Characterization of Generalized Pustular Psoriasis in Northwest China: A Single-Center Retrospective Study
Xiao-Na Li, Bin Peng, Song-Mei Geng*
Department of Dermatology, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi 710004, China.
Abstract Objective: This study was performed to investigate the clinical characteristics of patients with generalized pustular psoriasis (GPP) in Northwest China.Methods: The clinical data of patients with GPP were retrospectively collected in the Second Affiliated Hospital of Xi’an Jiaotong University from January 1, 2017, to December 31, 2021 and analyzed using the chi-square test and Mann-Whitney U test.Results: In total, 179 hospitalized patients were included. The male:female ratio was 1.16:1.00, and the mean age at onset was 35.05 ± 19.11 years. Psoriasis vulgaris was also present in 76.0% of patients, and a family history of psoriasis was present in 15.6%. The mean duration of hospitalization was 10.67 ± 4.31 days, and the mean duration of flares was 29.58 ± 24.32 days. Infections and suspected drugs were predisposing factors. A fever developed in 54.7% of patients, and pruritus developed in 70.9%. Some patients had involvement of the nails (38.0%), scalp(46.9%), and tongue (4.5%). Cardiovascular disease, hypertension, and gallbladder-related disease were common comorbidities. The efficacy of acitretin was 84.7%, that of methotrexate was 66.7%, and that of cyclosporine was 100%. Fifteen patients were treated with secukinumab or adalimumab and responded well. The mean response time was 6.34 ± 2.91 days based on the combination treatments. The mean duration of the treatment regimen was 111.35 ± 94.25 days, and approximately 46.6% (n = 131) of patients developed recurrence.Conclusion: Our retrospective study showed that most cases of GPP were accompanied by psoriasis vulgaris and associated with fever or pruritus. Acitretin had good therapeutic efficacy, but recurrence should be noted.Biologics are increasingly becoming effective treatments, but their superiority and safety need further research.
Keywords: generalized pustular psoriasis, clinical characteristics, treatments
Introduction
Generalized pustular psoriasis (GPP) is a rare and severe form of aseptic pustulosis that often presents with varying degrees of systemic symptoms, including fever, pain,fatigue, leukocytosis, and an elevated C-reactive protein concentration.1Infection, drugs, pregnancy, and hypocalcemia are common predisposing factors for GPP.2
GPP was previously considered a severe subtype of psoriasis, but current studies consider it a distinct clinical entity.3GPP may appear in the setting of previous or existing psoriasis vulgaris (PsV) or alone. These 2 conditions differ in terms of genetic and clinical characteristics.4-5
GPP exhibits heterogeneous disease severity and extracutaneous symptoms at onset. Arthritis and arthralgia, uveitis and conjunctivitis, neutrophilic cholangitis,interstitial pneumonia, and acute kidney injury are common extracutaneous manifestations of GPP.1-2The common complications of GPP included hypertension, peptic ulcers, osteoporosis, diabetes, gout, psoriatic arthritis,chronic obstructive pulmonary disease, obesity, thyroid disease, and other forms of psoriasis.6
There are no uniform criteria for the diagnosis of GPP.The consensus of the European Rare and Severe Psoriasis Expert Network (ERASPEN) defined GPP as the presence of primary, sterile, visible pustules occurring on non-limbic skin (excluding cases in which pustules are limited to psoriatic plaques). GPP can be present with or without systemic inflammation and with or without PsV, and it may be either relapsing (>1 episode) or persistent (>3 months).7Japanese treatment guidelines indicate that GPP can be definitively diagnosed in patients whose condition meets the following four characteristics and that patients with the second and third characteristics should be suspected to have GPP: (1) systemic symptoms such as fever and fatigue,(2) systemic or extensive flush accompanied by multiple sterile pustules that sometimes merge to form lakes of pus,(3) neutrophilic subcorneal pustules histopathologically characterized by spongiform pustules of Kogoj, and (4)repeated occurrence of the above clinical and histological features.8The recommended diagnostic criteria for GPP in China are consistent with the ERASPEN consensus.9
Treatment of GPP consists of 2 phases. Acute treatment is focused on improving skin and systemic symptoms and preventing potential complications, and long-term management is aimed at preventing the recurrence or progression of the disease and treating its comorbidities.10Acitretin is the first choice for GPP (although it is contraindicated in pregnant patients), and attention must be paid to its adverse effects when used in the long term; these include cheilitis,dry mouth, hepatotoxicity, hyperlipidemia, bone damage,and teratogenicity.8-10Methotrexate may be used in patients who experience poor efficacy of acitretin and severe arthritis. Common adverse effects include hepatotoxicity, bone marrow suppression, pulmonary fibrosis, and teratogenesis.8-10For patients with severe acute-stage disease who need rapid improvement, cyclosporine can be used and then switched to acitretin or methotrexate after the condition improves. Attention should be paid to its adverse effects such as nephrotoxicity, hypertension, and immunosuppression.8-10Oral steroids can be an effective adjunct but not first-line treatment when patients are severely ill or do not respond to other therapies.8Biologics have shed light on the proper treatment of GPP, but they have not been approved to treat GPP in most countries. Some of the biologics currently in use include tumor necrosis factor-α (TNF-α) inhibitors (adalimumab, infliximab, and certolizumab pegol),interleukin (IL)-17 and IL-17 receptor (IL-17R) inhibitors(secukinumab, ixekizumab, and brodalumab), IL-23 and IL-23/IL-12 inhibitors (guselkumab, risankizumab, and ustekinumab), and IL-1β and IL-1R inhibitors (canakinumab, gevokizumab, and anakinra).10
Studies of GPP have been published in different countries and regions, but few have been published in China.This study was performed to outline the clinical characteristics of patients with GPP from our center and compare the differences between patients who have GPP with PsV and patients who have GPP alone.
