolyte respectively. This proof of concept cell exhibited an open circuit voltage of 2.35 V and an average CE of 83% within the voltage range of 0-3.0 V. To further improve the energy density of the organic semi-solid electrodes, Chen and coworkers52proposed an innovative concept of organic multiple redox semi-solid-liquid (MRSSL) catholyte which combined the high soluble TEMPO in the liquid phase and the insoluble MPT in the solid phase suspension. By activating the liquid phase, the proposed Li hybrid MRSSL catholyte exhibited a small voltage gap of 0.1 V between liquid and solid phase, a high cell voltage of 3.4 V, a high volumetric capacity of 75 Ah·L-1, and a high energy density of 260 Wh·L-1.
4.4 Redox-targeting approach
Inspired by the concept of redox mediation, Wang et al.53proposed to use redox-targeting reactions to fully realize the capacity of solid active materials in a flow battery configuration.As shown in Fig. 7, the dissolved redox shuttle molecule (S) acts as the charge carrier exchanging electrons between the current collectors and the insoluble solid active materials. Specifically,S+cation is first generated at the current collector by the oxidation of S at an elevated potential during charging, then diffuses to the vicinity of LiFePO4particle. With equilibrium potential higher than the Fermi level of LiFePO4, S+is finally reduced back to S by LiFePO4accompanied by the delithiation of LiFePO4. During discharging process, the above process is reversed and S+will be first reduced to S at the current collector and transfers the electron to the delithiated LiFePO4. A pair of redox shuttle molecules with potentials sit astride the Fermi level of the targeted solid active material were often adopted for the charging and discharging process respectively in practical application54-60. The concept of redox-targeting RFB was firstrealized by using FcBr2and Fc as redox shuttle molecules for the charging and discharging of LiFePO4particles respectively61,62.The electrolytes are constantly flowing between the cell stacks where the redox shuttles get oxidized or reduced and the tanks where the targeted solid electrodes are deposited and the electron transfer happens between the redox shuttle molecules and the solid electrodes. The redox potentials of FcBr2and Fc are 3.55V and 3.25 V vs. Li/Li+respectively, which sits astride that of LiFePO4(3.45 V vs. Li/Li+). FcBr+2is first generated by the oxidation of FcBr2during charging and then circulated to the tank where LiFePO4particle is oxidized by FcBr2+accompanied by the regeneration of FcBr2. FcBr2then flows back to the cell stacks and initiates a new cycle. Consequently, the LiFePO4particle is remotely oxidized and the electrons are transferred to the current collector by the redox shuttle molecules as charge carriers. During discharging, Fc is first delivered to the tank and oxidized by the delithiated LiFePO4to form Fc+, which is then transported back to the cell stacks and reduced back to its neutral form Fc61. Following the same designing strategy, redoxtargeting of the anodes by two shuttle molecules could also be achieved, leading to the construction of a full redox-targeting flow cell54.

Adapted from Wiley publisher 53.
5 Achieving reversible multi-electron transfer
As discussed above, the energy density of RFBs could be efficiently improved by increasing the effective concentration of active materials in the electrolyte either by the molecular engineering route to improve the solubility or through the eutectic/semi-solid approaches. However, the viscosity of electrolytes increases sharply with the increase in the concentration of active materials, resulting in high voltage loss and low energy storage efficiency. In addition, electrolytes with highly concentrated active materials are usually thermodynamically unstable which may cause phase separation or salt precipitation. An alternative route to increase the charge storage capacity of the redox-active electrolyte without the undesired viscosity increase could be achieved by enabling the multielectron transfer63-69. Kang and coworkers63first developed a 5,10-dihydro-5,10-dimethyl phenazine (DMPZ) catholyte,which exhibited a stable two-electron transfer at -0.15 and 0.61 Vvs.Ag/Ag+. Coupled with the FL anolyte, the proposed all organic RFB achieved high energy density per mole DMPZ.However, the solubility of DMPZ in the MeCN is only 60 mmol·L-1, which is too low for practical application (Fig. 8)63.By substitute the two methyl groups with 2-methoxyethyl chains, they successfully improve the solubility of the newly synthesized 5,10-bis(2-methoxyethyl)-5,10-dihydrophenazine(BMEPZ) to over 0.4 mol·L-1. BMEPZ also undergoes a fast two-electron redox reaction at -0.29 and 0.50 Vvs.Fc/Fc+and the all organic RFBs with BMEPZ catholyte and FL anolyte achieved high capacity retention of 99.94% per cycle over 200 cycles with 0.1 mol·L-1BMEPZ catholyte65. Although phenothiazine also supports a two-electron redox process, it suffers from low solubility in non-aqueous electrolytes. By introducing one or two oligoglycol chains to the phenothiazine core, the newly synthesized phenothiazine derivatives become miscible with 0.5 mol·L-1TEATFSI/ ACN in its neutral form69.
