Degradation of Mg-Zn-Y-Nd alloy intestinal stent and its effect on the growth of intestinal endothelial tissue in rabbit model
Zongin Sun ,Zhnhui Wng,∗ ,Shokng Gun ,Shijie Zhu ,Tinghe Dun ,Qiuxi Zheng ,Shopeng Liu
a Luoyang Central Hospital affliiated to Zhengzhou University,Luoyang 471000,China
b School of Materials Science and Engineering,Zhengzhou University,Zhengzhou 45002,China
Abstract Biodegradable magnesium alloys have excellent properties with respect to biodegradability,biocompatibility,and biomechanics,which may indicate a possibility of its application in intestinal stents.Investigation of Mg-Zn-Y-Nd alloy’s application in intestinal stents has been performed.This study aims to investigate the degradation behavior of Mg-Zn-Y-Nd alloy intestinal stents coated with poly(L-lactide)/paclitaxel in the intestinal environment and its biocompatibility with intestinal tissue.In this paper,Mg-Zn-Y-Nd alloy’s corrosion properties were evaluated by the immersion test in human feces,SEM and XRD,and animal tests.In vitro results showed that when the Mg-Zn-Y-Nd alloy was immersed in human feces for two weeks,its corrosion resistance could be improved by micro arc oxidation (MAO) and poly-l-lactide(PLLA) dual coating.Additionally,this result was also confirmed in vivo experiments by rabbit model.And animal tests also demonstrated that the Mg-Zn-Y-Nd alloy with MAO/PLLA/paclitaxel dual coating drug-eluting stents could inhibit the proliferation of local intestinal tissue around the stents.However,in vivo studies illustrated that the intestinal stents gradually degraded in rabbit model within 12 days.Considering the degradation rate of the stent was faster than expected in rabbits,the support performance of the scaffold requires further improvement.
Keywords: Mg alloys;Degradability;Intestinal stent;Poly(L-lactide)/paclitaxel coating.
1.Introduction
Biomaterials have been widely employed in clinical medicine,including orthopedic implants or cardiovascular stents [1-6],including intestinal stents to treat intestinal stenosis [7-11].Stenting is considered as a novel approach for the endoscopic treatment of intestinal strictures,especially colorectal strictures.Stents can be used for both malignant and benign intestinal strictures,including neoplastic and inflammatory causes.Currently,the treatment of intestinal strictures include self-expanding metal stents (SEMS),self-expanding plastic stents (SEPS),and biodegradable stents(BDSs) [11-13].Ordinarily,those devices are made of stainless steel,titanium,and other metals.There are also reports on the use of biodegradable polymer materials such as scaffolds,but information on their mechanical properties and biocompatibility was insufficient [14].However,traditional medical implants are non-absorbable and long-term retention will cause foreign body rejection.Therefore,implants have to be removed in a second operation after successful healing [15].Moreover,these materials release toxic metal ions or particles,such as nickel and cadmium,which can cause inflammation and pathology [16-19],which limits its application.
In contrast to conventional metallic biomaterials,most investigations have shown that novel magnesium-based alloy materials have excellent biodegradable,biomechanical,and biocompatible properties.First,pure magnesium and its alloys gradually degrade in simulated body fluid or animal tissue with time [20-23].This feature helps to avoid a second operation to remove the implants.Secondly,in vivo studies have shown that the degradation process of magnesium alloys are not associated with obvious inflammation and necrosis and that degradation products do not change the serological index of vital organs [20,22,24-26].Additionally,when magnesium alloy is used as an implant material,the stress shielding effect of the bone implant material is greatly reduced [16,27],indicating that biodegradable magnesium alloy materials have good biomechanical properties.Therefore,biodegradable magnesium alloys have become a suitable alternative for intestinal stents,in theory.Due to the superiority of the degradable polymer coating,investigations have focused on improving the corrosion resistance of the Mg alloy,by modifying the surface using a biodegradable polymer coating [28-31].Previous reports have demonstrated that PLLA-coating significantly enhanced the corrosion resistance,compatibility [32-34],and mechanical properties of the magnesium alloy [35,36] and can be used in drug-eluting stents[37-39].Micro arc oxidation (MAO) treatment was another way to improve the corrosion resistance of magnesium alloys[40,41].It is well known that the external layer of the rough and brittle micro-arc oxidation layer has poor wear resistance,but its internal layer is hard,corrosion-resistant,and wearresistant.Paclitaxel is a widely used anticancer drug,extracted from the bark of a slow growing Western yew [42].Taking into account the anti-proliferative properties of paclitaxel[43],magnesium alloy stents treated with PLLA/paclitaxel coatings would be efficacious in the treatment of inflammatory and malignant intestinal strictures.However,there are few reports on the application of magnesium alloy drug-eluting stents in the intestinal environment;and the corrosion behavior and biocompatibility of magnesium alloy drug-eluting intestinal stents have not been published.
In this study,considering the excellent corrosion resistance,and the mechanical properties of the Mg-Zn-Y-Nd alloy[44,45],an extrusion Mg-Zn-Y-Nd alloy was processed into disk samples or scaffolds,followed by different treatments.Degradation behavior and biocompatibility of magnesium alloy drug-eluting intestinal stents were tested in vitro under simulated intestinal conditions and in vivo animal experiments(Fig.1).Corrosion morphology was evaluated through scanning electronic microscope (SEM) for the samples submerged in human feces (37 °C) for 0,2,7,and 14 days.Weightlessness tests were performed to evaluate the corrosion resistance of coatings.It is well known that the external layer of the rough and brittle micro-arc oxidation layer has poor wear resistance,but its internal layer is hard,corrosion-resistant,and wear-resistant [46].In view of this,one of the main purposes of this project is to investigate the effect of MAO coating on the corrosion resistance and support performance of magnesium alloy stents under support stress in vivo experiments.In addition,stents for different treatment were implanted into rabbits’ intestines.At different time,the stents were removed to evaluate corrosion behavior in vivo.The in vivo corrosion resistance of the stent was evaluated by x-ray and SEM and the effect on the proliferation of endothelial tissue biosafety was observed through immunohistochemical staining.

