Emerging natural hemp seed proteins and their functions for nutraceutical applications
Hihong Chn, Bing Xu, Yi Wng*, Wi Li, Dong H,Yn Zhng, Xizhn Zhng, Xinhui Xing,g,*
a Institute of Biopharmaceutical and Health Engineering, Tsinghua Shenzhen International Graduate School, Shenzhen 518055, China b Institute of Biomedical Health Technology and Engineering, Shenzhen Bay Laboratory, Shenzhen 440300, China c Key Laboratory for Industrial Biocatalysis, Ministry of Education, Institute of Biochemical Engineering, Department of Chemical Engineering,Tsinghua University, Beijing 100084, China
d School of Chemical Engineering and Energy Technology, Engineering Research Center of Health Food Design & Nutrition Regulation, Key Laboratory of Healthy Food Development and Nutrition Regulation of China National Light Industry, Dongguan University of Technology, Dongguan 523808, China
e Hebei Food Inspection and Research Institute, Shijiazhuang 050091, China f College of life Science and Technology, Guangxi University, Nanning 530004, China g Center for Synthetic and Systems Biology, Tsinghua University, Beijing 100084, China
Keywords:Hemp seed protein Extraction Purif ication Physicochemical properties Nutraceutical functions Food industry
A B S T R A C T With changing dietary habits and increasing awareness of the nutraceutical role of dietary foods, the demand for natural plant proteins and interest in non-traditional protein sources in the food industry are increasing.Industrial hemp, belonging to the plant family Cannabaceae, is cultivated for its f ibre and edible seeds. Due to its nutritional value, it has also been used in the food industry and medicine. In particular, hemp seed proteins have drawn considerable attention in both scientif ic and industrial f ields because of their excellent nutraceutical values, superior digestibility, low allergenicity and diverse techno-functional properties. In this review, we provide a summary of the current research progress on the extraction and purif ication processes,physiochemical properties, nutraceutical functions, and applications of hemp seed proteins. Perspectives in the application of advanced technologies for hemp seed bioactive peptide mining are also discussed. This review provides up-to-date insights into the nutraceutical values, health benefits, and future applications of this emerging plant source protein.
1. Introduction
Hemp (CannabissativaL.), belonging to the Cannabaceae family, is an annual plant that is native to central Asia and known for its high medicinal values and textile applications dating over 12 000 years [1]. Hemp is now widely planted in Europe,central Asia, the Philippines, and China. However, the high amounts of tetrahydrocannabinol (THC) in hemp seeds have long impeded the widespread use of hemp in the food industry, as it can neurologically alter a person’s sensory and psychological experiences, and may finally cause central nervous system depression [2]. Nonetheless, around 20 years ago, industrial hemp with a THC level lower than 0.3% became available in several countries, including Canada and China, which has promoted the commercialization of foods formulated with hemp seeds.According to the data recorded by the Food and Agriculture Organization (FAO), China supplied almost half of the world’s industrial hemp, with the majority of the remainder being cultivated in Canada, Chile, France, and Spain [3]. In China, hemp plants have been approved for medicinal food homology [4].
Hemp seeds are 2.5-3.5 mm in length and are brown with darker brown stripes. Hemp seeds can be consumed raw, cooked, or roasted,and because of their richness in nutritional values, they have been used in the food industry as well as in medicine [5]. Deferne et al. [6]reported that hemp seeds comprise 20%-30% carbohydrates with 10%-15% insoluble fibre, 25%-35% oil, and 20%-25% protein.According to a previous report, hemp seed protein mainly consists of albumin and legumin, with an essential amino acid content that is superior to that of soybean and sufficient for children(2-5 years old) [7]. In particular, hemp seeds are rich in arginine,which can be used as a nutritional ingredient to formulate foods and improve cardiovascular health [8].
To gain insights into research advances and trends pertaining to hemp protein, we searched the Web of Science database from 1921 to 2021, with the keywords “hemp”/“Cannabis sativa” and “hemp seed protein”/“hemp seed protein”/“hemp seed peptide”/“hemp seed peptide” and performed statistical analysis on the related publications.The results showed a large number of publications related to“hemp”/“Cannabis sativa” (n= 9 129, Fig. 1a), and a rapid upward trend in recent years. However, most of the publications were related to plant sciences, agronomy, and materials science disciplines, rather than hemp seed proteins (only 233 publications focused on hemp seed proteins/peptides; Figs. 1b and 1c). Owing to their excellent nutritive values, hemp seed proteins have attracted the attention of researchers,and the number of related publications has shown a remarkable upward trend from 2018 to 2020 (Fig. 1b). Of particular interest is the bioactive peptide produced from hemp seed protein and its functionality [9-11], as well as the application of hemp seed proteins.Although the application of hemp seed proteins is closely related to their structural and functional properties, studies on these aspects are scarce. Accordingly, in this review, we have summarised and discussed the extraction and purification processes, physiochemical properties, nutraceutical activities, and application of hemp seed proteins for the food industry to provide contemporary insights for future research on hemp seed proteins as nutraceutical products.
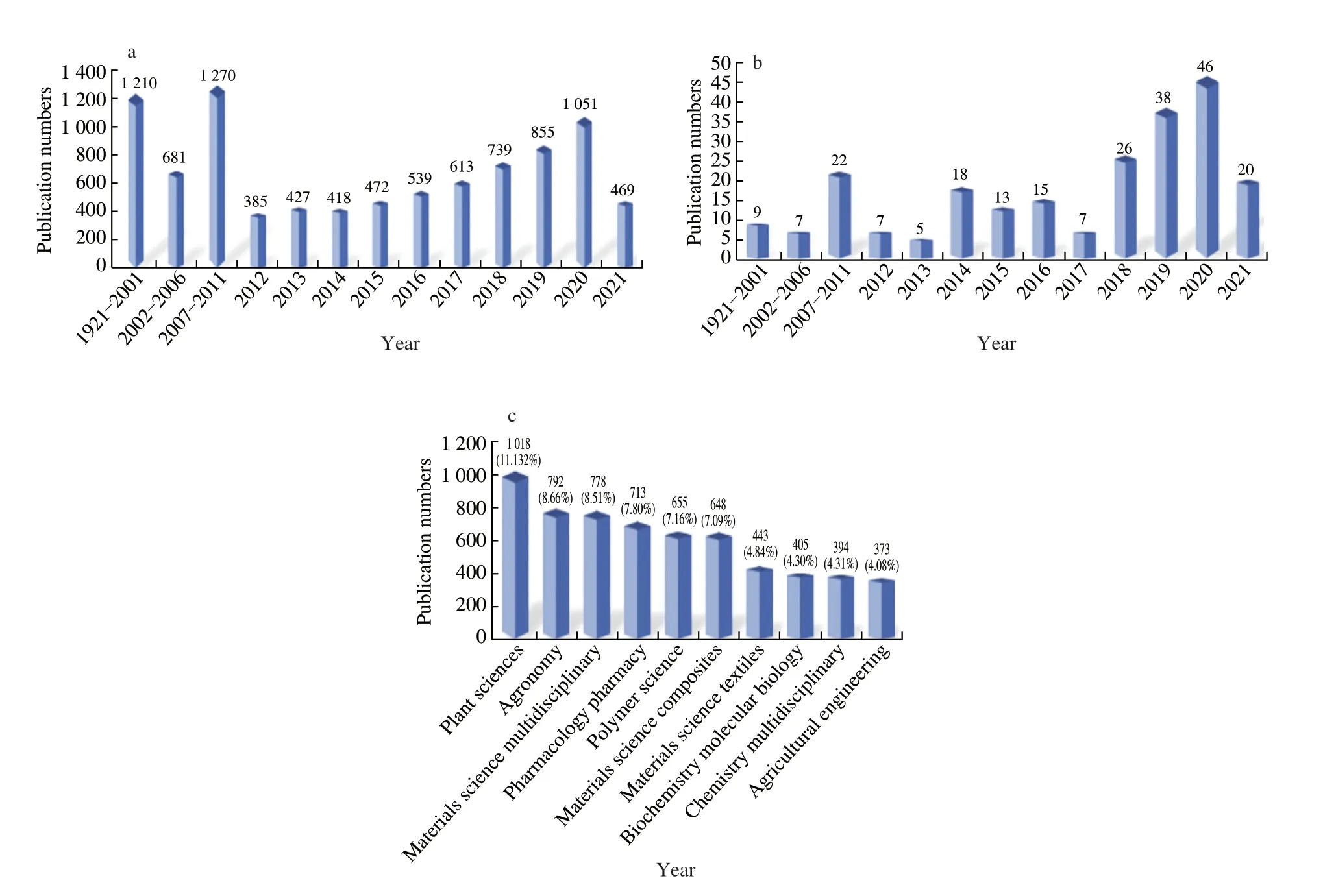
