负载Pt-WOx催化剂上甘油选择氢解合成1,3-丙二醇
李宇明,刘海超
北京大学化学与分子工程学院北京分子科学国家研究中心,北京 100871
1 Introduction
With the increasing demand for fossil fuels and their depleting reserve, development of renewable, eco-friendly and cheap energies has attracted much attention these years1. Naturallyoccurring biomass provides a renewable alternative to fossil fuels for the production of chemicals, fuels and materials2-4. One typical example is glycerol. As one of the top 12 biomassderived platform molecules5, it has been converted to a wide range of value-added products via different routes, such as glycerol hydrogenolysis to 1,3-propandiol (1,3-PDO), an important monomer for the synthesis of polytrimethylene terephthalate6.
The glycerol hydrogenolysis to 1,3-PDO requires the selective cleavage of the secondary C-O bond without attacking the C-C bonds and other C-O bonds of glycerol. A number of catalysts and particularly Pt-WOx-based catalysts have been extensively studied for this reaction7-18. In 2008, Kurosaka et al.19found that Pt/WO3/ZrO2catalyzed the glycerol hydrogenolysis to 1,3-PDO with a 24% yield at 170 °C and 8 MPa H2. Recent studies have reported the effects of different supports, WOxstates and additives on the properties of the Pt-WOx-based catalysts10,20-23. García-Fernández et al.24characterized the Pt-WOxcatalysts by in situ attenuated total reflection infrared spectroscopy, and proposed that the superior function of the WOxspecies for the formation of 1,3-PDO is attributed to their anchoring of the primary -OH groups of glycerol. Zhou et al. found that the addition ofincreased the surface negative charge and the Pt-WOxinteraction, leading to a higher catalytic activity22. Similar promoting effect was also observed upon introduction of a small amount of Au into a Pt/WOx/Al2O3catalyst, which increased the glycerol conversion from 66.3% to 77.5% while the 1,3-PDO selectivity remained unchanged (54.8% vs. 53.0%) at 180 °C and 5 MPa H2, as a result of the electron transfer from W to Au to weaken the Pt and WOxinteraction23. By varying the oxygen vacancies on the WO3surface, the WO3-supported Pt catalyst exhibited a 63.8% glycerol conversion with a 43.1% selectivity to 1,3-PDO under the conditions of 160 °C and 5 MPa H217, and the coordinatively unsaturated tungsten sites are proposed to participate in the reaction. Concerning the structures of the active tungsten sites,Fan et al.25proposed that the isolated WO4sites with Lewis acidity were the active WOxspecies for the glycerol hydrogenolysis on Pt/W-SBA-15 catalysts. Aihara et al.26reported that the WO3monolayer was important for the formation of 1,3-PDO. Zhao et al.27prepared the isolated tetrahedral WO4-dispersed Pt clusters or Pt single atoms, and proposed that their high activity in the glycerol reaction was related to the in situ formed Brønsted acid sites caused by the interaction between the Pt and WOxspecies in the presence of H2. Clearly, the active tungsten structures and their roles in the glycerol hydrogenolysis have still not been well established.
In this work, we prepare a series of Pt-WOxcatalysts with different WOxsurface densities on three different supports,including TiO2, ZrO2and Al2O3, and study the effect of the WOxdensities on the glycerol hydrogenolysis to 1,3-PDO. We also attempt to probe the Pt-WOxinteraction and reaction kinetics to get insights into the role of the WOxspecies and the glycerol reaction mechanism on the Pt-WOxcatalysts.
2 Experimental
2.1 Catalyst preparation
All the materials were purchased from Sinopharm Chemical Reagent Beijing Co., Ltd. and used as received, except TiO2,which was bought from Innochem Company (their purities were listed in Table S1, Supporting information).