Fig.1.Graphs depicting dynamic degradation of the uncoated,MAO treated,PLLA-coated,and PLLA/paclitaxel-coated Mg-Zn-Y-Nd alloy samples (37 °C ± 0.5 °C) as a function of immersion time in human feces:weight loss of the samples.
2.Experimental
2.1.Preparation of specimens and coating
The new Mg-Zn-Y-Nd alloy was provided by Henan Key Laboratory,China.A Mg-Zn-Y-Nd alloy was melted and extruded into a rod shape,then processed into disk samples and silk samples.Disk samples with a diameter of 10.0 mm and a height of 3.0 mm were turned and cut from the extruded rods for in vitro degradation tests.Mg-Zn-Y-Nd alloy samples were ground with SiC paper progressively at 200,400,600,and 800 grits,polished with ethanol,and dried at room temperature.Mg alloy stent silks were prepared by single screw extrusion.After annealing treatment,the silks were woven into mesh stents with a diameter of 10.0 mm and a length of 25.0 mm.The diameter of the silk was 0.3 mm,and the stents improved the cleanliness and oxidation resistance by electrolytic polishing.
Part of samples were treated by micro arc oxidation(MAO).The composition of the electrolyte for MAO is listed in Table 1.When all the reagents were dissolved,vv deionized water was used to determine volume and the sample was mixed evenly.High frequency single pulse power supply (YS9000D-30040) was used in MAO process.The magnesium alloy sample was used as an anode to connect with the positive electrode of the power supply,and the stainless steel plate was connected with the negative pole of the power supply as the cathode.Under constant voltage mode,the voltage increased from 0 V to 260 V at 1.6 V/s rate.The samples were treated for 20 minutes and then washed with deionized water.The samples were naturally air-drying.The morphology of the samples after being polished was observed by scanning electron microscope (JSM-6490LV,Japan).Human feces were obtained from a hospitalized patient on the fifth day after intestinal obstruction surgery.
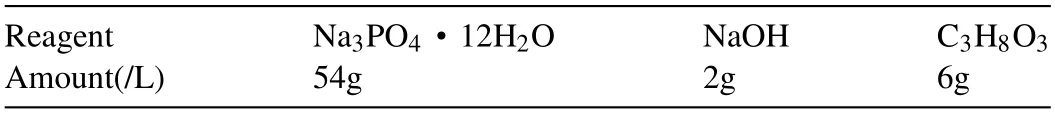
Table 1 Chemical composition of electrolytes for micro arc oxidation (MAO).
PLLA (molecular formula of PLLA: (C6H8O4) n,viscosity 3.4) and paclitaxel were respectively provided by the Science and Technology Company of Solarbio (Beijing,China)and the Academy of Pharmaceutical Sciences (Shandong,China).PLLA-coating,PLLA/paclitaxel-coating,MAO/PLLA coating,and MAO/PLLA/paclitaxel coating were all prepared by the dip coating method.The PLLA was dissolved in dichloromethane according to a 0.03 g/mL proportion,and paclitaxel (more than 98% content) was dissolved in the PLLA solution by a 0.008 g/mL proportion.The samples were soaked in the solution for 1 min,respectively,then pulled out slowly and evaporated for 12 h.In the evaporation process,the solvent temperature was maintained at 20°C to ensure that a volatilization rate of 24 h was 2 ml/h,making the thickness uniformity and adhesion of coating tend to standard.The PLLA and MAO/PLLA/paclitaxel coated Mg-Zn-Y-Nd alloy stents were also prepared this way.The thickness of PLLA coating is about 15.1 μm ± 3.1.Prior to the biocompatibility experiment,all magnesium alloy samples were sterilized for 1 h.
2.2.In vitro degradation studies
Human feces were used as soaking solvents to evaluate the degradation behavior of magnesium alloy stents in the human intestinal environment in in vitro degradation experiments.The corrosion morphology of Mg alloy samples was observed by SEM.
2.2.1.Microstructural analysis and weight loss test in human feces
The surface microstructure of the uncoated,MAO/PLLAcoated,and MAO/PLLA/paclitaxel-coated Mg-Zn-Y-Nd alloy disk samples was observed through scanning electronic microscope (SEM) after immersion in human feces for 0,2,7,and 14 days.The temperature of the feces was maintained at 37°C.The concentration of CO2was maintained at 5% and the experiment was carried out strictly in accordance with ASTMG31-72 (the ratio of surface area to solution volume was 1 cm2: 20 ml).Uncoated,PLLA-coated,and PLLA/paclitaxelcoated Mg-Zn-Y-Nd alloy disk samples were immersed in human feces by the same methods.The corrosion rate was observed by measuring the weight loss of Mg-Zn-Y-Nd alloy samples after 0,1,3,5,7,9,and 12 d.The corrosion rate was calculated by following equation [47]:
Corrosion rate=(K×W)/(A×T×D)
Where the coefficient K=8.76 × 10-4,W is weight loss(g),A is the sample area exposed to solution (cm2),T is the exposure time (h),and D is the density of the material (g/cm3).