Fig. 1 Number of publications reported in the Web of Science. (a) The number of publications containing the terms “hemp” and “Cannabis sativa” in the title,abstract, and keywords from 1921 to 2021 (as of June 20, 2021); (b) The number of publications containing the terms “hemp seed protein”, “hemp seed protein”,“hemp seed peptide”, and “hemp seed peptide” in the title, abstract, and keywords from 1921 to 2021 (as of June 20, 2021); (c) The top 10 Web of Science categories and the number of publications in each category containing the terms “hemp” and “Cannabis sativa” in the title, abstract, and keywords from 1921 to 2021 (as of June 20, 2021)
2. Extraction and purification of hemp seed proteins
Generally, protein products can be divided into three categories according to their origins: plant, microbial and animal source proteins. The protein extraction method was specific to these three different origins. Extraction methods for plant proteins typically include chemical extraction, physical extraction, and enzymeassisted extraction (Fig. 2). Chemical and enzymatic extraction assisted by physical techniques were commonly used for hemp seed protein extraction. Hemp seed was rich in oil. Therefore, the process of protein extraction for hemp seed usually includes three steps:defatting of the sample, extraction, and precipitation of the proteins.The workflow for the preparation and production of hemp seed proteins is illustrated in Fig. 3.

Fig. 2 Commonly used plant protein extraction methods.
2.1 Defatting of samples
Degreasing, or defatting, is considered a necessary step for the effective extraction of plant seed proteins, as the presence of oil leads to the formation of lipid-protein complexes and reduces the extraction rate. Cold-pressing and solvent extraction (including petroleum ether,n-hexane, andn-pentane) were the most commonly used methods for hemp seed oil extraction [11]. The degreased product of hemp seed was commonly considered as a hemp seed protein meal, and its protein content ranged from 30% to 50%, depending on the origin of hemp seeds, the degreasing method, and its efficiency [11].
2.2 Extraction and precipitation of the proteins
Since hemp seed protein meal includes various water-soluble and water-insoluble non-protein constituents, such as oligosaccharides,fibers, starch, and pigments, enzymatic and liquid-liquid extraction methods have been adopted to remove most of the non-protein constituents and concentrate the hemp seed proteins. Malomo and Aluko combined carbohydrase and phytase digestion with membrane ultrafiltration separation to purify and enrich the hemp seed proteins,and the protein content of the obtained fraction was up to 70% [12].Generally, hemp seed protein concentrate contained > 65% protein in dry weight [12].
As mentioned above, the protein content in hemp seed protein concentrate was approximately 65% [12]. However, the protein contents of most of the purified and enriched commercial protein products were higher than 90%. Alkaline extraction followed by acid or isoelectric precipitation and micellization extraction are commonly used to prepare hemp seed protein isolates with high protein content.It is worth noting that the selected extraction method affects the protein composition, functionality, and contents of the final protein isolate. Alkaline extraction (usual pH 9-10) followed by acid or isoelectric precipitation is the most common method for preparing hemp seed protein isolates [13]. By optimizing the extraction conditions (e.g. pH, temperature, and time), a protein purity of up to 94% was obtained [14]. Carne et al. [14] compared the effects of acid and alkali extractions on the physicochemical and functional properties of hemp seed protein isolates. The results showed that in comparison with the defatted seed cake sample both acid and alkali treatments resulted in a higher enrichment of protein content (> 90%), higher emulsifying activity and emulsion stability, and improved amino acid profiles [14].However, alkali extraction generated protein isolates with a higher waterholding capacity, whereas acid extraction protein isolates had the highest creaming stability and largest droplet size [14].
Alkaline extraction/isoelectric precipitation was an effective method for hemp seed protein extraction. However, researchers found that proteins obtained via alkaline extraction/isoelectric precipitation method were off-flavor and exhibited a greenish color [13], which was a critical factor and impedes the application of the purified hemp seed protein in the food industry. To overcome this problem and preserve the native state of the protein, the micellization technique has been adopted for hemp seed protein isolation. Hadnadev et al. [13]adopted alkaline extraction/isoelectric precipitation and micellization procedures for hemp seed protein extraction and showed that the protein contents of the extracted proteins using both alkaline extraction/isoelectric precipitation and micellization methods were higher than 90%; however, protein recovery via micellization extraction was lower than that via the isoelectric precipitation method.Additionally, the protein powder color extracted by the micellization technique was brighter than that of the protein powder extracted by the alkaline extraction/isoelectric precipitation [13]. Furthermore,it was also found that the physicochemical properties of the protein obtained using these two methods were different: the isoelectric point of the protein isolate obtained using alkaline extraction/isoelectric precipitation (HPI) was 5.0, whereas the micellization technique extracted protein isolate (HMI) had an isoelectric point of 6.0, and the transition enthalpy of HPI was higher than that of HMI [13].Dapčević-Hadnađev et al. [9] performed salt extraction using the micellization method to extract hemp seed proteins and obtained a protein isolate with a protein content of 98.9% in dry weight.
3. Physiochemical properties of hemp seed proteins
3.1 Composition and structure of hemp seed proteins
Globulin and albumin are two of the main components of hemp seed proteins. Hemp seed globulin protein, also called edestin, is the main storage protein of hemp seed, accounting for approximately 60%-80% of the total protein content, where albumin constitutes the majority of the remaining proteins [7].
