Impact of microsecond-pulsed plasma-activated water on papaya seed germination and seedling growth
Deng-Ke Xi(席登科) Xian-Hui Zhang(张先徽) Si-Ze Yang(杨思泽) Seong Shan Yap(叶尚姗)Kenji Ishikawa(石川健治) Masura Hori(堀勝) and Seong Ling Yap(叶尚凌)
1Plasma Technology Research Center,Department of Physics,Faculty of Science,University of Malaya,Kuala Lumpur 50603,Malaysia
2Fujian Provincial Key Laboratory of Plasma and Magnetic Resonance,Fujian Engineering Research Center for EDA,Fujian Provincial Key Laboratory of Electromagnetic Wave Science and Detection Technology,Xiamen Key Laboratory of Multiphysics Electronic Information,Institute of Electromagnetics and Acoustics,Xiamen University,Xiamen 361005,China
3Department of Physics,Xiamen University Malaysia,Selangor Darul Ehsan 43900,Malaysia
4Center for Low-temperature Plasma Sciences,Nagoya University,Nagoya 464-8603 Aichi,Japan
Keywords: plasma-activated water,non-thermal plasma,microbial inactivation,seed metabolism
1. Introduction
Many seed plants have been domesticated in modern agriculture as monoculture crops. Plants generated are susceptible to diseases, and seeds produced have high dormancy. Priming of seeds is often performed by commercial seed suppliers.In smaller scale,farmers could perform hydropriming by presoaking the seeds for a specific duration prior to germination.Papaya is a popular fruit crop grown from seed. Its global export was approximately 353 ktonne in 2020.[1]Papaya is a rich source of nutrients, including vitamins (A, C, E, and K),minerals(such as potassium and calcium),and papain(an enzyme that helps digest proteins and is mainly used in the food industry).[2,3]The possible health benefits of consuming papaya include reducing the risk of heart disease, aiding in digestion, lowering blood pressure, and improving wound healing.[4]
Papaya seed contains the seed coat,endosperm,and embryo. Seed germination is a prerequisite part of plant growth and crop production, being crucial for next-generation plant development. It is a complicated process,starting from breaking dormancy, undergoing testa and endosperm rupture, and ending up with radicle protrusion, and affected by many factors.[5]In the case of papaya,the seed can germinate when the fruit is ripe, but desiccation leads to seed dormancy, in which seed cannot temporarily germinate due to the isolation of water and oxygen.[6]Besides,the hard shell-like seed coat with poor penetrability further isolates water and oxygen absorption and resists radicle protrusion by its mechanical energy to suppress seed germination.[7,8]Once water absorption of papaya seed,metabolism takes place to provide energy for germination and overcome dormancy,but the inhibitorsin vivowill suppress it.[9,10]Abscisic acid(ABA)is a potent inhibitor in keeping seed dormancy and inhibiting embryo development by preventing the metabolism of sugars and proteins.[11]The above-mentioned factors result in a slow, erratic, and asynchronous germination of papaya. Therefore, the seed treatment is essential to promote germination for plant growth and crop production.
Seed treatments have been widely studied and applied to suppress detrimental microorganisms on seeds,to promote germination rate and enhance early seedling growth. In the treatment of papaya seeds, low or high temperature,[7,12,13]plant hormones,[14]and chemicals[15,16]were applied in many studies. For example, exposure to ultra-low temperature(−196◦C for 3 d) was reported to have enhanced the germination rate by 28%due to cracks in the seed coat, improving the seed imbibition and release of inhibitorsin vivo.[12]In general, papaya seeds responses to heat shock. Websteret al.[7]analyzed the heat shock (35◦C for 4 h) treated papaya seeds and concluded that the heat shock did not change the seed coat while the physiological changes induced within the seed was associated with protein synthesis. An improved germination of papaya seed triggered by heat shock (36◦C for 4 h) was also reported by Woodet al.[13]They suggested that the physiological change involving protein synthesis was possibly related to the activation of a heat-sensitive signaling pathway.Nitrate is an effective chemical in breaking seed dormancy and promoting papaya seed germination. Specifically, Furutaniet al.[15]used 1.0-M KNO3to treat papaya(Carica papaya L.) seeds for 15 min and found that the germination time reduced to 11 days–14 days for 50%germination rate compared with the control group. In the work of Zanottiet al.,[16]the germination rate of papaya increased by 34% after 30 days,and the starch and lipid mobilization distinctly increased after KNO3(1 M)soaking for 60 min. Anburaniet al.[17]found that the highest germination rate(78.3%)was obtained and the germination time was also shorter in the soaking treatment of GA3(200 ppm)for 12 h. Similar results can be found in other works.[13–16]Compared with KNO3treatment,the concentration of GA3 was lower, varying from 0.1 mM to 1 mM for promoting papaya seed germination. However, the synthesis and extraction of GA3 are complicated and costly.