2.3.In vivo study
In order to evaluate the corrosion resistance and biocompatibility of MAO/PLLA/paclitaxel-coated Mg-Zn-Y-Nd alloy eluting stent in vivo,stents with different drugs were implanted into the intestines of New Zealand rabbits for 2,5,8,and 12 days.New Zealand rabbits were randomly divided into three groups according to type of stent: uncoated Mg-Zn-Y-Nd alloy stents,PLLA coated Mg-Zn-Y-Nd alloy stents,and MAO/PLLA/paclitaxel coated Mg-Zn-Y-Nd alloy stents.In the same group,New Zealand rabbits were divided into different time points to study,with observation points of 2,5,8,and 12 days,respectively.The number of New Zealand rabbits in every group was no fewer than 5.In every group,the same part of intestinal tract was used for implantation of the different coated Mg-Zn-Y-Nd alloy stents.In this study,Ni-Ti alloy rings were used as biomarkers to track the position of stents in the intestine.The experiments were performed with 3% pentobarbital sodium to narcotize the animals at a dose of 2 ml/kg.
In this research,experiment and animal care was in accordance with the guidelines of the National Institutes of Health and the American Heart Association for animal care and use,and was approved by the animal research committee of Zhengzhou University.
2.3.1.Evaluation of corrosion resistance of stents in vivo
Implanted magnesium alloy stents were retrieved at different observation endpoints and observed for macroscopic morphology and external structure (2,5,8,12 days).The surface morphologies of bare,PLLA,and MAO/PLLA/paclitaxelcoated Mg-Zn-Y-Nd alloy stent silks were observed by SEM after 2 days of implantation.The corrosion morphology of the MAO/PLLA/paclitaxel-coated Mg-Zn-Y-Nd alloy stent silks were also observed after 5 and 8 days of implantation.In addition,in order to fully explore degradation behavior,x-rays were taken.
2.3.2.Immunohistochemistry test
The intestinal mucosa consists of the epithelium,lamina propria,and muscularis.The intestinal mucosa is the nearest tissue to the scaffold,therefore,the compatibility of scaffolds was evaluated by observing the status of the intestinal mucosa where the stents were located.Immunohistochemistry was performed on days 2 and 8.Specimens were immersed in 10% formaldehyde for 48 h,then washed through a series of gradient ethanol solutions (70%,80%,90%,and 100%)and lastly embedded in paraffin (30 and 50 microns).Paraffin sections were incubated in mouse proliferating cell nuclear antigen (PCNA) antibody (diluted 1:100;Absin Biotechnology Company,Shanghai,China),apoptotic genes Caspase-3(diluted 1:200),andα-smooth muscle actin (α-SMA) antibody (diluted1:200),and examined by immunohistochemistry.The tissues were observed by immunohistochemical staining.
3.Statistical analysis
Statistical analysis was performed to assess the differences between groups by analysis of one-way ANOVA.Step-wise regression analysis was conducted to elevate the dose effects.A p-value of less than 0.05 was considered statistically significant.Statistical values are shown in relevant experiments.
4.Results
4.1.Weightlessness tests of Mg-Zn-Y-Nd alloys in human feces
Fig.1 shows the dynamic degradation characteristics of the uncoated,MAO treated,PLLA-coated,and PLLA/paclitaxelcoated Mg-Zn-Y-Nd alloy samples (37 °C ± 0.5 °C) in human feces over time.As displayed in Fig.1,the weight loss of PLLA/paclitaxel-coated Mg-Zn-Y-Nd alloy specimens was significantly less than that of uncoated and MAO treated Mg-Zn-Y-Nd alloy specimens during the same period(P<0.05).It was also found that weight loss of PLLA/paclitaxel-coated alloy and PLLA-coated alloy was basically the same in the first 72 h of immersion(P>0.05),while the weight loss of PLLAcoated Mg-Zn-Y-Nd alloy specimens was slightly larger than that of PLLA/paclitaxel-coated Mg-Zn-Y-Nd alloy specimens after 72 h.This indicates that the Mg-Zn-Y-Nd alloy specimens modified with PLLA could improve corrosion properties strongly in human feces,and the PLLA/paclitaxel-coated Mg-Zn-Y-Nd alloy possessed better corrosion resistance.
Fig.2 shows that the corrosion rate of three alloys with different treatments after immersion in fences for up to 288 h.Fig.2 shows that the PLLA/paclitaxel-coated and PLLAcoated Mg-Zn-Y-Nd alloy specimens exhibit a much lower corrosion rate than that of the uncoated and MAO treated Mg-Zn-Y-Nd alloy specimens in human feces in the same period(P<0.05).There was no significant difference in corrosion rate between PLLA-coated and PLLA/paclitaxel-coated Mg-Zn-Y-Nd alloy in feces (P>0.05).From the results of Fig.2,over time,the corrosion rate of the former 72 h was obviously higher than that after 72 h;especially after 24 h,corrosion rate reaches its highest value.This is because during the initial period of immersion,the area of magnesium alloy samples exposed to immersion feces was larger and the samples had sufficient contact with feces,thus the corrosion rate was higher.Over time,corrosion products were deposited on the surface of the alloy,which led to insufficient contact with the feces and delayed corrosion.

Fig.2.Graphs depicting dynamic degradation of uncoated,MAO treated,PLLA-coated,and PLLA/paclitaxel-coated Mg-Zn-Y-Nd alloy samples(37 °C ± 0.5 °C) as a function of immersion time in human feces: corrosion rate of the samples.
The results of weight loss test in vitro show that the corrosion resistance of PLLA coating sample is better than that of MAO treated magnesium alloy sample,which may be related to the formation of porous oxide layer after MAO treatment.It can be inferred that PLLA coated magnesium alloy stent has better corrosion resistance than MAO and bare magnesium alloy stent,which will be further verified and explored in vivo experiments of rabbit model.