In 1994, Patel et al. [15] used X-ray diffraction to characterise the crystal structure of hemp seed edestin. Their results showed that edestin had 6 identical subunits, and each subunit consists of acidic and basic subunits linked by a single disulphide bond. Wang et al. [16]used the pH shifting technique to study the constituents of hemp seed edestin and found that 11S and 7S are the two main types of hemp seed edestins. Furthermore, Wang et al. [16] characterised the composition of 11S and 7S using sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) and found that 11S-rich hemp seed protein constituents consisted of acidic and basic subunits. The acidic subunit was approximately 34.0 kDa and possessed a good homogeneity, whereas the basic subunit was mainly composed of 20.0 and 18.0 kDa constituents [16]. Similarly,the 7S-rich constituents were mainly composed of basic subunits (a subunit of approximately 4.8 kDa) [16]. In addition, Kim et al. [17]combined acid precipitation with gel filtration chromatography to isolate and characterise the properties of hemp seed edestin from the Cheungsam (Korean) hemp variety and found that the purified edestin was approximately 300 kDa.
The albumin component took up approximately 25% of the total hemp seed storage proteins [10]. Compared to the globulin component,the albumin component in hemp seed had more flexibility but a less compact structure because it contained few disulphide-bonded proteins. And it was confirmed by Malomo et al. [10] via intrinsic fluorescence technology combined with circular dichroism analysis found that albumin has greater exposure to tyrosine residues than globulin. Additionally, they reported that albumins displayed a highly ordered secondary structure with minor tertiary conformation [10].However, with an increase in pH, the protein structure shifted more toward tertiary conformation. Hence, Malomo et al. [10] concluded that albumin has higher solubility and foaming capacity than globulin because of its higher degree of flexibility and ordered secondary structure. In contrast, globulin has a more compact or aggregated structure [10].
Additionally, hemp seed protein is rich in sulphate. Odani et al. [18]isolated a 10 kDa protein by using buffer extraction, ultrafiltration,and SDS-PAGE. Amino acid analysis revealed that this protein was rich in methionine and cysteine (accounting for 20 mol% of the total amino acids) [18]. Sequence analysis showed that the protein has two subunits composed of 27 and 61 residues, respectively [18].Moreover, the two subunits were held together by two disulphide bonds [18]. In addition, the authors observed that this methionineand cysteine-rich protein (2S albumin) exhibits no trypsin inhibitory activity [18]. Moreover, Ponzoni et al. [19] identifiedCs2S-1andCs2S-2as the genes encoding the 2S albumin precursor.Furthermore, Odani et al. [18] reported thatCs2Sis 97% similar to the mature 2S protein.
3.2 Amino acid composition of hemp seed proteins
Hemp seed protein is a desirable, health-promoting plant protein because it contains all the essential amino acids required for the human body in a balanced ratio (Table 1) [20]. The nutritional value of hemp seed in terms of amino acid contents was comparable to that of egg white and soybean proteins [20] and was sufficient to meet the nutritional requirements of children (2-5 years old), as suggested by the FAO and World Health Organization (WHO) [16].Furthermore, hemp seed proteins contain exceptionally high levels of health-promoting amino acids. For instance, arginine plays a positive role in blood pressure regulation as it is the precursor of nitric oxide (NO), and NO is a vasodilating agent that lowers blood pressure [8]. Lu et al. [21] reported that hemp seed protein contains around 12% arginine. This is notably higher than that in other plant proteins, in which arginine generally represents less than 7% [20,21].Furthermore, hemp seed proteins contain a high level of the sulphurcontaining methionine and cysteine amino acids, which contents ranged from 3.5% to 5.9% [20,22]. Limiting amino acids are those in the shortest supply relative to the demand for protein synthesis in the human body. The amino acid with the shortest supply relative to the demand of the human body is considered to be the first limiting amino acid. House et al. [7] reported that lysine was the first limiting amino acid in hemp seed proteins, which varied 0.50%-0.62%.
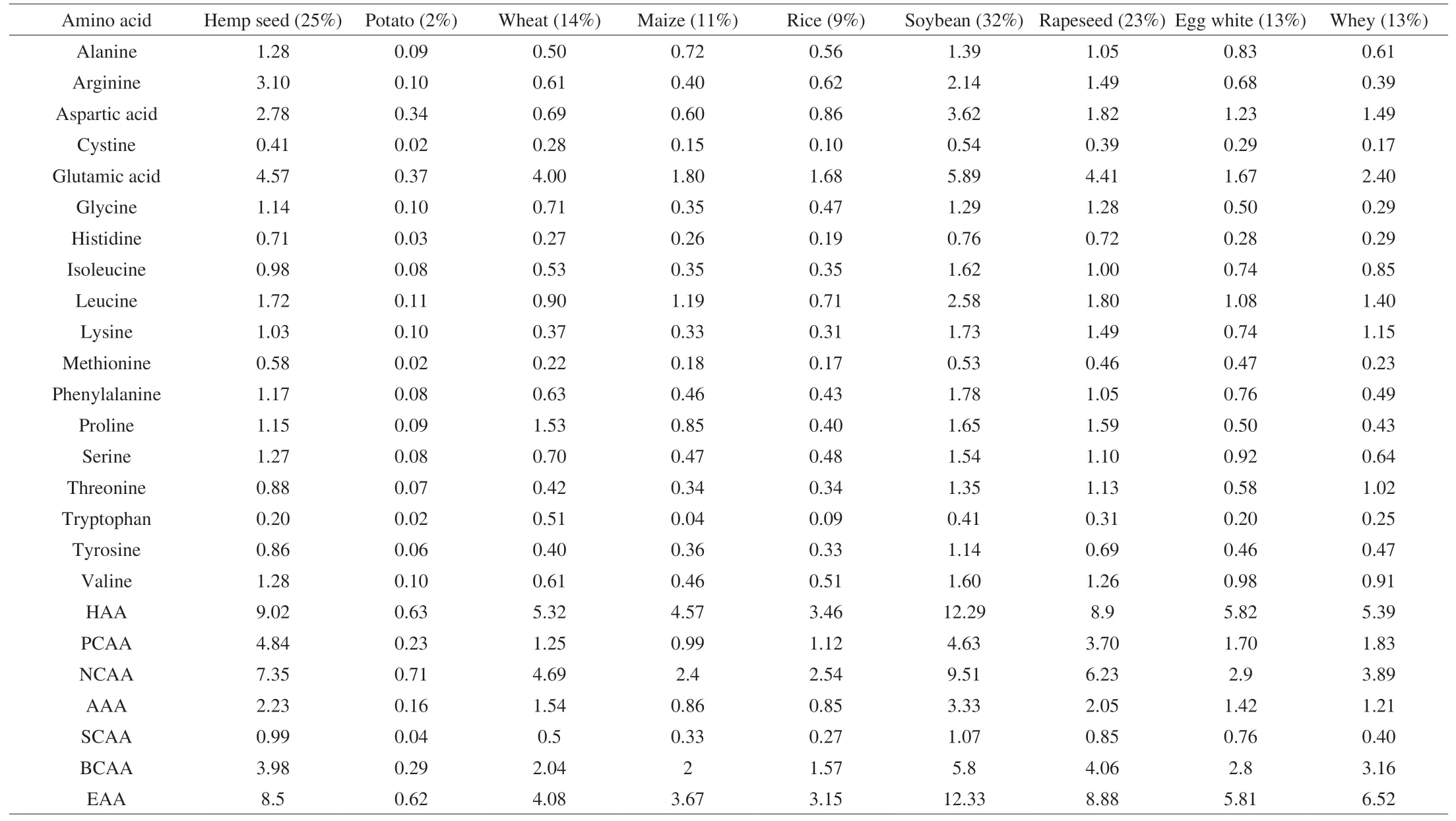
Table 1Percentage amino acid compositions of food protein hydrolysates.