Supported Pt-WO3catalysts were prepared by the wetness impregnation method. Typically, a certain amount of(NH4)6W7O24·6H2O was dissolved in deionized water, into which the support was added and impregnated at room temperature for 10 h with a magnetic Stirrer (IKA, Germany).Afterwards, the impregnated powder was dried at 110 °C overnight and consequently calcined at 500 °C in air for 3 h to obtain the supported xWO3sample, where x represents the theoretical weight percentage of WO3. Pt component was then loaded by impregnating the resulting xWO3/Support powder with an aqueous solution of H2PtCl6·6H2O (10 mg·mL-1), and the as-prepared sample was stirred at room temperature for 10 h,followed by evaporation to dryness. The impregnatedyPt/xWO3/Support samples were obtained after drying and calcination at 500 °C for 3 h, whereyis denoted as the theoretical weight percentage of Pt.
2.2 Catalyst characterization
Texture properties of the catalysts were characterized by N2adsorption at -196 °C on a Micromeritics ASAP 2010 analyzer(America) by the t-plot method. The sample was pretreated at 300 °C in vacuum for 2 h, prior to the measurement.
Crystalline structures of the catalysts were characterized by XRD on a Rigaku D/Max-2000 diffractometer (Japan) using CuKαradiation (λ= 0.15418 nm) at 40 kV and 100 mA. The scanning rate was 8 (°)·min-1from 10°-80° at a step of 0.5°.
TEM images of the catalysts were taken on Philips Tecnai F30 FEG-TEM (Netherlands) at 300 kV. The samples were dispersed ultrasonically in ethanol for 30 min and then dropped on a Cu mesh, followed by drying at room temperature. The average sizes of Pt particles and their size distributions were estimated by measuring about 200 particles in the TEM images.
CO-DRIFTs spectra were recorded in the wavenumber range 4000-400 cm-1at 298 K on a Bruker Tensor-27 spectrometer(Germany) equipped with a MCT detector (Germany) with a resolution of 4 cm-1. About 0.05 g of the Pt-WO3sample was loaded into the sample cell, andin situtreated in a N2flow at 300 °C for 0.5 h. After the sample was cooled to room temperature, a CO flow was introduced for 0.5 h, followed by flushing the cell with a N2flow for 0.5 h. All spectra were obtained as difference spectra by subtracting the background spectrum of the sample from the spectrum after exposure to CO.
Bruker-500 MHz NMR (Germany) was used for1H NMR measurement. The products with D2O as the solvent were separated by resin column and analyzed by Agilent 7890A(America) before test.
2.3 Glycerol hydrogenolysis reactions
The glycerol hydrogenolysis reaction was carried out at 50 mL stainless steel autoclave, equipped with a 40 mL polytetrafluoroethylene liner. In a typical run, 0.5 g of the asprepared catalyst and 10 mL of 10% (mass fraction) glycerol aqueous solution was added and sealed into the autoclave. After purging the residual air using H2for several times, the autoclave was pressurized with H2to 4.0 MPa, and then heated to the reaction temperature. During the reaction, the autoclave was agitated at a speed of 700 r·min-1to eliminate the external diffusion effect. When the reaction ended, the autoclave was cooled to room temperature quickly in ice water. The products were centrifugated and washed with deionized water to a constant volume.
The liquid products were analyzed by gas chromatography(Agilent 7890A) (America) equipped with a capillary chromatographic column (DB-WAXETR 30 m × 0.25 mm ×0.25 μm) and a FID detector. 1,4-Butanediol and 1-butanol were used as the external standards. The conversion of glycerol and selectivity to a given product were calculated by the following equations, and the carbon balances for all the reactions were 100% ± 10%.