Materials and methods
This study involved patients who fulfilled the Japanese diagnostic criteria for GPP8and were hospitalized in the Second Affiliated Hospital of Xi’an Jiaotong University from January 1, 2017, to December 31, 2021. The data were retrospectively collected and included sex, history of psoriasis, age at onset, duration of hospitalization, duration of GPP flares, skin biopsy, predisposing factors, accompanying symptoms, comorbidities, treatments, treatment response time, duration of the treatment regimen, and recurrence. According to the patients’ history of PsV, they were divided into a GPP+PsVgroup and GPP-PsVgroup. This study was approved by the Research Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University (Approval number: 2019072).
Statistical analysis
SPSS 23.0 software (IBM Corp., Armonk, New York)was used for the data analysis, the chi-square test and Mann-WhitneyUtest were the main statistical methods.The measurement data was presented as mean ± SD, andP< 0.05 was considered statistically different.
Results
General information of patients with GPP
In total, 179 patients with GPP with a male:female ratio of 1.16:1.00 were included in this study. Among them, 136(76.0%) patients had a history of PsV (GPP+PsVgroup),and 43 (24.0%) patients developed only pustules (GPP-PsVgroup). The age at onset in the GPP-PsVgroup was younger than that in the GPP+PsVgroup (P= 0.013). An onset age of <18 years was more common in the GPP-PsVgroup than in the GPP+PsVgroup (P= 0.024). Twenty-eight (15.6%)patients had a family history of psoriasis, including 27 in the GPP+PsVgroup and 1 in the GPP-PsVgroup (P= 0.006)(Table 1).
The mean duration of hospitalization was slightly longer in the GPP-PsVgroup than in the GPP+PsVgroup(P= 0.511). The mean duration of GPP flares was longer in the GPP-PsVgroup than in the GPP+PsVgroup (P= 0.133).Skin biopsy was performed in 48 patients, 5 of whom had a pathological diagnosis of psoriasis, and 1 patient was pathologically diagnosed with subcorneal pustulosis, but she had a 3-year history of psoriasis followed by recurrent erythematous pustules and rehospitalization. Still, all patients were clinically diagnosed with GPP (Table 1).
Predisposing factors
The predisposing factors for GPP included infection, especially upper respiratory tract infection (URTI); a suspected drug; and others such as food, mental stress, fatigue, and trauma. URTI was a more common predisposing factor in the GPP+PsVgroup than in the GPP-PsVgroup (P= 0.023).Suspected drugs included glucocorticoids, herbal preparations, antibiotics, and other unspecified drugs. The proportion was similar in the GPP+PsVand GPP-PsVgroups(P= 0.402); GPP in both groups was mainly caused by improper use of glucocorticoids or herbal preparations.One patient in the GPP+PsVgroup developed the disease 11 days after the second dose of the COVID-19 vaccine.The other 43.6% patients had no clear predisposing factors; this ratio was higher in the GPP-PsVgroup than in the GPP+PsVgroup (P= 0.027) (Table 1).
Accompanying symptoms
Ninety-eight (54.7%) patients developed a fever (>38°C)during the acute stage, with no difference between the GPP+PsVand GPP-PsVgroups (P= 0.849). A total of 127 (70.9%)patients also had pruritus, which was more common in theGPP-PsVgroup than in the GPP+PsVgroup (P= 0.337). Twelve(6.7%) patients also had arthralgia, and all of these patients were in the GPP+PsVgroup (P= 0.096) (Table 1).