Fig.1. A pictorial scheme showing the growth cycle of papaya. µPAW treatment could effectively inactivate microbes,modify the seed coat,and regulate the metabolism and proportion of ABA to break dormancy; germination underdoes the rapture of seed coat and endosperm, and radicle protrusion; in seedling developmental stage, roots absorb nutrients in different levels, and the ions’ content, SOD activity and MDA content are reflected from the metabolism of early seedling growth.
Water can be activated by non-thermal plasma treatment, generating some long-live species including H2O2,NO−2, NO−3, and NH+4, often named plasma-activated water (PAW).[18]PAW has an outstanding antibacterial ability,due to the presence of reactive oxygen and nitrogen species(RONS) and an acidic condition, and can efficiently inhibit a wide range of microorganisms.[19–21]Zhouet al.[22]used an atmospheric plasma jet to treat deionized water with the feeding gases of N2,He,O2,and air,and investigated the effect of plasma-treated water(PTW)on the germination and seedling growth of mung beans. In their study,PTW treatment with air as feeding gas obtained a germination rate of 93.67%at 24 h,significantly higher than that of He-PTW(51.33%),N2-PTW(42.33%), and the control group (29.67%). Meanwhile, Air-PTW also resulted in the highest plant length,vigor index,and diameter of the hypocotyl of the treated mung bean seedlings.These seedlings possessed the highest SOD activity and IAA content and lowest MDA and ABA contents, indicating that PTW treatment suppressed oxidative damage and increased antioxidant enzyme activities. Recently, Machadoet al.[23]applied PAW to treat alfalfa(Medicago sativa)and mung bean(Vigna radiata) seeds inoculated with Escherichia coli O157 and E.coli O104,respectively,to investigate the effect of PAW on antibacterial, seed germination, and plant growth. PAW was demonstrated to have an excellent antibacterial ability,with a reduction of Log101.67 CFU/g in alfalfa seeds inoculated with E.coli O104 and Log101.76 CFU/g in mung bean seeds inoculated with E.coli O157. And the germination rate and growth of alfalfa and mung bean were not influenced after the PAW treatment, but the control group had a lower germination rate due to the inoculation of Escherichia.
As the production of PAW does not use chemicals and not produce toxic side products, it is generally considered as a clean resource, without negative impact to the environment. Discharge in air introduces ionic nitrogen compounds into PAW serves as a nitrogen fertilizer when applied to plants.This has an added advantage as an antimicrobial agent and a potential stress effect promoting germination. However, the production of PAW by non-thermal plasma varied largely by the methods of plasma generation. The effect of PAW on papaya seed germination has not been researched,despite germination of papaya seeds remains a challenge. In this paper,we prepared the plasma-activated water by microsecond-pulsed plasma jets(µPAW)and reported its effect on papaya seed germination and seedling growth.The mechanism ofµPAW treatment on the papaya seeds was discussed based on the results obtained from the morphological and biochemical analysis.
2. Materials and methods
2.1. Papaya seeds
2.2. Production ofµPAW
The microsecond-pulsed discharge was implemented via an array of 13 plasma jets embedded in the tubes with gas flow,as shown in Fig.2(a).Each of the 13 plasma jets consisted of a pin electrode made of tungsten wire wrapped with Al2O3. The outer diameter of the tungsten wire was 1 mm,and each of the tip has been sharpened.The tungsten electrodes were enclosed in quartz tubes with an inner diameter of 1.5 mm. The array of the 13 plasma jets was arranged circularly in a quartz cup with an inner diameter of 60 mm. The quartz tubes enclosing the electrodes were connected to a pressurized gas, such that the tubes are always filled with the gas. The discharge took place at the tips of the electrodes in the gas medium.This is achieved by maintaining the gas flow such that the distance between the aperture of the plasma jet to the water environment was 3 mm,with a gas flow rate of 10 L/min. The microsecond-pulsed power supply applied at 20 kV and at a frequency of 5 kHz.Discharge at the electrode tips was observed as a plume. Figure 2(b)shows the typical waveform of the discharge voltage and currents. The reservoir of device was filled with 90 ml of deionized water to ensure full coverage of the 13 plasma jets enabling discharge beneath the water, and the treated µPAW was collected after each treatment.