4.2.Corrosion morphology analysis of Mg-Zn-Y-Nd alloy samples (SEM&EDS)
The results show the surface morphology of the bare,MAO,MAO/PLLA-coated,and MAO/PLLA/paclitaxel-coated Mg-Zn-Y-Nd alloy samples immersed in human feces for different time periods at 37±0.5°C in Fig.3,A-P.As shown in Fig.3A.,E.,I.,and M,the surface of each set of magnesium alloy samples with different treatments was smooth and clean,and was maintained intact before immersion in human feces.A large number of bulk corrosion and cracks could be found on the surface of the bare magnesium alloy after immersion in feces for 2,7,and 14 days as displayed in Fig.3,B-D.A porous ceramic oxide film formed on the entire surface of MAO-coated Mg-Zn-Y-Nd alloy samples as shown in the EDS spectrum (Fig.4A),presenting pores and cracks of MAOcoating from the magnified morphology (Fig.3E).A porous PLLA film also formed on the surface of the MAO coating for the MAO/PLLA-coated alloy and the MAO/PLLA/paclitaxelcoated alloy,sealing the pores of the MAO coating as depicted in the magnified morphologies (Fig.3 I.,M).After 7 days,the MAO coating surface became rough (Fig.3G),and after 14 days,a large number of corrosion products formed on the surface of the MAO-coated Mg-Zn-Y-Nd alloy as shown in Fig.3H,suggesting that the substrate of the Mg-Zn-Y-Nd alloy had corroded.However,besides the intestinal contents attached to the surface of the MAO/PLLA/paclitaxel-coated alloy(Fig.3N.,O.,and P),the alloy surface was very smooth,without obvious corrosion phenomenon and with minimal damage on the surface of the MAO/PLLA/paclitaxel-coated alloy and MAO/PLLA-coated alloy after soaking for 14 days(Fig.3I.,P).

Fig.3.SEM images of the surface morphologies of bare,MAO,MAO/PLLA-coated,and MAO/PLLA/paclitaxel-coated Mg-Zn-Y-Nd alloy samples after 0,2,7,and 14 days in dynamic human feces testing (37 ± 0.5 °C): (A-D) the bare,(e-h) MAO,(I-L)MAO/PLLA-coated,(M-P) MAO/PLLA/paclitaxel-coated Mg-Zn-Y-Nd alloy samples (magnifications are at original 100X;magnifications of enlarged pictures in Fig.E,I,and M are 500X).

Fig.4.The morphology and corresponding EDS spectrum: (A) high magnification of MAO coating after 7 days in dynamic human feces testing (37 ±0.5 °C) and (B) its EDS spectrum.(C) high magnification of the corrosion pit on the surface of the MAO coating after 14 days in Fig.4H,and its EDS spectrum(D).
The high magnification of Fig.4A showed that the pores of the oxide film extended from the inside to the outside due to corrosion.The cracks between the pores become larger.The EDS spectrum in Fig.4A illustrated that the elements of Mg,P,O,Ca,and P proved to be the main component of the MAO coating,as demonstrated in Fig.4B.The high magnification of Fig.4C presented the microscopic corrosion morphology of the ceramic oxide film after magnesium alloy samples immersed for 14 days.The EDS spectrum in Fig.4D also showed that the gray products formed on the surface of the alloy was mainly composed of O,P,Ca,Mg,C,Au,and a small amount of Na,N and Si.
These results of Figs.3 and 4 confirmed that the Mg-Zn-YNd alloy treated with MAO/PLLA coating possessed excellent corrosion resistance in a complicated intestinal environment.And in vitro immersion test also shows that MAO/ PLLA coated magnesium alloy sample has better corrosion resistance than MAO treated magnesium alloy sample.It can be inferred that MAO/ PLLA coated magnesium alloy stent has better corrosion resistance than MAO treated magnesium alloy sample.In conclusion,we speculate that MAO/PLLA coated magnesium alloy stents have better corrosion resistance than PLLA coated magnesium alloy stents.Therefore,in view of the results of in vitro experiments,we further designed and prepared MAO/ PLLA coated magnesium alloy stent as the main research object,and its degradation characteristics will be verified and explored in vivo experiments of rabbit model.
4.3.X-ray scanning evaluation of corrosion resistance of stents

Fig.5.X-ray films of the bare,PLLA,MAO/PLLA/paclitaxel-coated Mg-Zn-Y-Nd alloy stent after implantation in New Zealand rabbits,for PLLA/paclitaxelcoated Mg-Zn-Y-Nd alloy intestinal stents implanted in rabbits at 2,5,and 8 days: (A-C) bare stents;(D-F) PLLA-coated stents;(G-I) MAO/PLLA/paclitaxelcoated Mg-Zn-Y-Nd alloy stents.
Fig.5 illustrates x-ray films of the bare,PLLA,MAO/PLLA/paclitaxel-coated Mg-Zn-Y-Nd alloy intestinal stents implanted in rabbits for different time periods.Fig.5A,D.,and G shows a bright,high-density cylindrical tube in the intestines,which is the x-ray film of the magnesium alloy intestinal scaffold for 2 days.Fig.5B shows the bare stent degraded completely and disappeared after implantation in New Zealand White Rabbit for 5 days,leaving only the ring marker of Ni-Ti alloy.Fig.5E.and H.shows a weaker x-ray film of the PLLA/paclitaxel-coated Mg-Zn-Y-Nd alloy intestinal stents implanted for 5 days,compared with that of the intestinal stents implanted for 2 days.This is due to the degradation of stents in the intestines of New Zealand rabbits.Fig.5I shows that the x-ray film of MAO/PLLA/paclitaxelcoated Mg-Zn-Y-Nd alloy stents implanted for 8 days is significantly weaker than that of stents implanted for 5 days,which indicated that MAO/PLLA/paclitaxel-coated Mg-Zn-YNd alloy intestinal stents began to degrade in large quantities after implantion into rabbits for 8 days.In the case of the bare and PLLA intestinal stents implanted for 8 days,the x-ray film of intestinal stents was almost invisible.This is probably due to the almost complete degradation of the stent.