Despite the successes achieved in improving the solubility,the reversibility of the multi-electron transfer process is equally important for the design of high-energy NORFBs. High valence state organics such as dications and dianions are often chemically or electrochemically unstable, subjecting to nucleophilic and electrophilic attacks respectively. Odom and coworkers69,70optimized the phenothiazine derivatives to achieve a stable two-electron redox process.N-ethylphenothiazine (EPT) supports two-electron oxidation, however, the generated electron-deficient EPT dications undergo rapid degradation, resulting in the irreversible second-oxidation reaction. Functionalization of EPT with methyl and methoxy groups at 3 and 7 positions was found to be effective to stabilize the EPT dications. Computational study showed that the electron-donating methyl groups ofN-ethyl-3,7-dimethylphenothiazine (DMeEPT) and methoxy groups ofN-ethyl-3,7-dimethoxyphenothiazine (DMeOEPT) successfully delocalized the positive charges on the parent EPT motif. Similarly, the stability of dianions could be enhanced by the introduction of EWGs to share the negative charges. Unfortunately, the addition of EDGs to the dication catholytes leads to a negative potential shift and the functionalization of the dianion anolyte with EWGs causes a potential increase, which eventually results in a depressed cell voltage. A comprehensive study that takes into account the solubility, redox potential, and reversibility should be performed during the molecular modification of the ROMs.
6 Conclusion and outlook
The most significant challenge faced by the NORFBs is the low specific energy density. Cell voltage, the effective concentration of the ROMs, and the number of electrons transferred per ROM are the key parameters dictating the practical specific energy density of NORFBs. Strategies to increase these parameters have successfully improved the energy density of the NORFBs, however new issues are introduced as we tune these parameters and challenges still remain to be tackled before the practical deployment of the NORFBs. The key advantages and disadvantages of the representative strategies to improve the energy densities of NORFBs are summarized in Table 3.
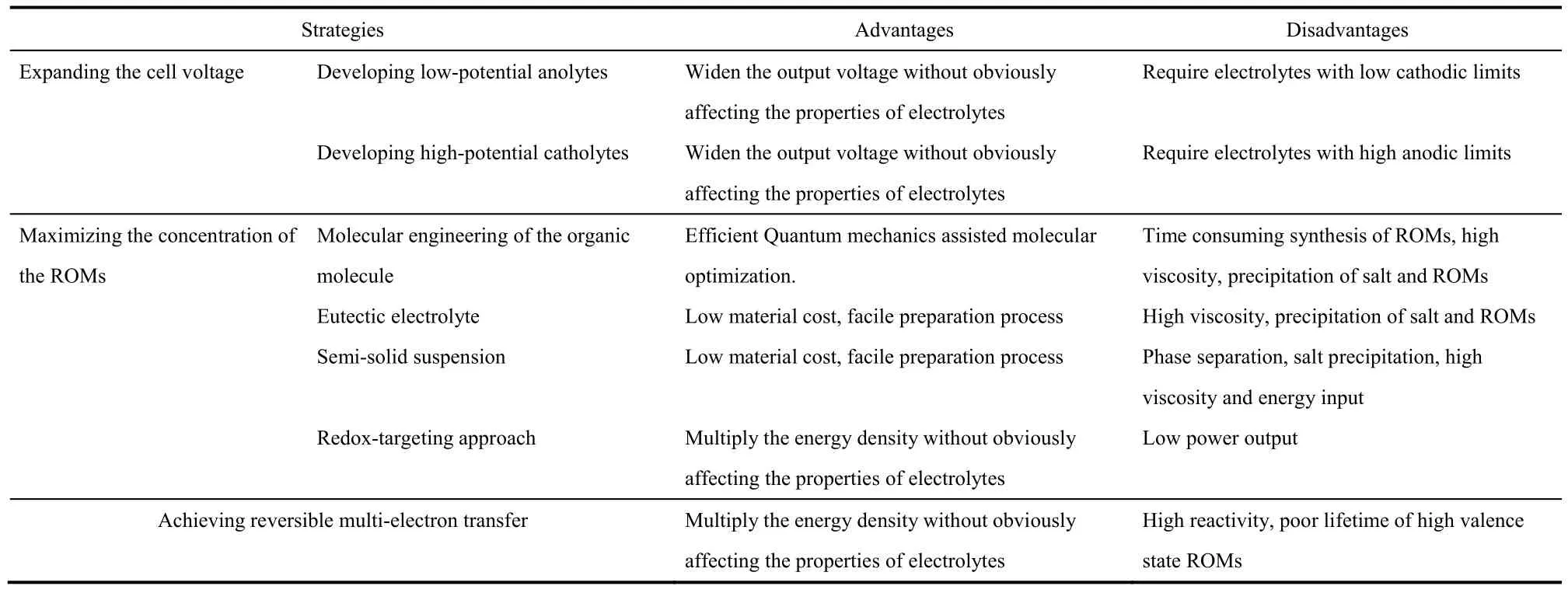
Table 3 Comparison of the representative strategies to improve the energy density of NORFBs.