Fig. 2. (a) The schematic diagram of the device and (b) waveform of the discharge voltage(red line)and current(blue line).
2.3. Seed germination and early seedling growth
Healthy seeds were selected manually from the seed lot.Each sample set was prepared with 50 seeds.The seed samples were treated with 40 ml ofµPAW in a 50-ml of sample tube,whereby all the 50 seeds were completely soaked in the tube.Sample sets were also prepared with deionized water and hot water with the same method, as shown in Fig.3. Seeds were soaked in the respective tubes for 48 h before transferring to the seed germination dish. Sterilized 9-cm diameter petri dish was used with gauze moistened with the corresponding liquid to 3 times the mass of dry gauze. The Petri dishes with seeds were placed in an incubator,preset to a temperature of 30◦C,a daylight of 6 h,alternate with 18 h darkness. The observation of the seed germination was conducted daily.
The germinated seeds were monitored and counted from the 5th day to the 15th day, daily at noon. Once the radicle grew to 2 mm,the seeds would be removed from the incubator to the seedling bag. The seedlings were sowed in soil bag and grew in a greenhouse at 30◦C in natural light.The growth performance of the seedlings was observed from day 2 to day 30.The experiments were performed with three replicates for each parameter. The data obtained for all the experiments were analyzed to give the germination rate,germination potential,germination index,and vigor index following the equations;
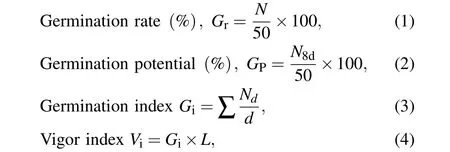
whereNis the number of total germinated seeds;N8dis the number of total germinated seeds on the 8th day;Ndis the number of germinated seeds on the day;dis the germination days;Lis the total length of the plant.
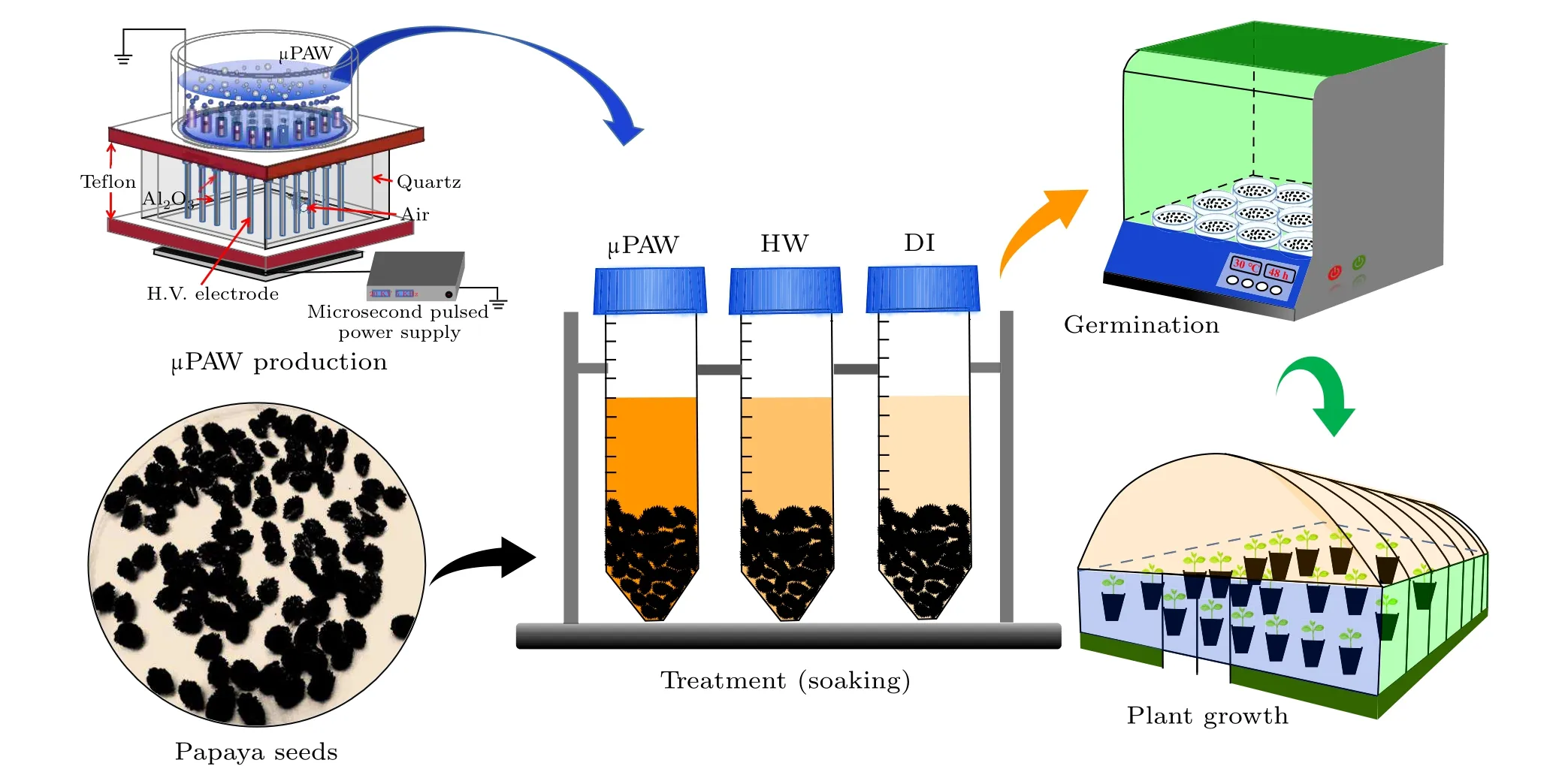
Fig.3. The preparation ofµPAW and the seed treatment process.
3. Characterizations and measurements
3.1. Characterization ofµPAW
The pH,electrical conductivity(EC),and temperature ofµPAW were measured by the SX-620 pH monitor (San-Xin,Shanghai), SX-650 conductivity detector (San-Xin, Shanghai), and Pt-100 thermometer (MaserAC, German), respectively. The concentrations of NO−3, NO−2, H2O2, and NH+4were quantitatively measured by using the ultraviolet spectrophotometer(UV-2600). NO−3was determined photometrically by spectroquant nitrate assay kit(SIGMA,06239-1KT),and the concentration was calculated with reference to the standard curve of NaNO3, which comprised of a set of standard concentrations of 6,60,150,250,400,700,and 900µM.Samples were pre-treated with sulfamic acid to eliminate nitrite interference. NO−2was measured by Griess reagent(Sigma-Aldrich, product number 03553). NaNO2solutions of known concentrations (2, 5, 10, 15, 20, 30, 40 µM) were used to prepare the NO−2calibration curve. The concentration of NO−2was obtained based on the absorbance of the solution. Hydrogen peroxide is an antimicrobial and oxidizing agent. The presence of H2O2in theµPAW was quantified using the titanium oxysulfate(TiOSO4,Sigma-Aldrich,product number 89532)colorimetric method. A standard curve of the known H2O2concentrations of 0.1,0.3,0.5,0.8,1.1,1.3,and 1.5 mM was plotted as a reference. The absorbance of the samples was converted into H2O2concentration according to the standard curve of H2O2. For measuring NH+4, Nesler’s reagent (Sigma-Aldrich, product number 72190) was used,
whereby the NH+4concentration was determined based on a standard curve of known NH4Cl concentrations(2, 6, 10, 30,50, 70, 90, 100 µM). The measurements of the temperature,pH, EC, and particles in the µPAW were conducted immediately after plasma treatment.Every measurement was repeated three times, where the standard deviations of the measurements were indicated by error bars in their respective places.
3.2. Measurement of microbial load
The microbial load of papaya seeds was evaluated from the crushed seeds upon removal from soaking.The seeds were weighed and crushed into powder form. About 1 g of the papaya seed powder was mixed with 200 ml of sterile physiological saline solution in a flask and shaken by hand. 1 ml of the solution was extracted aseptically and diluted to 10 ml with sterile physiological saline solution. For the enumeration of the microbial load, 1 ml of the mixture was serially diluted (1:10) in the sterile saline solution, and 100 µl of the diluted solutions were spread uniformly in triplicate on LB and PDA plates, respectively. The sample plates on LB and PDA were incubated at 37◦C and 30◦C, respectively. The microbial count was conducted after 24 h incubation,and the results were expressed as log colony forming units per gram(log CFU/g). The reduction of microbial count was obtained by comparing the log colony forming units per gram inµPAW treated sample to the control sample. The sterilization effect of the µPAW in the Log Reduction is given by the following formula:
1.3 观察指标 局部脑氧饱和度rSO2:记录时间点包括T1(麻醉诱导开始前)、T2(麻醉诱导后5 min)、T3(CPB开始)、T4(停循环开始5 min)、T5(下半身恢复循环即刻)、T6(CPB结束)。记录术后辅助通气时间、ICU停留时间、术毕至出院时间、各系统并发症情况及病死率等。