3.3 Digestibility of hemp seed proteins
It has been reported that different from other plant proteins, hemp seed protein contained very low amounts of anti-nutritional factors and was therefore more digestible [23]. A classic digestion study performed by House et al. [7] evaluated the digestibility of whole hemp seed, dehulled hemp seed and hemp seed protein meal by using an animal model and the FAO/WHO amino acid requirement of children (2-5 years of age) as reference. They found that protein digestibility of dehulled hemp seed varies from 90.8% to 97.5%,which is almost comparable to the digestibility of casein (97.6%) [7].Moreover, Wang et al. [16] compared the digestibility of hemp seed protein isolate and soybean protein isolate by using anin vitrodigestion model. It was found that the digestibility of hemp seed isolate protein was comparable to that of soybean protein isolates for pepsin digestion, but the digestibility of hemp seed isolate proteins (88%-91%) was significantly higher than that of soybean protein isolates(71%) for pepsin plus trypsin digestion [16]. Mamone et al. [23]explored the digestibility of hemp flour and hemp protein isolate by using anin vitrostatic model of gastrointestinal digestion and found that both hemp flour and hemp protein isolate showed a high degree of digestibility, with only a very limited number of peptides present in the simulated intestinal digestion.
4. Functionality properties of hemp seed proteins
Proteins are essential components in several food processes,and they play a critical role in improving nutrition and maintaining the stability of food. Functionality refers to the properties of food or food components, apart from its nutritional properties, including solubility, proteolytic activity, water-binding capacity, oil-binding capacity, thermostability, gelation, foaming, emulsification, and film formation. Interaction with other components in the food system is a characteristic of protein functionality, and these interactions play a critical role in maintaining the stability and sensory characteristics of the food system, and in determining protein utilization(Table 2) [24]. Due to the poor solubility of hemp seed proteins,the emulsifying activity index, emulsion stability index, and waterholding capacity of hemp seed proteins were inferior to those of soybean protein, while the fat adsorption capacity was similar [25].However, hemp seed protein isolate-based cast films had some superior characteristics, such as much lower total soluble mass and relatively higher surface hydrophobicity (support matrix side), as compared to soybean protein isolate-based films [26]. Moreover, hemp seed contained 25%-30% protein, and the protein content in defatted hemp seed powder was higher than 50%, indicating high development value for the food industry.

Table 2Functionality properties and underlying mechanisms for the application of proteins in food products.
4.1 Protein solubility
Protein solubility refers to the amount of protein that goes into the solution or colloidal dispersion, which affects protein dispersion. Protein solubility also greatly influences other functional characteristics of proteins, including water-binding capacity, oilbinding capacity, gelation, foaming, and emulsification. Hemp seed proteins have been reported to display a typical U-shaped pH-solubilityprofile with an isoelectric point at approximate pH 5.0 [13,25].At pH 7.0, hemp seed protein isolates generally displayed poor solubility, ranging from 8% to 38% (m/m), depending on the experimental conditions, including centrifugal force, solubility determination protocol, and extraction methods [10,12,13,25].Malomo et al. [10] compared the solubility of albumin and globulin fractions at pH values ranging from 3.0 to 8.0 and showed that the solubility of the albumin fraction ranged from 57% to 84%. This value was significantly higher than that of the globulin fraction,which ranged from 20% to 50% [10]. The propensity of edestin to aggregate might be attributed to the low solubility of the hemp seed protein isolate at pH < 7 [25]. However, the solubility of the protein increases from 65% to 90% at pH > 8.0 [13,25], indicating that hemp seed protein isolate, in a strict sense, was a type of alkali-soluble protein. The potential mechanism of protein solubilization at alkaline pH (especially at pH > 10.0) could be attributed to the dissociation of edestin [27], which was similar to the alkaline effect on soybean glycinin andβ-conglycinin [28]. In addition, the solubility of hemp seed proteins was higher than that of soy protein at pH < 8.0, whereas the solubility of the two types of proteins became similar at pH > 8.0.The difference in protein solubility at acidic pH might be attributed to the differences in the protein constituents and the extent of hexamers aggregation (glycinin or edestin). The high cysteine residue content in edestin might predispose it to intermolecular disulfide bond formation,thereby increasing the extent of aggregation [29].
4.2 Proteolytic capacity
The proteolytic capacity of dietary proteins depends on enzyme accessibility, which is affected by the molecular structure as well as other components associated with proteins. House et al. [7] evaluated the protein proteolytic capacity of hemp seed products (30 samples of hemp seed products grown in Western Canada), including whole hemp seed, hemp seed meal, and dehulled hemp seed using a rat bioassay model, and showed that the proteolytic degree of the three kinds of hemp seed products from the varying hemp seed sources were 84.1%-86.2%,90.8%-97.5%, and 83.5%-92.1%, respectively. Wang et al. [16]performed a proteolytic capacity study for hemp seed protein isolates (7S and 11S) and soluble protein isolates using anin vitrodigestion model by measuring nitrogen release. They found that edestin (consisting of acidic and basic subunits) was rapidly degraded by pepsin (within 1 min), which was similar to the digestion of the acidic and basic subunits of soybean glycinin [16]. Although the digestibility properties of the hemp seed protein isolate were similar to those of the soybean protein isolate when only digested by pepsin,the total proteolytic capacity (pepsin plus trypsin digestion) of the hemp seed protein isolate (88%-91%) was distinctly higher than that of the soybean protein isolate (71%), implying that the hemp seed protein isolate is a valuable source of protein nutrition for human consumption [16].
4.3 Water binding capacity (WHC)
According to the literature, the characteristics of water absorption,water holding, and water binding are manifestations of the interactions between proteins and water [29]. In particular, the ability to interact and entrap water is extremely important for the application of proteins in food products. WHC refers to the ability of proteins to retain water against gravity and is a common term that describes the amount of water entrapped in the protein matrix. It was reported that WHC was closely associated with many intrinsic and extrinsic factors,including the amino acid profile, protein concentration, conformation,hydrophobicity, pH, temperature, and ionic strength [30].A comparison study reported by Radočaj et al. [31] showed that for higher protein content, the WHC of hemp flour was higher than that of flours prepared from fava bean, buckwheat, green pea, and wheat,respectively. Malomo et al. [12] reported that hemp seed protein concentrate prepared using enzymatic digestion combined with membrane ultrafiltration possessed notably higher WHC than that of hemp seed protein isolate and hemp seed protein meals. Furthermore,Hadnadev et al. [13] indicated that the purification of hemp seed protein might favor the interactions between the protein hydrophilic groups and water, thereby increasing the protein WHC. Hadnadev et al. [13]performed a comparison study between HPI and HMI, and showed that the HPI had higher WHC than that of HMI. This could be presumed that the method of protein extraction using alkali solution resulted in more conformational changes and a higher amount of hydrophilic and hydrophobic groups in the protein extracts.