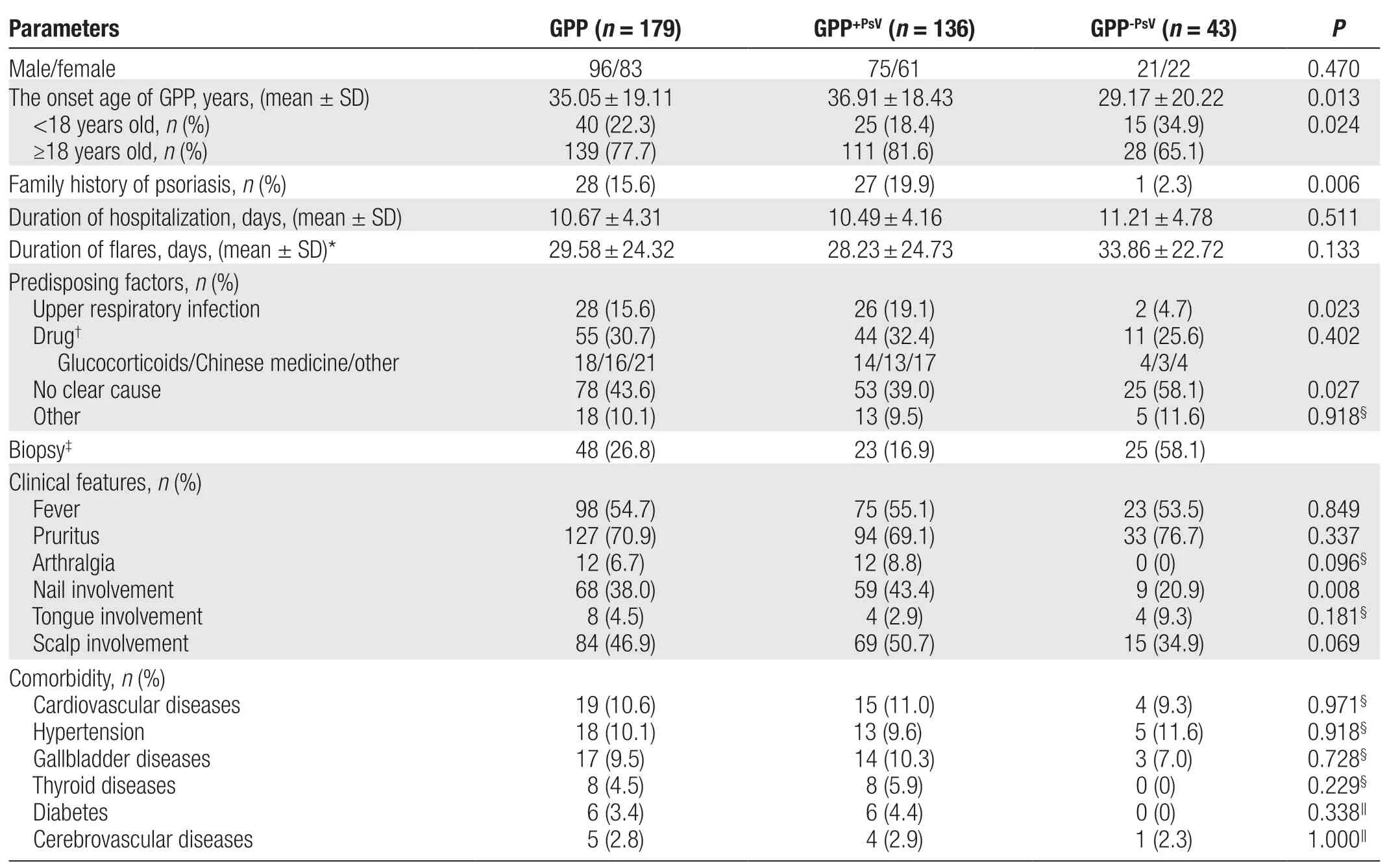
Table 1 Clinical characteristics of patients with GPP.
Involvement of special areas
Nail involvement, manifesting as nail thickening, deformation, punctate depression, or thimble-like nails, was present in 38.0% of the patients and was more common in the GPP+PsVgroup than in the GPP-PsVgroup(P= 0.008). Tongue involvement, manifesting as a fissured or geographic tongue, was present in 4.5% of the patients and was more common in the GPP-PsVgroup than in the GPP+PsVgroup (P= 0.181). Scalp involvement,manifesting as erythema, scales, or pustules, was present in 46.9% of the patients and was more common in the GPP+PsVgroup in the GPP-PsVgroup (P= 0.069) (Table 1).
Comorbidities
Comorbidities of GPP included cardiovascular diseases,hypertension, gallbladder-related diseases, thyroid-related diseases, diabetes, and cerebrovascular diseases.Cardiovascular diseases included coronary atherosclerotic heart disease, pericardial effusion, and lower limb or aortic plaques. Gallbladder-related diseases included gallbladder polyps, stones, and a coarse gallbladder wall. Thyroid-related diseases included hyperthyroidism,hypothyroidism, and thyroid nodules. Cerebrovascular diseases included cerebral infarction and cerebral aneurysm. These comorbidities were not significantly different between the GPP+PsVand GPP-PsVgroups, although there were no patients with thyroid-related disease or diabetes in the GPP-PsVgroup. Some patients had multiple diseases simultaneously, but whether these diseases were associated with GPP was unclear (Table 1).