3.3. Surface morphology, surface chemistry and functional groups of papaya seed
The surface morphology change in the seed coat after µPAW treatment was evaluated by scanning electron microscopy (SEM) (Hitachi 4800, under vacuum at 10 kV–15 kV).The papaya seeds were dried in an oven at 105◦C for 12 h after being removed from the soaking. The dried seeds were manually separated into seed coats and naked seeds. The inner and outer surfaces of the seed coat after soaking in PAW,hot water,and deionized water were scanned.
The surface chemistry of the seed coat was studied by xray photoelectron spectroscopy (XPS) (PHI Quantum-2000).XPS was used to give the quantitative measurements of the elemental composition of the sample surface (about 10 nm),and to identify the binding states of the elements. The samples were pressed into the 3 mm thin sheets and then analyzed by XPS with an Al mono performing at 28.5 W and 10 kV. The wide scan spectra were measured with a resolution of 1 eV at a pass energy of 187.75 eV, while the narrow scan spectra were carried out with a resolution of 0.1 eV at a pass energy of 58.70 eV.The C 1s spectra were obtained by fitting techniques using the MultiPak software.
The Fourier transform infrared spectroscopy (FTIR,Thermo Scientific Nicolet IS10)was performed to investigate the functional groups of the seed coat and naked seed by using the potassium bromide tableting method with a resolution of 0.5 cm−1for the wavenumbers from 4000 cm−1to 400 cm−1.
3.4. Measurement of abscisic acid content
The abscisic acid (ABA) content in the seeds after the treatments was extracted and measured by enzyme-linked immunosorbent assay(ELISA KIT,SP29775,Spbio)during the germination period. Seed samples were randomly selected from the germination plates on the zero (after soaking), the third, and the fifth day of the germination experiment in the incubator. These seeds were separated into the seed coat and naked seed and homogenized in pre-chilled 80%methanol in ice under low light condition. The homogenate mixture was centrifuged at 4000 rpm for 5 min at 48◦C,and the debris was cleaned with pre-chilled 80%methanol and centrifuged. The supernatant was purified with C18 columns. For ABA analysis, the absorbance of the samples was measured at 450 nm with a microplate meter,and the concentration of ABA in the samples was calculated according to the standard curve.
3.5. Determination of MDA content and SOD activity
MDA content and SOD activity of papaya seedling leaves were measured to evaluate the defense mechanism, indicating the growth performance. The malondialdehyde (MDA)content of papaya leaves was determined by the MDA Content Assay Kit (BC0020, Solarbio). MDA could react with Thiobarbituric acid(TBA)under acidic and high-temperature conditions to produce the brown-red trimethyltran (3, 5,5-trimethyloxazol-2, 4-dione) with a maximum absorption wavelength of 532 nm. Papaya seedling leaves (0.1 g) were homogenized by grinding with 1 ml of extracting solution before the samples were centrifugated at 8×103rpm for 10 min.The supernatant(0.1 ml)was mixed with 0.3 ml of Kit 1 and 0.5 ml of Kit 2. The mixture was heated at 95◦C for 30 min in the water bath and then cooled in an ice bath. After centrifugation at 104rpm for 10 min, the specific absorbance of the sample was read at 450,532,and 600 nm.Equation(6)is used to determine the content of MDA[24]in the papaya seedling,

whereA450,A532, andA600represent the absorbance read at 450, 532, and 600 nm, andWis the mass concentration (in units g/ml)of the leaves used.
The superoxide dismutase (SOD) activity of papaya leaves was determined by the SOD activity Assay Kit(BC0170, Solarbio). Papaya seedling leaves (0.1 g) prepared were mixed with 1 ml of extraction solution and ground in an ice bath. The supernatant obtained by centrifugating at 8×103rpm for 10 min at 4◦C, was used for SOD analysis and stored in the ice bath. 18 µl of the supernatant (or distilled water acting as the control group)was added into a test tube and mixed with 45 µl of Kit 1, 100 µl of Kit 2, 2 µl of Kit 3, and 35 µl of Kit 4. The samples were thoroughly mixed and left to rest at room temperature for 30 min. The absorbance of the sample solutions and control group were determined at 560 nm. One unit of SOD activity was defined as the amount of enzyme that would inhibit 50% of NBT photoreduction. The total activity of SOD was calculated based on the following expression:

whereA0is the absorbance of the control group at 560 nm;A1is the absorbance of the samples at 560 nm;Wis the weight of the papaya seedlings used(in unit g);Dris the dilution ratio of samples.
3.6. Ion concentration in the papaya seedling
The papaya plants grown for 30 days were harvested and processed for ion concentration measurement. The concentration of P, K, Ca, Mg, Fe, Mn, Zn, and Cu ions in the papaya plant sample was determined using ICP-MS(Agilent 7700 Series). The samples were prepared following the procedure:1 g of papaya plant was ground, mixed in 100 ml of deionized water, and stirred for 24 h. The as-obtained mixture was filtered,and 1 ml of solution was obtained after filtration,which was diluted with 9 ml of deionized water and ultrasonicated for 10 min. The thus-obtained solution was centrifuged for 10 min at 3800 r/min. The supernatant has to pass through a C18 treatment column to ensure that any organic matter has been removed. The remainder of the solutions was prepared for the ICP-MS characterization.
4. Results and discussion
4.1. Physicochemical properties and sterilization ofµPAW
TheµPAW was applied as a priming medium to treat papaya seeds. First, the physicochemical properties of µPAW were characterized, and the electrical conductivity (EC), pH,temperature, and concentration of the reactive species were obtained (Fig. 4). The pH of deionized water was 6.96,dropped to 3.86 at 8 min and 2.65 at 30 min after plasma treatment (Fig. 4(a)). In contrast, EC increased markedly from 3µS/cm of deionized water to 72µS/cm at 10 min and 142 µS/cm at 22 min after plasma treatment. The decrease in pH and increase in EC were mainly due to various reactive chemical species derived from the dissolution of particles from cold plasma and chemical reactions between water molecules and plasma active particles.[25]The temperature of µPAW increased gently from 28◦C to about 40◦C after 30 min of the plasma treatment. The temperature rose owing to the heating effect by a collective process involving water molecules and other physicochemical processes, such as ionion recombination.[26,27]
The concentration of the reactive species, NO−3, NO−2,NH+4,and H2O2were obtained according to the standard curve(Fig. 5), and the results are plotted in Fig. 4(b). The concentrations of NO−2and NH+4were much lower than that of NO−3and H2O2. NH+4concentration was observed to have increased linearly with treatment time and reached 44.04µM at 30 min. The concentration of H2O2registered 606.24 µM at 15 min and 1048.58 µM at 30 min. NO−2concentration increased with treatment time before 15 min and whereafter increased slower until 30 min,with the maximum concentration of 291.99µM.The concentration of NO−3increased markedly during the treatment and reached 791.96 µM at 30 min. The standard curves are included in Fig.5 for reference.
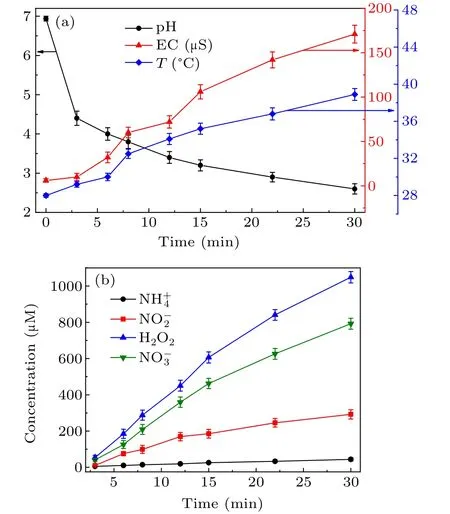
Fig. 4. The physicochemical properties of µPAW (a) the pH (black line),EC(red line),and temperature(blue line),(b)concentrations of NO−2,NO−3,H2O2,and NH+4 (red,green,blue,and black lines respectively)ofµPAW as a function of plasma treatment time.