3 Results and discussion
3.1 Effect of WO3 surface density on Pt/WO3/TiO2 catalysts
To understand the effect of WO3on the formation of 1,3-PDO,WO3was first examined by physically mixing with the Pt/TiO2and Pt/ZrO2catalysts, in comparison with the sequentially impregnated Pt-WOxcatalysts. As listed in Table 1, 0.5 g of 2Pt/TiO2showed only 5.4% glycerol conversion and 13.8% 1,3-PDO selectivity with higher selectivities to 1,2-PDO (42.3%)and 1-PO (43.0%) after reaction for 4 h at 180 °C and 4 MPa H2(Entry 1). Upon addition of commercial WO3(10 mg), the glycerol conversion dramatically increased from 5.4% to 45.5% while the 1,3-PDO selectivity increased from 13.8% to 39.8% and the 1,2-PDO selectivity decreased from 42.3% to 4.8%(Entry 2). After WO3was dispersed on TiO2, the activity and selectivity of the Pt catalyst were further improved. For example,using only 0.2 g of 2Pt/4WO3/TiO2containing only 8 mg WO3,the glycerol conversion reached similar level of 47.9% (Entry 3),although the two catalysts, as shown in Fig. 1, possessed similar Pt particle sizes (2.3 nm vs. 2.0 nm). Moreover, the selectivity to 1,3-PDO increased to 51.2%. Similar promoting effect of WO3was also observed with the Pt/ZrO2catalyst. On 2Pt/ZrO2, the glycerol conversion was lower than 2% (Entry 4), which increased significantly to 26.8% by physically mixing with WO3(0.1 g), and the selectivity to 1,3-PDO reached 36.6%. It was noticed that the 2Pt/8WO3/ZrO2catalyst (Entry 6) showed a much higher glycerol conversion of 76.3% with a similar selectivity to 1,3-PDO (33.1%). Such comparison clearly shows that the presence of WO3, even physically mixing with the Pt catalysts, can dramatically improve the glycerol conversion to 1,3-PDO, although the underlying reason has not been clarified.These results also show the more significant promoting effect of WO3on the supported Pt-WOxcatalysts.