4.4 Oil-binding capacity (OAC)
OAC, defined as the amount of oil absorbed per gram of protein,has been used to describe oil binding by plant proteins, such as quinoa protein isolate [32]. Malomo et al. [12] reported that hemp seed protein concentrates and hemp seed protein isolates showed similar OACs. However, hemp seed protein concentrates had a higher OAC than hemp seed protein meals [12]. The higher OACs of hemp seed protein concentrates and hemp seed protein isolates when compared to hemp seed protein meals might be attributed to their higher protein content. Conversely, Carne et al. [14] found that hemp seed protein isolated by acid or alkaline extraction has a lower OAC than hemp seed protein meals. In addition, their studies observed that, compared to alkaline extraction, acid extraction results in hemp seed protein isolates with lower OACs [14]. The influence of extraction conditions on the OAC of hemp seed protein isolate was also studied by Hadnađev et al. [13] who observed that alkaline-extracted hemp seed protein isolates had higher OACs than micellised hemp seed protein isolates. This might be attributed to partial protein denaturation(unfolding) by alkaline extraction, which caused an increase in surface hydrophobicity [28]. The OAC of hemp seed protein isolates was comparable to that of soybean protein isolates [25] and significantly superior to that of flaxseed and canola isolates [14].
4.5 Thermostability
Stability against thermal denaturation is an important quality characteristic of proteins because conformational changes induced by heat play a crucial role in the functionality of proteins for the food industry.It has been reported that hemp seed protein isolates displayed a typical endothermic peak with a denaturation temperature (Td) from 92 °C to 95 °C when analyzed at pH 7.0 and a heating rate of 5 °C/min [13,16,25].This peak corresponded to the edestin component, especially the hexamer form. Wang et al. [16] found that the 11S fraction of hemp seed proteins exhibited a melting curve similar to that of the hemp seed protein isolates, but no thermal transition signals were detected in the 7S fraction. A much lower Td (68.3 °C) was observed for the hemp seed protein isolate by Carne et al. [14],who found that the hemp seed protein isolates obtained by alkaline(or acid) extraction-isoelectric precipitation had a lower Td than that of defatted hemp seed cake. Furthermore, Hadnadev et al. [13]found that HMI has a slightly higher Td (96.2 °C) and enthalpy(ΔH= 21.7 mJ/mg) than those of HPI, which had a Td and ΔHof 93.5 °C and 11.8 mJ/mg, respectively. This might be attributed to the milder extraction conditions of micellization compared to those of the alkaline extraction-isoelectric precipitation method, which had minimal effects on the protein native structure, thereby allowing edestin to largely maintain its higher structural order and thermal stability [13].
4.6 Gelation capacity
Gel formation is a complex process involving multiple kinetic steps, including denaturation, dissociation, association, and aggregation of gel-forming substances. Heating, pH change, additional salts, pressure changes or shearing, and the presence of various solvents may lead to protein gelation. Heat induction is the most commonly used approach for protein gels, and it plays a critical role in affecting the structure and properties of many food products. Malomo et al. [11]performed a study to explore the effects of the protein concentration of hemp seed protein products on hemp seed protein gelation property. They found that a higher protein concentration was required to form gel for hemp seed protein isolate prepared by using alkali extraction-isoelectric precipitation than that of hemp seed protein meals, which was 22% for the former and 12% for the later [11]. The potential mechanism might be attributed to isoelectric precipitation resulting in a high level of protein aggregates, which reduces the molecular flexibility required for gel network formation. Moreover,non-protein materials, such as sugars and polysaccharides, might also promote the formation of superior gelling properties of hemp seed protein meals [11]. However, Isinguzo et al. [33] reported that hemp seed protein isolates prepared using isoelectric precipitation formed a self-supporting gel at 12% protein concentration. The difference in hemp seed source, extraction condition, and preheating treatment might have contributed to the inconsistency in the critical gelling concentration of hemp seed proteins.
4.7 Foaming properties
Protein foaming properties, including foam expansion (FE),foaming capacity (FC), and foam stability (FS), commonly refer to the ability of the proteins to be readily adsorbed at the air-water interface, undergo rapid conformational change and rearrangement at the interface, and form a cohesive viscoelastic film via intermolecular interactions [34,35]. These properties play a crucial role in affecting the rheological properties of many foods, including the texture of bread, cakes, whipped cream, ice cream, and beef froth. Protein composition, structure, conformation, amino acid sequence, net charge, charge distribution, solubility, concentration, and surface hydrophobicity were intrinsic factors that significantly affect the hemp seed protein foaming properties [11]. Ajibola et al. [24] isolated the hemp seed protein fractions 11S, 7S, and 2S by pH shifting and compared their foaming properties at different pH. And the authors found that the foaming capacity of the 2S fraction was higher than that of the 11S and 7S fractions, as well as the hemp seed protein isolate,at all pH values [24]. Malomo et al. [11] studied the differences in foaming properties among different hemp seed protein products,including hemp seed protein meal, hemp seed protein concentrate,and hemp seed protein isolate, and showed that hemp seed protein concentrates had a significantly higher FC than that of the other two products at all test conditions (pH ranging from 3.0 to 9.0 and protein concentrations ranging from 20 mg/mL to 60 mg/mL). The higher FC of hemp seed protein concentrate might be attributed to their flexible structure with a minimal level of protein aggregation, resulting in higher protein solubility [11]. Although not as strong a foaming agent as hemp seed protein concentrates, the hemp seed protein isolates showed a higher foam volume than the hemp seed protein meals at pH 3.0 and 5.0 and had a notably higher solubility at pH 3.0 [11]. In addition to forming thick and strong interfacial membranes favoured by the aggregation nature of proteins, the hemp seed protein isolate foams were more stable than those of hemp seed protein concentrate or hemp seed protein meals [11]. The lower protein content and presence of non-protein substances in both hemp seed protein concentrate and hemp seed protein meal might have positive effects on foam forming, as they could decrease the interfacial membranes against the gravitational force, thereby resulting in higher foam drainage in hemp seed protein concentrate than that in hemp seed protein isolate [11,12].Moreover, Malomo et al. [10] also evaluated the FC of hemp seed albumin and globulin fractions and observed that the albumin fraction possessed a notably higher FC than that of globulin fraction at broad pH and protein concentration ranges. The higher solubility and flexible structure of albumin might be attributed to the higher FC [10]. However, a higher FS for globulin than that of albumin at most pH values and protein concentrations were observed [10].The higher percentage of hydrophobic amino acids capable of promoting the protein-protein hydrophobic interactions and favouring a cohesive interfacial membrane at the air-water interface might attribute to the higher FS in globulin.