Fig.5. Standard calibration curve of(a)NaNO3,(b)NaNO2,(c)H2O2,and(d)NH4Cl.
Microorganisms on the seed surface often threaten seed germination and plant health.[28]The sterilization effect ofµPAW was evaluated by measuring the microbial load of seeds after soaking in µPAW for 48 h. The observation plates prepared with crushed seeds after soaking in deionized water(28◦C), hot deionized water (40◦C), and µPAW (15 min)are shown in Figs. 6 and 7. It is apparent that the bacterial counts in the control group and hot water treatment were much higher thanµPAW treatment(Fig.6). Bacterial counts of papaya seed reached as high as 2.41 log CFU/g and 2.42 log CFU/g in the control group and hot water treatment, respectively. The Log Reduction of the sample treated by µPAW was 1.80 log CFU/g.In addition,no mold colony was found in the PDA plate of the seed treated by PAW treatment(Fig.7).The µPAW prepared with great sterilization effect could reduce bacterial stress during germination. The sterilization effect of µPAW may be ascribed to the active species of H2O2and NO−2and the formation of oxidative species,such as peroxynitrous acid (ONOOH) and peroxynitric acid (O2NOOH)that are known to have strong bactericidal effects under an acidic condition.[19,29]

Fig.6. Bacterial colonies observed on LB plates derived from crushed seed after soaking in(a)deionized water,(b)hot deionized water,and(c)µPAW.

Fig.7. Mold colonies observed on PDA plates, derived from crushed seeds after soaking in(a)deionized water,(b)hot deionized water,and(c)µPAW.
4.2. Papaya seed germination and early seedling growth
Five groups of papaya seeds have been investigated, including three groups withµPAW(8,15,22 min)treatment,a control group with deionized water(28◦C),and a hot deionized water(40◦C)treatment group. The 40-◦C deionized water simulates the effect of heat shock on papaya seed germination and early seedling growth.The germination rates obtained on different days were summarized and shown in Fig. 8(a).The germination rates of the control group(28◦C)were shown in a black curve, being the lowest compared with all other conditions, with a final germination rate of 64%. The seeds treated with hot deionized water (40◦C) performed slightly better than the control group, with a final germination rate of 70%. The result indicated that heat shock was beneficial to seed germination. In the case ofµPAW,the seeds treated withµPAW(8 min)germinated earlier and had a germination rate of 62% at day 9versus46% of the control group. The germination rate of the seeds treated with the 15 min and 22 minµPAW soared to 76%and 78%at day 9,respectively. The final germination rate of seeds treated by 15-minµPAW reached 90%, registering about 26% improvement. The germination potential and index also conformed to above observation(Table 1),showing a significant benefit ofµPAW treatment on the papaya seed germination.
The papaya seedlings developed from the treated seeds were grown for 30 days. The growth performance of the seedlings was evaluated by recording the plant height, vigor index, and dry mass after harvest. The plant heights developed from the seeds under different treatments are plotted in Fig.8(b). Five plants were cultured in each condition to give the measured data. The plant height of the control group was 58.65 mm on the 30th day. The plant height of the hot water treatment has increased by 5.71% compared with the control group. TheµPAW group significantly increased to 71.00 mm on the 30th day and the increment was amounted to 21.06%.After treatment, the vigor index increased by 16.17% in the hot water group and 72.74%in theµPAW treatment. The dry mass of the control group was 0.1235 g after 30 days, and it increased by 64.62%after theµPAW treatment. It is demonstrated that the µPAW treatment has significantly enhanced the growth performance of papaya seedlings. The heat shock simulated by hot water treatment gave a mild improvement in seedling growth performance.

Table 1. Effect ofµPAW(15 min)on papaya seed germination and seedling growth.

Fig. 8. The germination papaya seed (a) germination rate for control, hot water,8,15,22 min(black,red,blue,magenta,and green lines)and(b)plant height on different days for control, hot water, and 15 min (black, red, and blue lines).
4.3. Surface morphology of papaya seed
In our experiments, papaya seeds were soaked in deionized water, hot deionized water, and µPAW for 48 h. The liquids changed to brown color and darkened gradually. The coloration in the deionized water,hot deionized water,and 15-minµPAW is shown in Fig.9. The darker color inµPAW indicated that a higher proportion of pigments has been released from the µPAW treated seeds. The reactive particles in PAW might affect the seed coat pigmentation,[30]which was also associated with germination. The pigments in a seed coat are usually released when cell membrane ruptures and associated with the seed permeability and imbibition. The weight of the seeds after soaking was measured and compared to the weight before soaking, to give a direct indication of imbibition. The results showed that the mean mass for seeds after deionized water, hot water and µPAW treatment was 2.81±0.03 mg,2.85±0.03 mg, and 2.99±0.09 mg, respectively, that theµPAW treated seeds absorbed more water and gained about 60%of weight in the imbibition process.