4.4.SEM evaluation of corrosion resistance of stents in vivo
Fig.6 shows the local corrosion morphology of the magnesium alloy stent silks implanted for 2 days.It was found that the structure of the stent for uncoated Mg-Zn-Y-Nd alloy was intact and a few cracks and flaking formed on the surface of the silks in Fig.6A,D.The cracks and flaking on the surface of silks in the PLLA-coated stent group were less than that of the bare stent group.However,there were much smoother and smaller corrosion pits on the surface of silks in the MAO/PLLA/paclitaxel-coated stents compared to that of uncoated Mg-Zn-Y-Nd alloy stents.This indicates that MAO/PLLA/paclitaxel-coated stents have better properties of corrosion resistance than the bare Mg alloy stents,as shown in Fig.6C and F.Fig.6F showed that villous microbes were attached to the surface of the stent.An interesting phenomenon was that silk corrosion at the junction point was more serious than that of the non-junction point,as revealed in Fig.6.

Fig.6.SEM images of the surface morphologies of the bare,PLLA,and MAO/PLLA/paclitaxel-coated Mg-Zn-Y-Nd alloy stent silks after implantion in New Zealand rabbits for 2 days: (A,D) the silks of bare stents,(B,E) the silks of PLLA-coated stents,(C,F) the silks of MAO/PLLA/paclitaxel-coated stents.(A-C) silks located in the non-junction;(D-F) silks located in the junction.

Fig.7.SEM images of the local corrosion morphology of the MAO/PLLA/paclitaxel-coated Mg-Zn-Y-Nd alloy stent silks after implantation in New Zealand rabbits for 2,5,and 8 days: (A-C) silks located in the non-junction;(D-F) silks located in the junction.
Fig.7 shows the local corrosion morphology of the magnesium alloy stent silks implanted for different periods.No obvious corrosion was found on the surface of the stent after implantation into New Zealand rabbits for 2 and 5 days in Fig.7A,B,D,and E.A small amount of cracks (shown by the red arrow) appeared on the surface of the support wire after implantation in New Zealand rabbits for 8 days,as illustrated in in Fig.7C and F.It could be also found that large defects and more cracks formed on the surface of silks located in the junction,rather than in the non-junction in Fig.7C and F (P<0.05).The generation of cracks and defects may be related to the change of support stress.
4.5.Immunohistochemistry evaluation of intestinal tissue around the stent
Fig.8 shows the immunohistochemical staining results for PCNA,caspase-3,andα-SMA.Two days postoperatively,the expression level of PCNA in the MAO/PLLA/paclitaxelcoated Mg-Zn-Y-Nd alloy stents (MPPS) group is slightly higher than that of the control group in Fig.8A.At eight days postoperatively,the expression level of PCNA in the MPPS group shows a significant decrease compared with that two days postoperatively,and the expression level of PCNA in the MPPS group is lower than that in the control group during the same period.However,it could be seen that the expression levels of PCNA in the uncoated group were always significantly higher than in the MPPS group and control group over time in Fig.8A.For caspase-3 expression,the expression levels of caspase-3 in the MMPS group were significantly higher than in the uncoated group and control group at all of the experimental periods in Fig.8C.However,it was found that there were no significant differences in caspase-3 expression between uncoated and control groups during the experimental period.Forα-SMA expression,the expression levels ofα-SMA in the uncoated group were significantly higher than in the MPPS and control groups in all experimental periods.The expression levels ofα-SMA in the MPPS group were significantly lower than in the control group at 2 or 8 days postoperatively in Fig.8E.The results also showed that fewer collagen fibers were found in the MPPS group compared with the other two groups in Fig.8F.
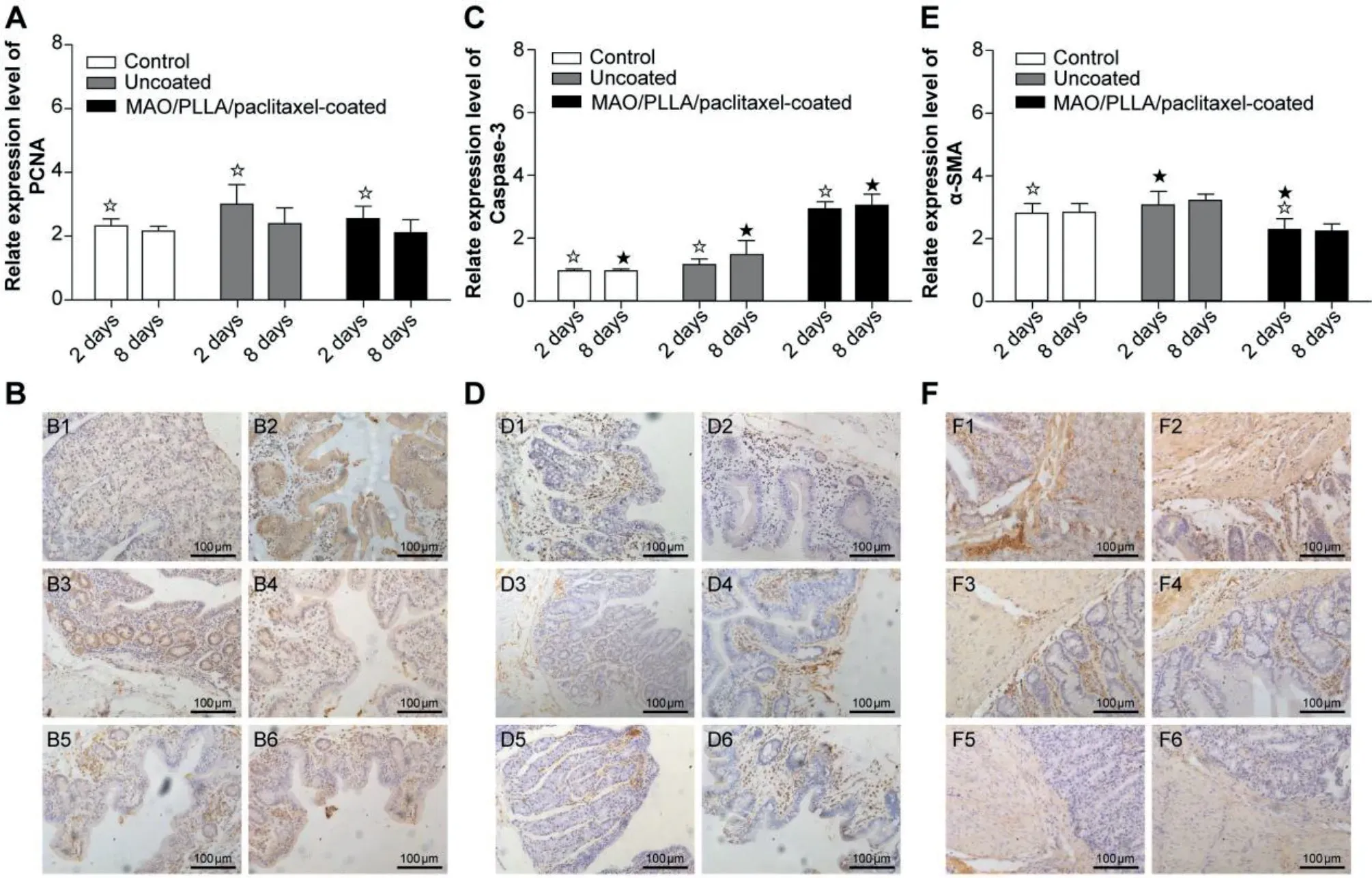
Fig.8.The expression of PCNA(A),Caspase-3(C),and α-SMA(E) antibody in the process of degradation after stenting with different components by immunohistochemistry.Statistical significance is indicated by ☆or ★(P <0.05).The right pictures showed histopathological changes of PCNA(B,magnification x200),Caspase-3 (D,magnificationx200),and α-SMA (f,magnification x200) antibody 2 days postoperatively (B1,B3,B5,D1,D3,D5,F1,F3,F5) and 8 days postoperatively (B2,B4,B6,D2,D4,D6,F2,F4,F6).In this test,the symbol B1-2,D1-2,and F1-2 represent the control group,B3-4,D3-4,and F3-4 represent the uncoated group,and B5-6,B5-6,and F5-6 represent the PLLA/paclitaxel-coated group.