4.8 Emulsification capacity
Emulsion refers to a dispersion/suspension of two immiscible liquids, in which one liquid was dispersed as small spherical droplets in the other liquid [36]. Food emulsions commonly include two types:oil in water (milk, creams, soup, etc.) or water in oil (margarine,butter, etc.). The emulsion system is thermodynamically unstable due to the increased interfacial surface tension; hence, it requires emulsifiers/stabilizers at the oil/water interface to reduce interfacial surface tension and prevent the coalescence of the dispersed small droplets. Protein is a desirable choice for stabilizing food emulsions in the food industry because of its amphiphilic nature and filmforming characteristics. This amphiphilic nature enables protein bonding to the water/oil interface, forming an interfacial layer around dispersed oil droplets, lowering the surface tension, and preventing structural changes in the emulsion, such as coalescence, creaming,flocculation, or sedimentation during processing or storage [37]. The physicochemical properties of proteins, including the rate of protein adsorption at the oil-water interface, the amount of protein adsorbed(loading), the conformational rearrangement at the interface, the extent of interfacial tension reduction, and the rheology of the cohesive film, have a crucial effect on their emulsifying capacities [38].Emulsifying activity (EA), emulsifying capacity (EC), emulsion stability (ES), and droplet size are commonly used to evaluate the emulsifying properties of proteins. A comparison among hemp seed protein fractions showed that the 2S fraction has the best emulsifying properties and that the emulsification capacity of the three types of fractions is better than that of the hemp seed protein isolate; the potential mechanism underlying its flexible structure allowed it to undergo rapid rearrangement at the oil and water interface [24].Malomo et al. [11] found that hemp seed protein concentrate had a generally lower EC than that of hemp seed protein meal and hemp seed protein isolates and that hemp seed protein meals had the highest EC. The size of oil droplets for hemp seed protein concentrates was typically 6-15 μm, whereas hemp seed protein meal and hemp seed protein isolate formed emulsions with oil droplet size < 1 μm [11]. A partially unfolded structure with more hydrophobic groups exposed,which led to stronger interactions between proteins and the oil core,could contribute to the high EC of hemp seed protein concentrates [11].By contrast, the existence of non-protein components, such as high levels of insoluble polysaccharides, might be responsible for the high EC of hemp seed protein meals, because they were more interactive with oil rather than water [11]. It has been reported that emulsions formed by hemp seed protein meal and hemp seed protein isolates were extremely stable; however, the stability of hemp seed protein concentrate emulsions was dependent on the protein concentration because of the molecular flexibility. Additionally, the emulsion was stable at the protein concentration from 10 mg/mL to 25 mg/mL,while became less stable when the protein concentration was higher than 50 mg/mL [12]. The potential mechanism might be attributed to that the higher protein concentration could result in protein crowding and eventually disrupt the ordered protein-protein interactions.
4.9 Film formation capacity
Proteins that undergo appropriate treatment, such as heating and acid/base/organic solvent/oxidizing agents, can develop an extended structure and many can form a cohesive protein film via hydrogen,ionic, hydrophobic, and covalent bond interactions [39,40]. It has been reported that films developed using soybean proteins possess excellent mechanical properties [26]. A comparison study between hemp seed protein isolate and soybean protein isolates showed that the former formed films exhibited higher tensile strength and smaller elongation at break, and that the total soluble mass in hemp seed protein isolate films was markedly lesser than that in soybean protein isolate films [26]. The potential mechanism might lie in more disulfide bonds of hemp seed protein isolate films which were the predominant interactive force in the network, whereas, in the case of soybean,the protein isolate film formation was the result of the collective contribution of disulfide and hydrogen bonds along with hydrophobic interactions.
As mentioned above, the hemp seed protein isolate generally displays poor solubility at neutral pH. Protein solubility has a marked effect on the development of colloidal structures, including gelation, foaming, and emulsification; therefore, its application in food manufacturing is extremely limited. To widen protein utilization, protein degeneration might be an effective approach, and enzymes, chemicals, or physical denaturation are the most commonly used methods to enhance the functionality of proteins for protein processing. However, according to the literature, these three kinds of traditional protein denaturation methods have drawbacks, including altered protein functionality and harmful health influences, food allergy, impaired nutrition, and high-energy consumption [41]. By contrast, novel methods, including high-pressure treatment, pulsed electric field, ultrasound, microwave, gamma irradiation, supercritical fluid extrusion, tribomechanical activation, ultrafiltration, and cold atmospheric plasma processing, were more desirable because they generally offer low-temperature and waste-free technologies for quality enhancement and high energy efficiency as well as maintaining the protein functionality [41].
5. Bioactivities of hemp seed protein hydrolysates
Foodborne proteins from different origins could have beneficial effects on human health via degradation by digestive enzymes in the gastrointestinal tract, producing bioactive peptides, which were integral parts of their native structure. Compared with medication,foodborne protein hydrolysates possess higher specificity and affinity and were associated with fewer side effects and demonstrate higher tissue penetration [42]. Enzymatic extraction and microbial fermentation methods were used to prepare the hemp seed hydrolysates. Enzymatic methods that use protamex, pepsin, alcalase,flavourzyme, neutrase, and trypsin were the most commonly used methods for the hydrolysis of hemp seed proteins [43]. Tang et al. [43]usedin vitroantioxidant evaluation systems to explore the antioxidant properties of hemp seed protein hydrolysates that had been enzymatically degraded by six kinds of proteases. In addition, Lu et al. [21]separated alcalase-catalysed hemp seed protein isolate hydrolysate with macroporous adsorption resin, then identified the amino acid sequence of the hydrolysates by using MALDI-TOF/TOF MS/MS,and found the peptides of NHAV and HVRETALV. Lin et al. [44]usedBacillus subtilisfor the liquid fermentation of hemp seed meal,which resulted in 50.5% peptide production.
In addition, several studies have reported that hemp seed protein hydrolysates possess various bioactivities including antioxidant,antihypertensive, renal disease modulation, acetylcholinesterase(AChE) inhibition,α-glucosidase inhibition, anticancer, anti-fatigue,immunomodulation against hypercholesterolemia, neuroprotection,and lipid metabolism disorder regulation effects (Fig. 4) [29,45-48].
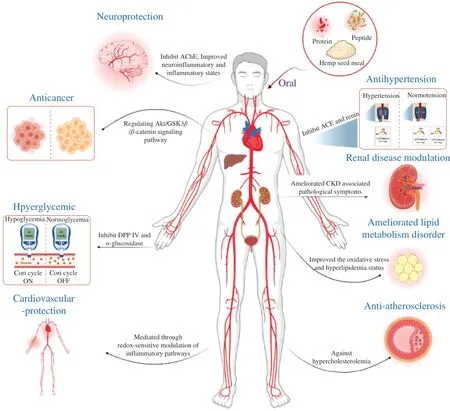
Fig. 4 Biological activities of hemp seed protein/peptide and the potential mechanisms. AChE: Acetylcholinesterase, ACE: Angiotension converting enzyme;CKD: Chronic kidney disease.
5.1 Antioxidant activity
Bothin vitroandin vivoexperiments indicated that hemp seed protein hydrolysates exhibited strong antioxidant effects. Tang et al. [43]enzymatically hydrolyzed hemp seed proteins by six different proteases, namely alcalase, flavourzyme, pepsin, neutrase, protamex,and trypsin, to evaluate and compare the antioxidant activities of the hydrolysates. All the hydrolysates exhibited, to a variable extent,antioxidant activities, including DPPH radical scavenging activity,reducing power, and Fe2+chelating ability, depending on the type of proteases and the period of hydrolysis time; among which pepsin was the most active in hydrolyzing the hemp seed protein at the acidic pH of digestion [43]. Furthermore, Xu et al. [49] reported that the hemp seed hydrolysates prepared using the 13 hemp showed different DPPH scavenging activities, ranging from 0.37% to 28.78%. Hence, the antioxidant properties of protein hydrolysates were largely dependent on the type and specificity of proteases, hydrolysis conditions, degree of hydrolysis, and hemp seed variety [49]. In addition, Girgih et al. [50]found that the treatment of young growing spontaneously hypertensive rats treated with hemp seed protein hydrolysates resulted in a notable amelioration in plasma superoxide dismutase and catalase activities.Interestingly, treatment of spontaneously hypertensive rats treated with unhydrolyzed hemp seed protein could also decrease oxidative stress injury, but the effect was inferior to that of hemp seed protein hydrolysates, indicating that hemp seed protein hydrolysates could serve as a more efficient nutriceutical aid than the whole protein for oxidative stress reduction in mammalian cells [50,51].