(1) To expand the cell voltage and make full use of theenlarged EPWs of the non-aqueous electrolyte, high-potential catholytes and low-potential anolytes are highly desired. Much progress has been made on developing ROMs with extreme redox potentials, which enables the construction of high-voltage NORFBs. However, the redox potentials of the catholytes and anolytes can’t exceed the anodic and cathodic limits of the electrolytes respectively. Unlike the solid electrodes which generate solid electrolyte interphase (SEI) when their potential exceeds the cathodic or anodic limit of the electrolyte and prevent further decomposition of the electrolyte, no SEI forms when the redox potentials of the catholytes and anolytes get close to the decomposition limits of the electrolyte and parasitic reactions will prevail over the redox reactions, leading to reduced cycling stability. Mechanistic studies on the degradation of the ROMs and optimization of the electrolyte compositions are urgently needed to improve the cycling stability of the high voltage NORFBs.
(2) The solubility of the ROMs in the non-aqueous electrolyte could be effectively improved via chemical modification of the ROMs and optimization of the compositions of the electrolyte.A collective strategy that takes into account the solubility, redox potential, reversibility, and other performance metrics should be performed when tailoring the ROMs. The emerging eutectic electrolyte and the semi-solid suspension approaches greatly enhance the effective concentration of the ROMs, however,these methods share the same disadvantage of high viscosity,which requires high energy input during battery operation. In addition, although the OEEs and semi-solid suspensions remain uniform in the initial state, phase separation may occur during cycling. The ROMs may form homogeneous OEEs with other components in their original valence state but may precipitate when their valence states are changed during cycling. For the semi-solid approach, if the lattice energies of the ROMs are too high and the interactions between the ROMs and the conductive matrix are too weak, the ROMs may congregate and separate from the conductive matrix during cycling. Further optimization of the compositions of the OEEs and the semi-solid systems to reduce the viscosity and improve the cycling stability is needed.By depositing the high energy density solid electrodes in the tanks and circulating the redox shuttle molecules in the liquid phase, the redox-targeting approach elegantly breaks the solubility limitation without dealing with the high viscosity problems. However, the power output of the redox-targeting system may be limited by the relatively low concentration of the redox shuttle molecules and the sluggish kinetics of the reactions between the redox shuttle molecules and the solid electrode material. Besides the potential differences between the redox shuttle molecules and the solid electrodes which are necessary to drive the chemical reactions between them to happen represent permanent voltage losses. Further optimization of the redox shuttles to reduce their potential differences and enhance the reaction kinetics with the targeted solid electrode is required to improve the feasibility of the redox-targeting approach.
(3) Enabling the second-electron transfer of the ROMs doubles the theoretical charge storage capacity without negatively affecting the physical properties of electrolyte, e.g.,high viscosity, thermodynamically instability. However, the ROMs at high oxidation/reduction states are highly unstable and suffer from rapid decomposition or parasitic reactions with the electrolytes. Rational design of the ROM structures by introducing conjugation units to delocalize the second electron/hole or bulky motifs to enhance the steric hindrance effect could effectively stabilize the high valence ROMs.
(4) Developing high-performance membranes. Compared with conventional batteries systems based on solid electrodes,the flowable nature of liquid electrodes endows the RFBs with the prominent feature of decoupled energy and power, and meanwhile induces the parasitic crosstalk between the anolytes and catholytes, which is powered by electric filed or concentration gradient. This scenario becomes even worse when high concentration anolytes or catholytes are used. Thus highperformance membranes are indispensable to avoid the mixing of the anolytes and catholytes while allowing the rapid transportation of supporting ions to balance the charge during operation. The most frequently used membranes in NORFBs fall into two classes: ion-selective polymers and ion-conducting ceramics. The polymer membranes suffer from poor compatibility with the non-aqueous solvent while the fragile ceramics are plagued by low ionic conductivity. The development of membranes with high ionic selectivity,improved conductivity, excellent chemical and mechanical stability is critical for the design of high-performance NORFBs.
(5) Computational tools for the optimization of ROMs and indepth understanding of reaction mechanisms. The high tenability and vast molecular diversity of ROMs provide a library of abundant candidates for the application of NORFBs. How to single out the optimum material for a specific application is like looking for a needle in a haystack. Unlike the traditional timeconsuming and inefficient trial-and-error approach, it is now possible to apply quantum mechanical calculations combined with machine learning and artificial intelligence techniques to pre-screen and identify the most promising molecule for a specific application. Besides, computational studies could also be adopted for the in-depth understanding of the reaction mechanisms and degradation pathways of ROMs, as well as the optimization of electrolytes (e.g., developing solvents and supporting salts with high anodic limits for high-potential catholytes).
- 物理化学学报的其它文章
- 一体化电极电催化二氧化碳还原研究进展
- 锂离子电池氧化亚硅负极结构优化和界面改性研究进展
- Challenges and Opportunities for Seawater Electrolysis: A Mini-Review on Advanced Materials in Chlorine-Involved Electrochemistry
- 锂离子电池隔膜的功能化改性及表征技术
- 三维大孔/介孔碳-碳化钛复合材料用于无枝晶锂金属负极
- NH2-MIL-125 (Ti) Derived Flower-Like Fine TiO2 Nanoparticles Implanted in N-doped Porous Carbon as an Anode with High Activity and Long Cycle Life for Lithium-Ion Batteries