5.Discussion
As an ideal medical biomaterial used in intestinal stenosis,magnesium alloy materials should have certain characteristics,including a suitable degradation rate and good mechanical properties.Previous studies regarding stents for intestinal stenosis mainly focused on non-absorbable metal materials,such as stainless steel or nitinol stents [48].However,the application of biodegradable magnesium alloy drugeluting stents as an intestinal scaffold for intestinal stenosis was rarely reported,and the degradation behavior of magnesium alloy drug-eluting stents under intestinal conditions was not well known.In this study,in vitro experimentation with human feces (rather than simulated body fluids) were used for the first time as a corrosive environment,to investigate the degradation of magnesium alloy stents under intestinal conditions.The results of the in vitro experiments indicated that PLLA/MAO/paclitaxel Mg-Zn-Y-Nd alloy drug-eluting stents possessed good corrosion resistance compared to the bare Mg-Zn-Y-Nd alloy stents and could inhibit the proliferation of local epithelial cells to prevent endothelialization in the intestinal tract.
5.1.Degradation of Mg-Zn-Y-Nd alloy drug-eluting stents under intestinal conditions
In addition to water,fecal components include protein,fat,undigested food fibers,inorganic salts,and other elements.Confronting high concentrations of buffering agents in human feces,the degradation rate of the magnesium substrate obviously increased.It has been demonstrated that inorganic components and proteins affect the corrosion rate [49].Magnesium was still the dominant component in the Mg-Zn-Y-Nd alloy system.When magnesium alloy is exposed to human feces,the following reaction would take place [50]:

At present,bare metal stents and drug-eluting stents are mostly made of non-degradable metals and polymers,which may have negative effects on surrounding tissues in the stage of chronic pathological reaction [51].The biggest defect of stainless steel and titanium materials is non-absorbability.Moreover,some studies have confirmed that long-term retention of non-absorbable materials in the body may lead to foreign body rejection and damage human life and health[15].Therefore,after healing,these non-absorbable materials have to be removed.Otherwise,patients need to face the pain of second operation and the long-term harm of implant materials to human life and health.In addition,magnesium alloys have great support strength.Christoph [52] et al.compared the strength of implant-bone interface of a new degradable magnesium alloy (Mg-Y-Nd-HRE,based on WE43) with that of Ti-6Al-7Nb,which is currently used clinically.The results show that the magnesium alloy group has higher tensile force and shear strength than the titanium alloy group.Heat treatment is one of the important ways to improve the mechanical properties of magnesium alloys through refining precipitates and grains.It has been proved that heat treatment can optimize the microstructure and mechanical properties of extruded Mg-Zn-Y-Nd alloy [53].Therefore,annealing treatment was applied to the preparation of stents in this research.
In this study,extrusion Mg-Zn-Y-Nd alloy was applied to the preparation of disk samples and the degradation performance was explored through weight loss experiments.And in one study,weight loss experiments confirmed that the corrosion resistance of Mg-Zn-Y-Nd alloy could be improved by increasing extrusion ratio and extrusion pass [54].The weight loss of PLLA/paclitaxel-coated Mg-Zn-Y-Nd alloy was significantly lower than that of uncoated Mg-Zn-Y-Nd alloy specimens at the same period (Fig.1) in this study.The corrosion rate of PLLA/paclitaxel-coated Mg-Zn-Y-Nd alloy reached a maximum in the first 24 hours,and subsequently the corrosion rate gradually decreased with time and finally stabilized(Fig.2).Although the corrosion rate of PLLA/paclitaxelcoated Mg-Zn-Y-Nd alloy was lower than that of the PLLA-coated Mg-Zn-Y-Nd alloy,there was little difference in the corrosion rate between the two groups.It was indicated that under intestinal conditions the Mg-Zn-Y-Nd alloy specimens modified with PLLA/paclitaxel could effectively improve corrosion properties.