Fig.9.(a)Deionized water without papaya seeds and the liquids of(b)deionized water, (c) hot deionized water, and (d) µPAW (15 min) after soaking papaya seeds for 48 h.
The modification of seed coat enables better absorption of water and oxygen, which can accelerate seed metabolism and promote germination.[7,8]The outer and inner surfaces of the seed coats were prepared separately and scanned by SEM to show the surface morphology(Fig.10). Figure 10(a)shows the exterior surface sculpture of the papaya seed coat without treatment,with irregular holes varying from 10µm to 40µm.In Figs. 10(b)–10(d), the linings of these holes were broken(the red dotted circles). The SEM image of theµPAW treated sample (Fig. 10(d)) showed that all the linings of the holes were broken, forming a fissure. The hot water treatment obtained a similar physical etching as the control group, which indicated that the heat effect of µPAW did not affect the surface morphology of papaya seed. Therefore, the RONS presented in µPAW were believed to play a significant role in the cleavage of cell wall polymers. It may be that changes inside the seed coat,in turn,modify the outer layers. The inner surfaces of the seed coats are shown in Figs.10(e)–10(h).The inner surface of the seed coat without treatment has many irregular polygons, whereby cracks were seen at their edges(indicated by the arrows). The features have changed and appeared differently in the treated samples.The edges were filled with substance presumably from the seed metabolism. All the edges of the polygons shown in Fig.10(h)were filled up most significantly corresponds to the sample treated byµPAW.The substance generated in the inner surface is presumably the secondary metabolites,resulting in the consumption and destruction of linings of holes in the outer surface. In the imbibition process,water is absorbed into the seed,metabolism takes place, and some secondary metabolites leaches out into the surrounding medium.[7]In the observed SEM images,the secondary metabolites were accumulated more in µPAW-treated sample.

Fig.10. SEM images of the outer surface of the papaya seed coat under(a)no treatment,(b)deionized water,(c)hot water,and(d)µPAW treatment and the inner surface of the papaya seed coat under(e)no treatment,(f)deionized water,(g)hot water and(h)µPAW treatment.
4.4. Surface chemistry of seed coat
The surface chemistry of the seed coat of the four selected samples (no treatment, deionized water soaking, hot water soaking,andµPAW soaking(15 min))was investigated via XPS.The results are presented in Fig.11. C KLL,O KLL,O 1s,N 1s,and C 1s were found in the seed coats at 1224,1000(and 976),531,400,and 284 eV,respectively.It is evident that the intensity of O and N peaks increased after treatment,especially theµPAW soaking. The relative atomic percentages of C,N,and O are given in Table 2.The content of O and N in the seed coat of treated samples increased.Soaking of seed has altered the elemental composition of the seed coat, resulting in the chemical modification. The changes were most significant in µPAW-treated sample, with O and N content increased to
45.4%and 4.2%,compared to deionized water soaking 30.1%and 1.1%and without treatment 21.1%and 0.7%,respectively.
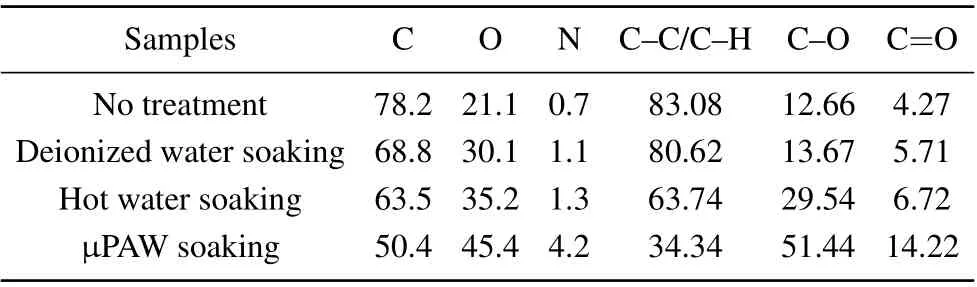
Table 2. Relative atomic percentage of elements and their corresponding components.

Fig.11. The XPS spectrum of the seed coat(a)no treatment, (b)deionized water soaking,(c)hot water soaking,and(d)µPAW soaking(black,red,blue,and green lines respectively).

Fig. 12. The C 1s spectra of the seed coat (a) no treatment, (b) deionized water soaking,(c)hot water,and(d)µPAW treated.
Figure 12 shows the C 1s spectra of the four samples.The C1s spectra were fitted by three peaks corresponded to–C–C or –C–H, –C–O, and –C=O. The –C–C or –C–H was dominant in the no-treatment seed coat (83.08%) and deionized water treatment(80.62%),showing the abundance of carbon in these two samples. The relative content of –C–O increased in the sample treated by the hot water, and more in the µPAW-treated sample. This may be due to the hydrolysis of organics in the seed coat (–C–O–C–+H2O→–C–OH+HO–C–).[31]The–C=O percentage was also highest in the µPAW treated sample might be because of the oxidation effect due to the presence of ROS in µPAW (C–OH+O or OH→C=O+H2O).[32]
4.5. Functional groups of papaya seed
The metabolism of the seeds was studied by FTIR. The FTIR results showing the functional groups of papaya seed coat and naked seed are presented in Figs.13(I)and 13(II),respectively. Table 3 lists the typical wavenumbers assigned for the possible functional groups or structures in the FTIR spectra according to available references.[33–36]Both the dry and treated seeds had an intense O–H stretch (3400 cm−1) peak in the seed coat, indicating that they were rich in hydroxyl substances, such as proteins, sugars, and lipids (Fig. 13(I)).Compared with the dry seeds without any treatment, the area of O–H stretch peaks of treated samples was larger inµPAW treated sample, with highest content of hydroxyl substances.This suggested that µPAW treatment promoted the seed coat metabolism and generated more hydroxyl substances.Furthermore, the hydrolysis of protein, lipid, and carbohydrate produced some small molecule compounds that have filled up the lining of the inner seed coat, as shown in the SEM images.An acidic condition in µPAW has accelerated the hydrolysis of these compounds,resulting in more hydroxyl substances as shown in Fig. 13(I), in the deep absorption at the wavenumber of 3400 cm−1. Other peaks at 1631 cm−1, 1512 cm−1,1233 cm−1, and 1040 cm−1were assigned to the ketones (or aldehyde and ester), bonded benzene ring group, lipase (or aliphatic ester), and polysaccharose, respectively. The intensity of these peaks increased with µPAW treatment, indicating activated metabolism. The effect of NO−3on overcoming seed dormancy was observed from the changes in the concentrations of reserve compounds of seed,such as sugars,amino acids,lipid,and others.[37]NO−3can also play a role in the pentose phosphate pathway and oxidative decomposition of glucose,which provide the energy required during the early stage of germination.[38]However, the absorptions at 2855 cm−1and 1748 cm−1(=N–H2+) vanished after µPAW treatment,which might be associated with the methyl substituent of C–H stretching vibration.[33]Other bands beside the above mentioned did not change compared to the untreated seeds.
On the other hand,all peaks in the spectra of naked seeds did not change after treatment compared with dry seeds without treatment, which suggested that the metabolism hardly happened in the seed endosperm and embryo during soaking.
A hypothesis explaining the metabolism of seed coat upon µPAW treatment is illustrated in Fig. 14. The hydrolysis of protein,lipid,and carbohydrate produced the secondary compounds to fill up the inner surface of seed coat. Secondary compounds were produced and possessed more hydroxyl substances.