5.2 Antihypertensive activity
Thein vitroandin vivoantihypertensive effects of hemp seed protein hydrolysates and peptide fractions were also tested. The ability to inhibit renin and angiotensin-converting enzyme (ACE)activities were commonly used to evaluate the antihypertensive activity of the molecule [52]. Girgih et al. [50] found that the hemp seed protein hydrolysates exhibited stronger ACE- and renininhibitory activities in comparison with that of the peptide fractions.The IC50values of ACE and renin for hemp seed protein hydrolysates and peptide fractions were 0.67 and 0.81 mg/mL, respectively [50].Furthermore, the authors found that the IC50values for Mw < 1 kDa peptide fractions against ACE and renin were 1.05 and 2.52 mg/mL,respectively, whereas the ACE and renin fractions, whose Mw ranged from 1 kDa to 3 kDa, had IC50values of 1.17 and 1.89 mg/mL,respectively [50]. The potential mechanisms might be attributed to the peptides against ACE and renin was a mixed-type pattern, in which peptides bind to both the active and non-active sites to reduce renin and ACE enzymatic catalysis. In addition, the authors also evaluated antihypertensive activityin vivousing an animal model [50].The spontaneously hypertensive rats were treated with hemp seed protein hydrolysates and different peptide fractions, and systolic blood pressure was measured using tail-cuff plethysmography at 2,4, 6, 8, and 24 h [50]. Systolic blood pressure in the group treated with hemp seed protein hydrolysates was significantly lower than that in the peptide fraction groups [50]. However, another study performed by Sunday et al. [53] found that the untreated proteins exhibited no measurable antihypertensive effects, suggesting that the digested peptide fractions, not the native protein, could facilitate the antihypertensive effects.
5.3 Renal disease modulation
Aukema et al. [54] used an animal model of Weanling Han:SPRD-cy rats with experimental polycystic renal disease to evaluate the effects of hemp seed, pea, and soybean protein sources on renal health. The rats were fed diets rich in hemp seed, pea, and soybean protein, and rats treated with the standard AIN 93G diet with casein as the protein source was used as the control [54]. The authors observed that the progression of renal disease was delayed in rats fed with soybean or hemp seed protein-containing diet [54]. Furthermore,compared to rats fed with a casein-based diet, soybean and hemp seed protein-containing diet-treated rats had lower fluid content, smaller cyst volumes, less fibrosis, lower chemokine receptor 2 levels, and normal serum creatinine level as compared to those in rats fed with a casein-based diet [54]. In addition, the study also reported that hemp seed protein treatment could mitigate the symptoms of cardiomegaly [54].These reports suggest that hemp seed protein possessed potential renal and cardiovascular protective effects [54].
5.4 AChE inhibition
Acetylcholine (Ach) is an indispensable neurotransmitter of the parasympathetic nervous system, a part of the autonomic nervous system (a branch of the peripheral nervous system). It is involved in smooth muscle contraction, blood vessel dilation, bodily secretion increase, and heart rate reduction. However, AChE, a serine hydrolase, breaks down Ach, thereby resulting in serious neurological diseases [55,56]. Therefore, research on AChE inhibitors capable of decreasing the degradation of Ach is becoming a popular topic [57]. Malomo et al. [58] used 6 different proteases (pepsin,papain, thermoase, flavourzyme, alcalase, and pepsin+pancreatin)to hydrolyse hemp seed protein isolates and investigated the AChEinhibitory characteristics of the enzymatically prepared hydrolysates.Results showed that hemp seed protein hydrolysate prepared by 1%pepsin exhibited the highest AChE inhibitor activity with an IC50value of 6 µg/mL, which was significantly lower than that of the other hemp seed protein hydrolysates with IC50values varying from 8 µg/mL to 11.6 µg/mL [58]. LC-MS profiling analysis showed that most of the peptide fractions possessed low molecular weight with maximum masses of less than 1 200 Da [58]. However, compared with papain and alcalase treatment, hemp seed proteins treated with pepsin exhibited a wider peptide molecular weight range, which varied from 244 Da to 1 009 Da [58]. Similarly, the peptide molecular weight distributions of hemp seed proteins treated with papain and alcalase were 246-758 and 246-607 Da, respectively. The higher AChE inhibitor activity for pepsin hydrolysates might be attributed to their wider distribution of peptide molecular weight [58].
5.5 α-Glucosidase and dipeptidyl peptidase IV (DPP IV) inhibition
Diabetes mellitus is a lethal disease and remains an important medical challenge around the world. Especially, impaired glucose homeostasis is a major threatening factor. The enzymes,α-glucosidase and DPP IV/CD26 play a critical role in maintaining glucose homeostasis in human bodies. Inhibition ofα-glucosidase and DPP IV activities have been identified to benefit the regulation of glycaemia.
Peptides are a promising source of safe hypoglycaemics in nutraceuticals with highα-glucosidase and DPP IV inhibitory activities. Yao et al. [59] used alcalase to treat hemp seed protein and investigated theα-glucosidase and DPP IV inhibitory activities of the enzymatic hydrolysates of hemp seed protein. The hemp seed protein hydrolysates exhibited highα-glucosidase inhibitory activity,which might be attributed to two kinds of peptides whose sequences were identified to be Leu-Arg (287.2 Da) and Pro-Leu-Met-Leu-Pro(568.4 Da) [59]. In addition, Lammi et al. [60] evaluated the DPP IV inhibitor activity of hemp seed protein hydrolysates derived from trypsin and pepsin treatments. Both of the two hydrolysates exhibited DPP IV inhibitory activities, however, the hydrolysate treated with pepsin was 2-fold higher activity than that treated with trypsin at 1.0 mg/mL, indicating that the specificity of the enzymes used to release the peptides from the parent proteins would affect the bioactivity of generated peptides [60].
5.6 Anticancer activity
Wei et al. [61] used neutral protease (3%,m/V)-treated hemp seed meal and separated the hemp seed protein hydrolysates using ultrafiltration (collected the fraction that was < 1 Ku). Then, the Hep3B liver cancer and L02 normal hepatocyte cell lines were treated with different concentrations (0, 0.5, 1, 2, 5, and 10 mg/mL) of hemp seed protein hydrolysates. Subsequently, their cell viability wound healing, reactive oxygen species (ROS) production, and mitochondrial depolarisation were investigated to evaluate the anticancer effects of hemp seed protein hydrolysates. Increased cell apoptosis and decreased cell viability and migration were observed in Hep3B human liver cancer cells pre-treated with hemp seed hydrolysates,while no effects on the normal liver cell line L02 was detected. The observed effects might be attributed to the induction of Akt and GSK-3βphosphorylation by hemp seed protein hydrolysate treatment,with a subsequent downregulation ofβ-catenin [61].