Under intestinal conditions,the magnesium substrate treated without MAO or polylactic coating shows that some cracks were found with time,as shown in Fig.3C and D.This illustrated that the bare Mg-Zn-Y-Nd alloy exposed to the intestinal environment began to corrode strongly in the first week of implantation.Song et al.found that the polylactic acid coating on high purity magnesium during dynamic degradation in SBF could be dissolved and washed away by exposed solution[55].In this study,after exposure to the feces for a period of time,the PLLA film degraded and swelled,and then a high concentration of solvent permeated into the magnesium substrate,when the above reaction took place(formula 1 and 2).Hydrogen was produced in solvent,and the locally aggregated hydrogen was likely to push away the polymer films during its escape.In addition,the thickness of the film can also affect the corrosion resistance of the magnesium alloy,especially the film prepared by the dip coating method,which is uneven.Some fields of the film were so thin that they easily degraded and the solvent in feces easily permeated into the magnesium substrate through the micro-pores in the MAO coating,leading to the above reaction in formulas 1,2,and 3,presenting the film as having fallen off in the strong intestinal corrosion environment.Finally,the stents were completely degraded.Therefore,the corrosion phenomenon could possibly be improved by increasing the thickness of the coating [56].
The cracks between the pores of the ceramic oxide film on the MAO coating magnesium alloy without the PLLA coating become larger when immersed for 14 days,which indicated that the micropores of MAO layer were harmful to long term stability of the implants.In this study,polylactide coatings could effectively block the contact between the magnesium body and buffer agent,and protected the magnesium substrate from corrosion for the MAO/polymer group as revealed in Fi.3.The occurrence of this phenomenon is closely related to the MAO layer between the polymer and the magnesium substrate.Previous studies demonstrated that the MAO layer enhanced the bond force between the organic and the magnesium substrate,making the adhesion strength more stable[41,57],and the dual coating significantly enhanced the corrosion resistance of the Mg-Zn-Y-Nd alloy under intestinal conditions.In the in vivo study,it was found that the microcosmic visual field of silks in the MAO/PLLA/paclitaxel-coated Mg-Zn-Y-Nd alloy stents showed better corrosion resistance compared to that of two other groups without MAO,as shown in Figs.6 and 7.The EDS spectrum results of the MAO coating in Fig.4B illustrate that the main corrosion products of the MAO coating are insoluble phosphates,carbonates,and insoluble calcifications besides Mg(OH)2and MgO.Fig.4D showed that the deposit sediment on the surface of the MAOcoated alloy samples may be the part of the intestinal content besides the corrosion products of the Mg-Zn-Y-Nd alloy.
The maintenance of structural integrity over time is one important factor of all the factors that influence the performance of mesh magnesium alloy intestinal stents[58].In vitro immersion experiments shows that,in addition to intestinal sediment adhering on the surface of the alloy,the alloy surface is smooth and uniform,which indicates that Mg-Zn-Y-Nd alloy sheets treated by a PLLA/MAO/paclitaxel coating have fair resistance to corrosion under intestinal conditions within 14 days.However,in vivo experiments in rabbits showed that the fracture of a large number of fibers on the scaffold leads to the complete collapse of the scaffold structure after implantation for 12 days in rabbits.Meanwhile,the x-ray films of intestinal stents were almost invisible after implantation for 12 days.Both results demonstrated that PLLA/MAO/paclitaxelcoated Mg-Zn-Y-Nd alloy intestinal stents have been almost completely degraded,without enough to support the intestinal framework after implantation for 9-14 days.So-Ra Son et al.revealed that all PLGA/Gelatin intestinal stents degraded within 14 days in the rat model,when the internal environment of the wound returned to normal,and the intestinal muscles began to move normally [59].However,the degradation rate of the stent designed in this study was slightly faster,and the time of degradation was slightly shorter than the healing time of the intestinal wound.Due to the effect of support stress [60],the corrosion degree of silk at the junction point was higher than that of the non-junction point,as revealed in Fig.7C and F.
Although brittleness increases in the Mg alloy after microarc oxidation,there are no reports on the use of micro-arc oxidation for stent research in the literature at present.In view of the fact that the effect of brittleness of the MAO-coating on corrosion resistance and support of stents remains unclear,it is known through the in vivo study that the corrosion resistance of the stent increases after micro-arc oxidation,which provides a good experience for future design of stent.
5.2.In vivo biocompatibility
Excellent biocompatibility of biomedical materials plays an important role in the application of intestinal stents.T.A.GrU Newald [61] and Carolin [62] had also confirmed that magnesium alloys have good biocompatibility compared with traditional biomedical materials (stainless steel,titanium and its alloys,etc.).However,the main challenge of stenting technology is to prevent restenosis (coronary restenosis).Restenosis is mainly caused by excessive growth and migration of underlying smooth muscle cells caused by damage to the arterial wall [63].Antiproliferative drugs such as sirolimus (SIR)and paclitaxel (PAT) released from drug-eluting cardiovascular stents are currently available to treat neointimal hyperplasia [64].Paclitaxel can destroy the M-phase of cell cycle,thereby inhibiting cell proliferation by enhancing very stable microtubules.Moderate release kinetics has been shown to inhibit the proliferation of HCASMCS (human coronary artery smooth muscle cell) and reduce the risk of necrosis through the release of paclitaxel [65].However,at present,there are few reports on the effect of paclitaxel-eluting magnesium alloy stent applied in the treatment of intestinal stenosis on the proliferation of intestinal endothelial tissue.