Table 3. Assignments of signals in FTIR spectrum to functional groups.
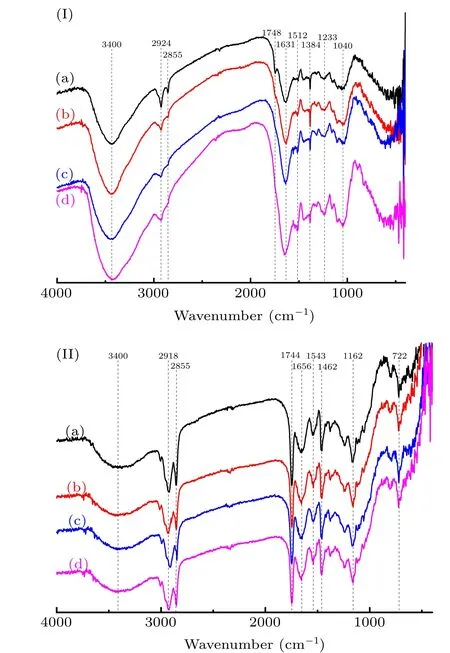
Fig.13. The FTIR spectra of(I)the seed coat and(II)the naked seed under different treatments: (a)dry seeds,(b)deionized water soaked,(c)hot deionized water soaked,and(d)µPAW soaked(black,red,blue,and magenta line respectively).

Fig. 14. Metabolism of seed coat upon µPAW treatment (hydrolysis of protein, lipid, and carbohydrate produces secondary compounds, as seen in the SEM images. These secondary compounds possess more hydroxyl substances).
4.6. Concentration of abscisic and effect of temperature
Abscisic acid (ABA) is a critical plant hormone regulating seed dormancy, germination, and seedling growth. The initiation of seed germination depends on the degradation of endogenous ABA and desensitization of embryo to ABA.[5]Three groups of seeds were extracted for chemical analysis to determine the ABA concentration. Group 1 was soaked with deionized water served as the control group, group 2 was the seeds soaked with hot water,and group 3 was soaked inµPAW(15 min). Table 4 shows the concentration of ABA in the seed coat and the naked seed of the three groups of samples extracted after sowing in greenhouse at day 0 (after soaking),day 3,and day 5.Hot water soaking provided a heat shock that has slightly reduced the ABA concentration. TheµPAW treatment induces lower ABA concentration leading to the breaking of dormancy.The ABA in the seed coat decreased by about 40%to 182.46 ng/g and 59%in the naked seed to 24.27 ng/g at day 0. The reactive species in µPAW has collectively resulted in the decrease of ABA in the seed coat and naked seed.The effect of chemicals breaking dormancy and reducing ABA was reported in the barley seed germination.[38]The results reported that H2O2and NO−3gave rise to the decrease of endogenous ABA content and promoted the activities of enzymes associated with the synthesis or degradation of ABA.After 5 days, the ABA content dramatically decreased in all seeds,and the seeds began to germinate. The current observation resembled the effects of chemical treatment,achieved by theµPAW treatment.
In our work, the hot water deionized water (40◦C) was used as a reference group. Our results showed that hot water treatment has also enhanced the germination rate of papaya. However, the heat effect of hot water and µPAW had little impact on sterilization and physical etching of the seed coats, but it has induced an increase in the content of O and N,and the functional groups of−OH,−C=O−,and−C–O–C−.The results indicated that the metabolism of papaya seeds during soaking has increased,involving the synthesis and consumption of protein. This is in accordance with the results of other reports.[7,12,13]Furthermore, we found that the heat effect slightly decreased the ABA inhibitor content to promote the germination of papaya,which was not reported elsewhere.