5.7 Anti-inflammatory activity and other benefits
Rodriguez-Martin et al. [62] reported the inflammation modulatory activities of hemp seed protein hydrolysates on lipopolysaccharide(LPS)-activated primary human monocytes in 2020. In their study,LPS-stimulated human primary monocytes were treated with hemp seed protein isolates (obtained using the alkali extraction and acid precipitation methods) and hydrolysates (hydrolysis with proteases,such as alcalase or flavourzyme). The results showed that hemp seed protein isolates exhibited excellent anti-inflammatory actives by downregulating the expression of pro-inflammatory mediators (TNF-α,IL-1β, and IL-6) and upregulating the expression of anti-inflammatory mediators (IL-10 and IL-4). Moreover, the expression of the M1 polarisation marker genesCCR7andiNOSwas downregulated by hemp seed protein isolates. By contrast, the expression of M2 polarisation marker genesCD200RandMRC1were upregulated.The expression of the chemotaxis genesCCR2andCCL2were also downregulated by the treatment with hemp seed protein isolates.These suggested that the anti-inflammatory activity of hemp seed protein products might be attributed to the regulation of M1 polarisation marker gene expression [62].
In addition to the bioactivities mentioned above, hemp seed proteins/peptides were also reported to have several regulatory effects,including anti-fatigue and immunomodulatory effects [45], effects against hypercholesterolemia [47], neuroprotective effects [63], and effects on lipid metabolism disorder [64].
6. Opportunities and challenges of hemp seed proteins in the food industry
With changing dietary habits and a higher demand for dietary foods of nutraceutical value, the demand for natural plant proteins and the exploration of non-traditional protein sources for the food industry are increasing. According to statistics, the global plant protein market size was 5.322 billion dollars in 2019 and is predicted to reach up to 8.946 billion dollars in 2025. Hemp seed proteins have attracted increasing attention in both scientific and industrial fields for their excellent nutraceutical value, superior digestibility,and physicochemical properties. Firstly, hemp seed proteins have high nutritional value and have been found to be a desirable nutritive additive and functional ingredient in formulated foods in terms of enhancing the product quality attributes. Secondly, hemp seed protein contains all the essential amino acids in a balanced ratio. Additionally,the amounts of arginine and sulphur-containing amino acids in hemp seed proteins are substantially higher than those in most plant proteins, including soybean and wheat proteins. The high amino acid content makes it valuable as a nutritional food ingredient suitable for postoperative, burn, hypertension, and cardiovascular disease patients [8].In addition, hemp seed proteins show lower allergenicity than most plant proteins, suggesting that they are safer. Thus, hemp seed protein is a better substitute in protein-related food products (Fig. 5).Currently, the application of hemp seed protein products in the food industry mainly includes five main categories: bakery products,extruded products, beverages, dairy and infant formula, and processed meat products. However, its application in healthcare food or special medical food still needs to be explored.
However, by changing the extraction procedures, the quality of hemp seed protein can be improved greatly. For instance, the color of hemp seed protein products may range from light tan to dark brown as the extraction pH, temperature, and drying method change. The color of the protein influences the appearance of hemp seed protein products and consumer acceptance, and therefore, their applicability in the food industry (Fig. 5). To overcome this issue, an optimized and stable extraction procedure should be established. The understanding of chemical composition, nutritional and health benefits, processing properties, and functional behavior of hemp seed proteins in food processing has been increased, but much nutraceutical aspects remain unknown. For instance, amino acid sequence, crystal structure, and functional annotation of most of the hemp seed proteins are not characterized yet. Therefore, more systematic studies are needed to explore the chemical composition, structure, functionality, and nutriceutical characteristics of hemp seed proteins. Furthermore,hemp seed proteins possess high nutritional value, but their poor solubility at neutral pH restricts their application in the food industry.Hence, structural modification methods need to be performed.Nowadays, the utilization of hemp seed proteins in the native protein conformation is common; however, these proteins are inactive until they are degraded by enzymes during digestion or food processing.Once the native protein is digested into peptides, the peptides are absorbed from the intestine into the circulatory system and display various biological activities. However, studies on the nutriceutical benefits and the mechanism of hemp seed-based food products for human consumption are still lacking. It is necessary to explore the relationship between protein structure and its health-promoting effects by combining the molecular, cellular, and animal models.
Plant bioactive peptides are becoming a hot topic in the cosmetic, medical, and food industries, because peptides have been identified to possess various bioactive properties, including antioxidant, antibacterial, immunoregulatory, anti-fatigue, antianxiety,anti-obesity, anti-inflammation, anti-atherosclerosis [65,66],antihypertensive [67], and anti-diabetic activities [48]. Enzymatic degradation of proteins was the most widely used approach in the mining of bioactive peptides; however, this traditional method has drawbacks, including the need for protease screening for protein degradation, the progression of bioactive peptide separation and purification and function discovery, which are complex and labour-,time- and money-consuming processes. Therefore, it is imperative to establish a new methodology to accelerate the mining of bioactive peptides. In recent years, with the development of omics technology,researchers have attempted to develop a multi-omics technology-based bioactive peptide mining strategy, such as adopting transcriptomics to obtain the protein sequence and using it as a database for proteomics identification, combining simulated enzyme digestion and functional annotation to obtain the structure and function of annotated peptides in a more effective manner (Fig. 6). Compared with the traditional top-down enzymatic degradation methods, the multi-omics-based method can greatly reduce the number of experiments for screening the target peptides, shorten the research cycle, and increase research outcomes. In addition, the use of rapidly developed molecular simulation techniques and artificial intelligence technology can accelerate the elucidation of the structure and function relationship of annotated biopeptides, thereby the mining efficiency of the biopeptides produced from various natural protein sources, including hemp seed biopeptides.

Fig. 5 Strengths, Weaknesses, Opportunities, and Threats (SWOT) analysis for hemp seed protein application in the food industry.

Fig. 6 Schematic diagram of the advanced technologies that can be applied to develop hemp seed protein based food products.
Moreover, there is still a lack of awareness regarding the differences between “industrial hemp” and “drug hemp”, especially among the public. Therefore, increased basic and applied research on the potential health benefits and application of hemp seed as well as public dialogue are needed for the prevention of human diseases,especially chronic diseases.
7. Conclusions
In summary, hemp seed protein is becoming an important alternative plant protein source in the food and nutraceutical industries for its high nutritive values, processing capability and medicinal food homology. With the changing in dietary habits and increasing demand for natural food ingredients, the need for hemp seed proteins is expected to grow rapidly. However, to make full use of the competitive advantages of the hemp seed proteins the following key issues need to be solved for promoting its application in the food industry. The first challenge is how to extract and isolate the proteins from hemp seed with retained nativity and functionality by developing green and economic methods at an industrial scale. Besides, in-depth fundamental and applied studies are indispensable for clarifying the relationship between protein structure and tech/bio-functionality,innovation of bioactive peptide mining and manufacturing technologies, and health-promoting mechanism of hemp seed protein and peptides based on evidence-based medicine.
Conflict of interest statement
The authors report no conflict of interest.
Acknowledgments
The financial support received from the Shenzhen Science and Technology Innovation Commission (KCXFZ20201221173207022),Youth Science Foundation Project (32101936) and China Postdoctoral Science Foundation, No.15 Special Fund (In-Station) (2022T150366)are gratefully acknowledged.
- 食品科学与人类健康(英文)的其它文章
- A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
- Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases
- The role of f lavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness
- The hypoglycemic potential of phenolics from functional foods and their mechanisms
- Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents
- Flowers: precious food and medicine resources