The in vivo study results revealed that during the early period after the stents were placed into the rabbit intestines,mechanical damage could stimulate intestinal tissue proliferation.That is why the expression level of PCNA in the MPPS (MAO/PLLA/paclitaxel-coated Mg-Zn-Y-Nd alloy stent) group was slightly higher than that of the control group two days postoperatively in Fig.8A.The paclitaxel released by MPPS inhibited tissue proliferation,which caused a decrease in expression level of PCNA in the MPPS group 8 days postoperatively.Instead,the bare stents did not release products that inhibited intestinal tissue proliferation.On the basis of expression levels for PCNA and caspase-3 in the uncoated groups for the entire experimental period in Fig.8C,it suggests that uncoated Mg-Zn-Y-Nd alloy stents do not induce apoptosis in the in vivo environment.It is related to the release of magnesium ions during stent degradation.There are also previous reports that excessive magnesium ion concentration or alkaline stress could produce negative stimuli for the cell population [66].Moreover,low magnesium diet may lead to higher intra-cellular ratio of Ca:Mg,leading to hypertension and insulin resistance[67].A precious study revealed that although low magnesium intake is related to constipation,high doses of oral magnesium have a defecation effect [68].This effect of magnesium may be related to the following facts: they are absorbed from the intestinal cavity and play a penetrating role to maintain water,thereby increasing the fluidity of the lumen content [69].Local delivery of paclitaxel has been demonstrated to inhibit hyperplasia,and have a prolonged effect on cells after a brief exposure time without causing systemic toxicity at a therapeutic dose [43].In addition,in vivo immunohistochemistry results also proved the superiority characteristics of paclitaxel for drug eluting stents for preventing inflammation and scar formation (Fig.8B,D,F).To sum up,the MAO/PLLA/paclitaxel-coated Mg-Zn-YNd alloy intestinal stents presented less tissue inflammation and collagen fiber proliferation compared with uncoated Mg-Zn-Y-Nd alloy intestinal stents.
5.3.Prospect of magnesium alloy surface modified
To date,in order to improve the surface properties to allow better adaptation to the physiological surroundings,surface modification of Mg-based biomaterials including substrateinvolving coatings,Ca-P based coatings,polymer-based coatings,composite coatings,and ion implantation were applied to enhance surface corrosion resistance,biomechanical properties,and biocompatibility of Mg-based biomaterials [70].Wang found that the addition of Nd led to homogeneous and equiaxed grains,the improvements in mechanical and corrosion properties of Mg-2Zn-0.46Y-xNd alloys [44].In this study,Mg-Zn-Y-Nd alloy treated with PLLA/MAO/paclitaxel coating revealed excellent corrosion resistance.
Degradation rates of stent is influenced not only by temperature,pH,and type of body tissue/fluid,but also pulse frequency,treatment time,duty cycle of micro arc oxidation[41],among others.In conclusion,designing biodegradable magnesium alloys with good biodegradability,biomechanical properties,and biosafety is the key to developing suitable intestinal stents.
6.Conclusion
In this study,in vivo studies illustrate that the PLLA/paclitaxel coating on the Mg-Zn-Y-Nd alloy has an obvious inhibitory effect on increment of intestinal endothelial tissue.This mechanism can effectively inhibit the proliferation of local intestinal tissue to help to solve the problem of intestinal stenosis.In addition,the in vitro study demonstrates that the Mg-Zn-Y-Nd alloy with the MAO coating and PLLA dual coating has better corrosion resistance in the intestinal environment within 14 days,and the Mg-Zn-Y-Nd alloy with MAO/PLLA/paclitaxel drug-eluting coating showed a significant delay in corrosion of the intestinal environment.Besides Mg(OH)2and MgO,insoluble phosphates,carbonates,and calcifications were also deposited on the surface of the Mg-Zn-Y-Nd alloy with MAO coating with the extension of immersion time.However,the in vivo study in the rabbit model illustrates that the intestinal stents gradually degraded in vivo within 12 days.The degradation rate of the stent was faster than expected,and the support performance of the scaffold needs further improvement.Considering that the effect of brittleness increases in the Mg alloy after MAO on corrosion resistance and support of stents under research conditions,this study provides experience for next steps in optimizing design of scaffolds.At present,the controllability of degradation of the alloy scaffold is being studied in the next step.
Declaration of Competing Interest
There is no conflict of interest.
Acknowledgments
We sincerely thank Scientific committee of the national natural fund of China for the research funds support and the help of professor Shijie Zhu,Dr.Yongxin Yang and Hu Chen in the School of Materials Science and Engineering,Zhengzhou University.The funding of this research come from the National Natural Science Foundation of China(No.U04825),the Key Scientific and Technological Projects of Henan Province (No.2102310012),the Natural Science Foundation of Henan Province (No.2300410241),the National Key Research and Development Program of China(2018YFC1106703) and the Science and Technology Development Projects of Luoyang City (No.03006A-3).
 Journal of Magnesium and Alloys2022年8期
Journal of Magnesium and Alloys2022年8期
- Journal of Magnesium and Alloys的其它文章
- Improving the Young’s modulus of Mg via alloying and compositing -A short review
- Surface modifciation of magnesium alloys using thermal and solid-state cold spray processes: Challenges and latest progresses
- Preparation,structure and properties of Mg/Al laminated metal composites fabricated by roll-bonding,a review
- A review on recent advancements in biodegradable Mg-Ca alloys
- Analysis of element loss,densification,and defects in laser-based powder-bed fusion of magnesium alloy WE43
- Pore structure of porous Mg-1Mn-xZn alloy fabricated by metal-gas eutectic unidirectional solidification✩