Table 2 Hydrogenolysis of glycerol and propanediols on 2Pt/4WO3/TiO2

Fig. 1 TEM images and histograms of Pt particle size distribution of(a) 2Pt/TiO2, (b) 2Pt/4WO3/TiO2 and (c) 2Pt/10WO3/TiO2 catalysts, and(d) 2Pt/4WO3/TiO2 after recycling glycerol experiments.

Table 1 Effect of WO3 on glycerol hydrogenolysis on Pt catalysts.
Such promoting effect depends sensitively on the WO3surface density. Al2O3, ZrO2and TiO2were chosen as the supports with a specific surface area of 150, 102 and 60 m2·g-1,respectively. As shown in Fig. 2, for the 2Pt/xWO3/TiO2catalysts, 9 different WO3surface densities in the range of 0 to 4.3 W·nm-2, corresponding to the loadings of WO3from 0 to 10%, were examined in the glycerol hydrogenolysis at 140 °C.TEM images (Fig. 1 and Fig. S1) for these catalysts show that they possessed the average Pt particle sizes of ~2 nm with the similar size distributions, ensuring no influence of the Pt dispersions on the glycerol hydrogenolysis. As the WO3surface density increased from 0 to 1.7 W·nm-2(i.e., 4% WO3), the selectivity to 1,3-PDO increased to the maximum value of 49.9%, and then decreased to 27.3% at 4.3 W·nm-2. The glycerol conversion monotonically increased to 61.5% with increasing the WO3density to 2.2 W·nm-2, which then decreased to a very low level (< 5%) by further increasing the WO3density to 4.3 W·nm-2. Similarly, the glycerol conversion and 1,3-PDO selectivity also showed the volcano-type change with the WO3density, and reached the maximum values around 1.5-2.0 W·nm-2on the 2Pt/xWO3/Al2O3and 2Pt/xWO3/ZrO2catalysts.The highest glycerol conversion of 31.2% and 1,3-PDO selectivity were obtained at 2.1 W·nm-2(i.e., 12% WO3) on 2Pt/xWO3/Al2O3, while for 2Pt/xWO3/ZrO2, the maximum conversion reached 76.2% with a selectivity to 1,3-PDO of 33% at the WO3density of 2.0 W·nm-2(i.e., 8% WO3).
From these results, it is notable that the tungsten species on the support surfaces exert tremendous effect on the glycerol hydrogenolysis reaction, leading to the highest conversion and 1,3-PDO selectivity at around 2.0 W·nm-2, corresponding to the 1/3 monolayer coverage. For the catalysts examined in Fig. 2,their WO3surface densities were in the range of 0-4.3 W·nm-2,below the monolayer coverage. Characterization of these catalysts by XRD, as represented by the patterns for the 2Pt/xWO3/TiO2catalysts (Fig. 3 and Fig. S2), shows no detectable diffraction peaks of WO3, indicating that the tungsten species were highly dispersed and no WO3crystalline phases were formed on the support surfaces. From the previous study28,as the WO3loadings are lower than 10%, i.e., below a monolayer coverage, the coordination structures of the tungsten species tend to change between the tetrahedral WO4and pentahedral WO5species. Such highly dispersed tungsten species are assumed to be strongly interact with the Pt particles and support surfaces,leading to the observed promoting effect on the catalytic glycerol reaction.
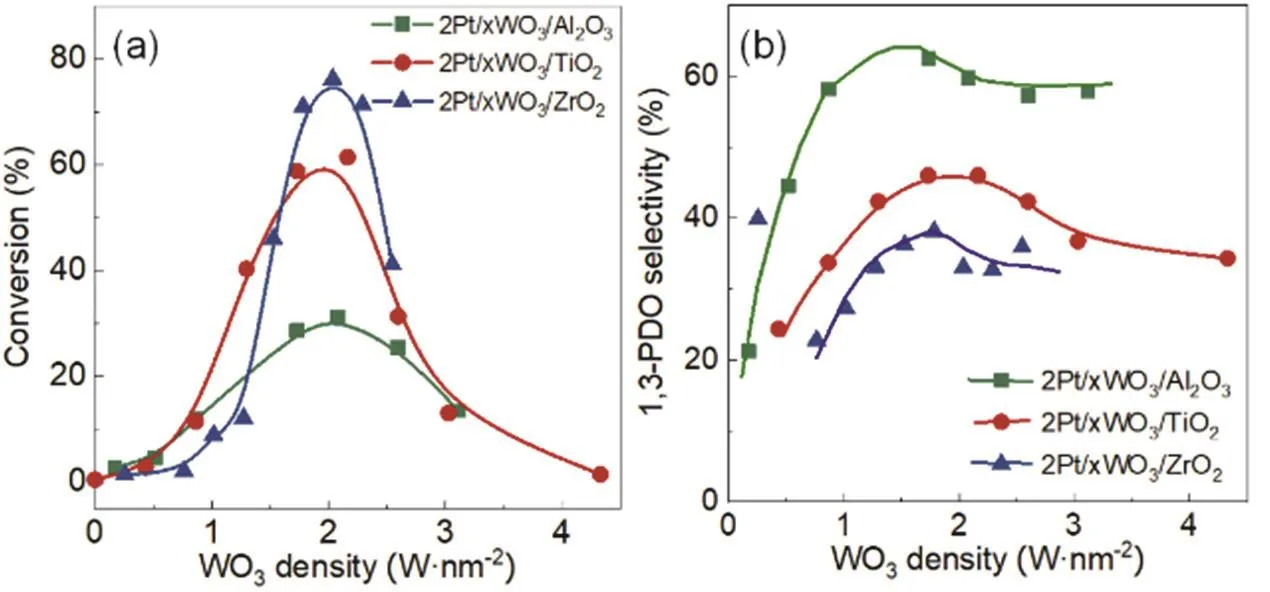
Fig. 2 Effect of WOx surface density of Pt/WO3/TiO2, Pt/WO3/ZrO2 and Pt/WO3/Al2O3 on (a) glycerol conversion and (b) 1,3-PDO selectivity.

Fig. 3 XRD patterns of 2Pt/xWO3/TiO2 catalysts(x represents WO3 loading).