Table 4. Concentration of ABA of papaya seeds under different parameters.
4.7. Malondialdehyde,superoxide dismutase and ion content of the papaya seedling
The growth of the papaya seedlings was observed in the greenhouse for up to 30 days. Papaya seedling begin to developed leaves from day 2, and each seedling was observed to have 4 leaves by day 5. Three groups of samples were extracted at day 5, day 14, and day 30. The measured samples would be extracted from the papaya seedlings for the measurement of malondialdehyde (MDA), superoxide dismutase(SOD)and ion content at the stipulated time. MDA is the final lipid peroxidation product and reflects the degree of peroxidation of the plant cell membrane.[39]Usually, a high MDA content indicates an elevated peroxidation and severe damage to the plant cell membrane. Scavenging enzymes are required to detoxify ROS in the cell.SOD is the primary O2−scavenger and provides the first line of defense against cellular injury.[40]
The MDA concentration and SOD activity of the papaya seedling leaves measured are plotted in Fig.15. MDA content were remarkably reduced by 58.29%after 5 days and 51.79%after 30 days in theµPAW treated samples.The reduced MDA accumulation was related to the reduced membrane lipid peroxidation damage. The hot water treatment demonstrated no effect on MDA and SOD.Therefore,the observed effects were corresponded to the reactive species in the µPAW. The SOD activities in the papaya seedling leaves were highest inµPAWtreated sample while remained unchanged in the hot water treated samples.The SOD activity in theµPAW treated sample significantly increased by 94.33% after 14 days and 89.31%after 30 days. The reactive species of µPAW played a vital role in reducing oxidative damage and helped maintain normal metabolic activities, being beneficial to papaya seedling growth.
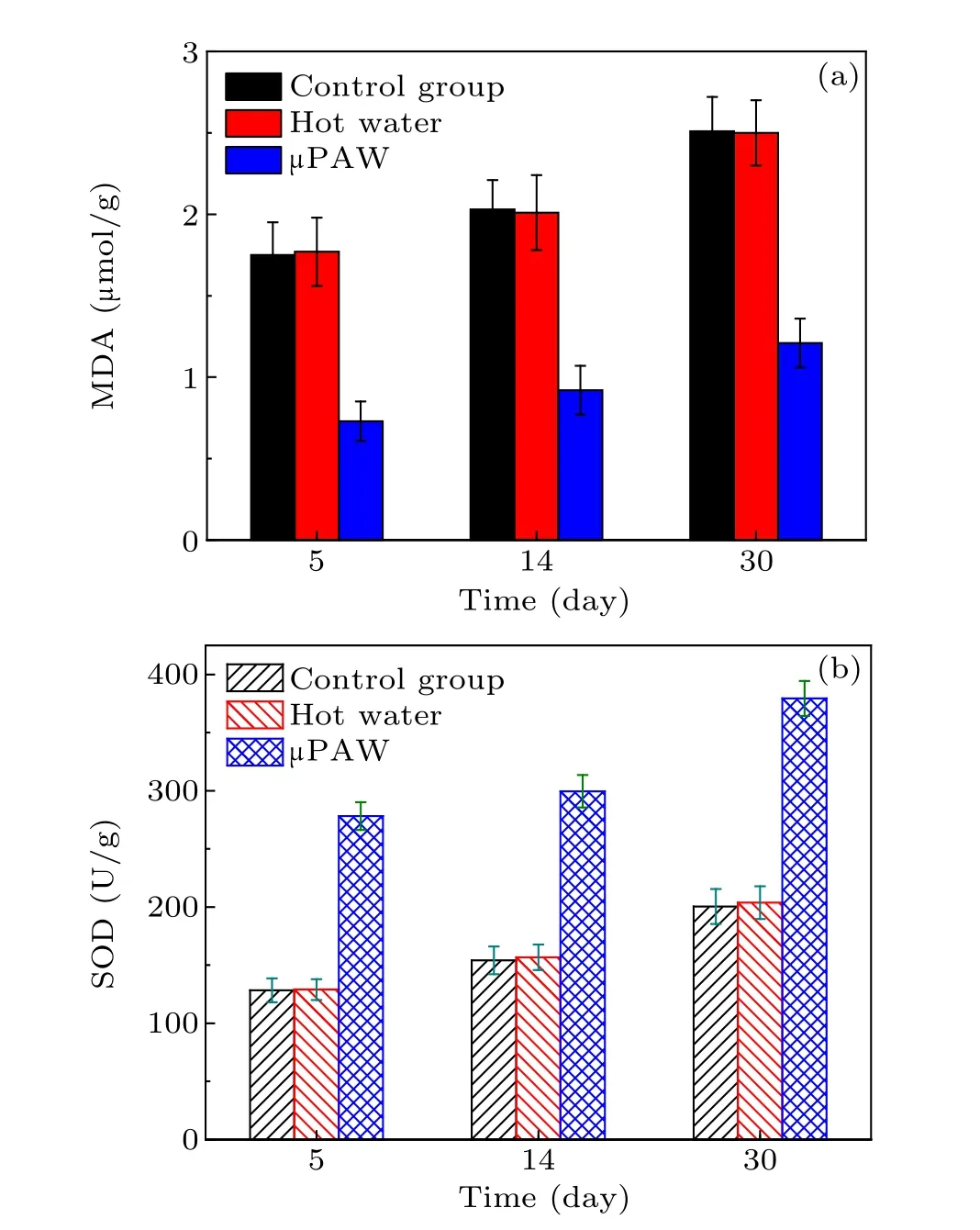
Fig.15.The comparison of control(black bar),hot water(red bar)andµPAW(blue bar)treated groups,(a)MDA concentration(b)SOD activity of papaya seedlings on different days.
On the other hand,papaya roots absorb nutrients and ions during plant growth. Ions play variously crucial roles in plant growth.[41]For example, phosphorus is an essential nutrient for plant growth and promotes photosynthesis and root development,being critical for early seedling growth. Potassium is an activator of enzyme that promote photosynthesis and protein synthesis,and improve the ability of the crops to resist adversity. Manganese is one of the indispensable trace elements for plant growth,improves the energy supply of material transportation, and promotes carbohydrate synthesis and transport in the plant.The ion content in papaya seedlings was measured to reveal the effect ofµPAW on papaya seedling growth. The list of ions analyzed is shown in Table 5. All the ions’ contents have higher reading in the µPAW treated samples. The major ions especially the K, Ga, and Mg were increased by 81.29%, 92.11%, and 213.97%, respectively, compared with the control group.

Table 5. The ions content of papaya seedlings under different treatments.
5. Conclusion
The microsecond-pulsed plasma efficiently produced suitable µPAW as priming medium for papaya seed. The results comparingµPAW treatment with hot water treatment and deionized water treatment have identified the important contribution of the plasma activation. µPAW soaking has significantly promoted the germination rate of papaya seed and accelerated the early seedling growth, shown in the healthy plants with more height, higher vigor index and average dry mass after 30 days. The reactive species ofµPAW eliminated microbial stress on the seeds. The physical etching effect and chemical modification of the seed coat were observed accompanied by the enhancement of water and oxygen absorption,accelerating metabolism in seed to break dormancy. Measurements of the papaya seedling from the seed treated byµPAW possessed the highest SOD activity and lowest MDA contents,indicating a reduced oxidative damage and enhanced antioxidant enzyme activities that were beneficial to seedling growth.The mineral contents in the plants also confirmed their wellbeing with significantly higher concentration of ions including K,Ga,and Mg.
Acknowledgments
The authors from University of Malaya acknowledge the support from the Ministry of Higher Education Malaysia for the Fundamental Research Project(Grant Nos. FRGS/1/2018/STG02/UM/02/8 and IIRG006A-19FNW). Project supported by the National Natural Science Foundation of China(Grant No.51877184).
- Chinese Physics B的其它文章
- Editorial:Celebrating the 30 Wonderful Year Journey of Chinese Physics B
- Attosecond spectroscopy for filming the ultrafast movies of atoms,molecules and solids
- Advances of phononics in 20122022
- A sport and a pastime: Model design and computation in quantum many-body systems
- Molecular beam epitaxy growth of quantum devices
- Single-molecular methodologies for the physical biology of protein machines