Fig. 4 CO-DRIFTs spectra of reduced (a) 2Pt/TiO2 and(b) 2Pt/4WO3/TiO2.
To probe the Pt-WO3-TiO2interaction, CO-DRIFTs spectra were recorded for the 2Pt/TiO2and 2Pt/4WO3/TiO2catalysts. As shown in Fig. 4, the 2Pt/TiO2sample after treatment in H2at 140 °C shows a strong band at 2063 cm-1with two weak bands at 2114 and 2085 cm-1, which are assigned to the CO adsorption on the metallic Pt0and unreduced PtOxsites, respectively29. For 2Pt/4WO3/TiO2, it also exhibits three bands, including a strong band at 2071 cm-1and two weak bands at 2125 and 2094 cm-1,but these bands shifted to the higher wavenumbers, compared to those for 2Pt/TiO2. Such blue shifts in the presence of WO3indicate the electron transfer from the Pt sites to the WOxspecies,confirming their strong interaction on TiO2.
Notably, 1,3-PDO is stable on 2Pt/4WO3/TiO2under the conditions employed in this work. As shown in Fig. 5, by prolonging the reaction time from 10 min to 6 h, the conversion of glycerol increased from 6.9% to 46.7% at 120 °C and 4 MPa H2, and the selectivity to 1,3-PDO maintained essentially constant at 47%-51%. The main by-product was 1-PO, and it was also stable (40%-42%), while only trace amounts of 1,2-PDO were always detected during the whole reaction.

Fig. 5 Glycerol conversion and product selectivity with reaction time in glycerol hydrogenolysis on 2Pt/4WO3/TiO2.
The stability of 1,3-PDO is verified by its separate hydrogenolysis reaction on 2Pt/4WO3/TiO2. As shown in Table 2, the 1,3-PDO conversion was only 4.9% after reaction for 4 h at 120 °C and 4 MPa H2. Under the same conditions, the glycerol conversion (38.0%) and 1,2-PDO conversion (35.0%) were much higher, which confirm that 1,3-PDO is much less reactive than glycerol and 1,2-PDO on 2Pt/4WO3/TiO2. Such different reactivity of glycerol, 1,2-PDO and 1,3-PDO is more apparent from the detailed analysis of their rate constants (k) in the hydrogenolysis reaction on 2Pt/4WO3/TiO2(Fig. S3), which decreased in the sequence ofk1,2-PDO(4.6 × 10-3min-1) >kglycerol(2.7 × 10-3min-1) >k1,3-PDO(9.7 × 10-4min-1). Taken together,these results can well explain the observed relatively high selectivity to 1,3-PDO and 1-PO and low selectivity to 1,2-PDO on the Pt-WOxcatalysts (Table 1 and Fig. 5).
The stability of the 2Pt/4WO3/TiO2catalyst was also examined. As shown in Fig. 6, after recycling for 3 times at 180 °C and 4 MPa H2, the glycerol conversion decreased from 47.9% to 41.3%, while the selectivity to 1,3-PDO slightly decreased from 51.2% to 48.5%. The TEM images (Fig. 1)showed that the average size of the Pt particles slightly increased to 2.9 nm from 2.0 nm after 2Pt/4WO3/TiO2was recycled for 3 times. These results show that the catalyst is relatively stable during the glycerol hydrogenolysis under the conditions in this work.

Fig. 6 Glycerol conversion and 1,3-PDO selectivity during recycling tests of 2Pt/4WO3/TiO2 catalyst.

Fig. 7 Effect of reaction temperature on glycerol hydrogenolysis on 2Pt/4WO3/TiO2 catalyst.
3.2 Effects of reaction parameters on glycerol hydrogenolysis on Pt/WO3/TiO2 catalyst
Next, the effects of the reaction parameters on the glycerol reaction to 1,3-PDO were examined on 2Pt/4WO3/TiO2. As shown in Fig. 7, compared within the kinetically-controlled regime (i.e., 10%-30% glycerol conversion), when the reaction temperature increased from 80 °C to 160 °C, the activity increased dramatically from 1.6 h-1to 109.8 h-1, while the selectivity to 1,3-PDO decreased from 63.4% to 40.7%,currently with the increase in the selectivity to 1-PO from 30.0% to 49.3%. These results show that the higher temperatures are not favorable for the formation of 1,3-PDO, which can still undergo further hydrogenolysis to 1-PO on 2Pt/4WO3/TiO2.

Fig. 8 Effect of glycerol concentration on glycerol hydrogenolysis on 2Pt/4WO3/TiO2 catalyst.

Fig. 9 Effect of H2 pressure on glycerol hydrogenolysis o n 2Pt/4WO3/TiO2 catalyst.
Fig. 8 shows the activities and selectivities on 2Pt/4WO3/TiO2as a function of glycerol concentrations in the range of 1% to 40% at 120 °C and 4 MPa H2. Increasing the glycerol concentration led to a monotonical increase in the activity from 2.8 h-1to 37.8 h-1, indicative of a first-order dependence on the glycerol concentration. During the process, selectivity to 1,3-PDO and 1-PO maintained constant at around 50% and 40%,respectively. Such effect illustrates that 2Pt/4WO3/TiO2possesses the potential for the glycerol hydrogenolysis to 1,3-PDO at high glycerol concentrations.
Variation of the H2partial pressures also led to the change in the production distribution and glycerol conversion. As shown in Fig. 9, when the H2pressure increased from 1 MPa to 5 MPa,the glycerol conversion increased from 23.0% to the maximum value of 46.2% at 3 MPa H2, and then decreased to 29.5%. With increasing H2pressure, the selectivity to 1,3-PDO monotonically increased from 38.7% to 52.3%, accompanied by the decrease in the 1-PO selectivity from 50.2% to 39.0%. The lower reaction activities at the higher H2pressures above 3 MPa H2indicate that the glycerol hydrogenolysis involves its dehydrogenation as the kinetically-relevant step on 2Pt/4WO3/TiO2.
This proposition is consistent with the glycerol hydrogenolysis results in the presence of D2and D2O, instead of H2and H2O, on 2Pt/4WO3/TiO2.1H-NMR was used for analyzing the different deuterated products after their separation(Figs. S4-S11). It is noted that after the reaction at 140 °C for 2 h, the glycerol conversion reached about 30%, within this kinetically-controlled regime, the ratio of the H atoms on the secondary carbon to the H atoms on the primary carbons in the 1,3-PDO product was estimated to be 1 : 2. If 1,3-PDO was directly used as the reactant, after its hydrogenolysis reaction with D2and D2O, the H-D scrambling at the C-H bonds were negligible. These results allow us to propose that the glycerol hydrogenolysis to 1,3-PDO follows the mechanism, as depicted in Scheme 1, involving the dehydrogenation of glycerol to the glyceraldehyde intermediate, and the subsequent dehydration and hydrogenation of the intermediate on 2Pt/4WO3/TiO2.Further, differently, if the reaction follows other mechanism,such as dehydration and direct hydrogenolysis, the ratio of the H atoms on the secondary carbon to the H atoms on the primary carbons in the 1,3-PDO product should be 1 : 3 and 1 : 4,respectively (Scheme S1), which were indeed not consistent with the observed ratio (1 : 2).

Scheme 1 Proposed glycerol hydrogenolysis mechanism on Pt/WO3/TiO2.
4 Conclusions
The supported Pt-WOxcatalysts are efficient for the glycerol hydrogenolysis reaction, while the activity and selectivity sensitively depend on the WO3surface density. Irrespective the identity of the supports, the highly dispersed tungsten species at the similar surface densities of 1.5-2.0 W·nm-2lead to the maximum glycerol conversion and 1,3-PDO selectivity. Such strong dependence demonstrates that the highly dispersed WOxdomains play a key role in deterring the properties of the Pt-WOxcatalysts, as a result of their strong interaction with the Pt sites.The hydrogenolysis reactions of glycerol, 1,3-PDO and 1,2-PDO on 2Pt/4WO3/TiO2show that the rate constants of 1,2-PDO and glycerol are much higher than that of 1,3-PDO, indicative of the promising potential for further improving the selectivity to 1,3-PDO on the Pt-WOxcatalysts.
Supporting Information:available free of charge via the internet at http://www.whxb.pku.edu.cn.