Treatments of patients with GPP
In addition to symptom-based medication, 98 patients were treated with acitretin and 83 (84.7%) responded well. Among them, 1 patient requested to be discharged after improvement, 2 patients still had a few pustules at the time of discharge, and 1 patient was transferred to the intensive care unit because of sudden respiratory failure after the pustules had basically subsided. Fortyone patients with severe disease were treated with glucocorticoids on admission, followed by a combination of conventional treatments. Three patients were treated with methotrexate, and 2 were effectively controlled;6 patients were treated with cyclosporine, and all were effectively controlled (Table 2).
Fifteen patients whose condition responded poorly or who were unable to tolerate immunosuppressants were treated with biologics and responded well, including 5 patients treated with secukinumab and 10 patients treated with adalimumab (Table 3). Six patients experienced relapse after stopping treatment.
The mean response time to treatment was 6.34 ± 2.91 days (median, 6 days) and was slightly longer in the GPP-PsVgroup (7.14 ± 3.73 days) than in the GPP+PsVgroup (6.08 ± 2.56 days) (P= 0.159). The treatment of GPP consisted of acute and maintenance treatment, with hospitalization to control acute symptoms as needed. The duration of treatment was calculated by following up the patients’ postdischarge outpatient follow-up data; however, 48 patients had no follow-up data. The mean duration of the treatment regimen was 111.35 ± 94.25 days(median, 81 days) and was slightly longer in the GPP-PsVgroup (126.39 ± 109.04 days) than in the GPP+PsVgroup(106.18 ± 88.64 days) (P= 0.363). A total of 46.6% of patients (n= 131) developed relapse during or after discontinuation of maintenance treatment [44.3% in the GPP+PsVgroup (n= 97) and 52.9% in the GPP-PsVgroup(n= 34)] (Table 2).
Discussion
Previous studies have suggested that female patients seem to be more common in GPP11-16; however, the data vary according to geographical and racial differences(Table 4). Our study included more male than female patients (96/83), which is similar to the study performed by Ohataet al.18(53/49). Zhenget al.17included significantly more male than female patients (80/30), but the ratio was equal in the GPP-PsVgroup (8/8); this is consistent with our results (21/22).
In our study, the mean age at onset of GPP(35.05 ± 19.11 years) was younger than that reported by Zhenget al.17(43.4 ± 19.2 years) and Ohataet al.18(45.3 ± 20 years), and these findings are consistent with the results reported by Tosukhowonget al.11(38.3 ± 17.1 years) and Kara Polatet al.14(36.1 ± 20.3 years). The differences may be related to the proportion of pediatric/adolescent patients in the different studies. The age at onset of GPP-PsVwas younger than that of GPP+PsVin the present study, which is consistent with the results of previous studies.17-18
In total, 76.0% of patients in our study had a history of PsV, with a previously reported proportion of 10.7%to 85.5%.12,14-20Studies have shown that female patients seem to be more common in GPP-PsVthan GPP+PsV,13,17-18which is consistent with our results. However, there was no statistically significant difference in the sex composition between the 2 groups.
The incidence of a family history of psoriasis ranged from 0 to 31.2% in previous reports.12-14,17-18,21In our study, 15.6% of patients had a family history, which is consistent with the findings of Zhenget al.17(14.5%). In our study, a family history of psoriasis was more common in the GPP+PsVgroup than in the GPP-PsVgroup, whereas Zhenget al.17showed opposite results but with no statistically significant differences.
The mean duration of hospitalization for 110 patients with GPP in a study by Okuboet al.6was 12 ± 7.4 days,which was 2 to 4 days longer than for patients with plaque psoriasis. The mean duration of hospitalization for 110 patients with GPP in the study by Zhenget al.17was 16.2 ± 6.5 days. In our study, the mean duration of hospitalization was 10.67 ± 4.31 days. These differences may be related to the patients’ conditions and treatment regimens.
URTI and drugs are common causes of exacerbations or predisposing factors for GPP. In our study, drugs were a more common predisposing factor than other factors in both groups, and URTI was more common in the GPP+PsVgroup than in the GPP-PsVgroup. In the study by Ohataet al.,18GPP-PsVwas more commonly induced by infection,and the contributions of drugs and infection were comparable in the GPP+PsVgroup, although neither was significantly different. However, about 43.6% of patients had no apparent predisposing factors; this was more commonin the GPP-PsVgroup, which is consistent with the results reported by Zhenget al.17
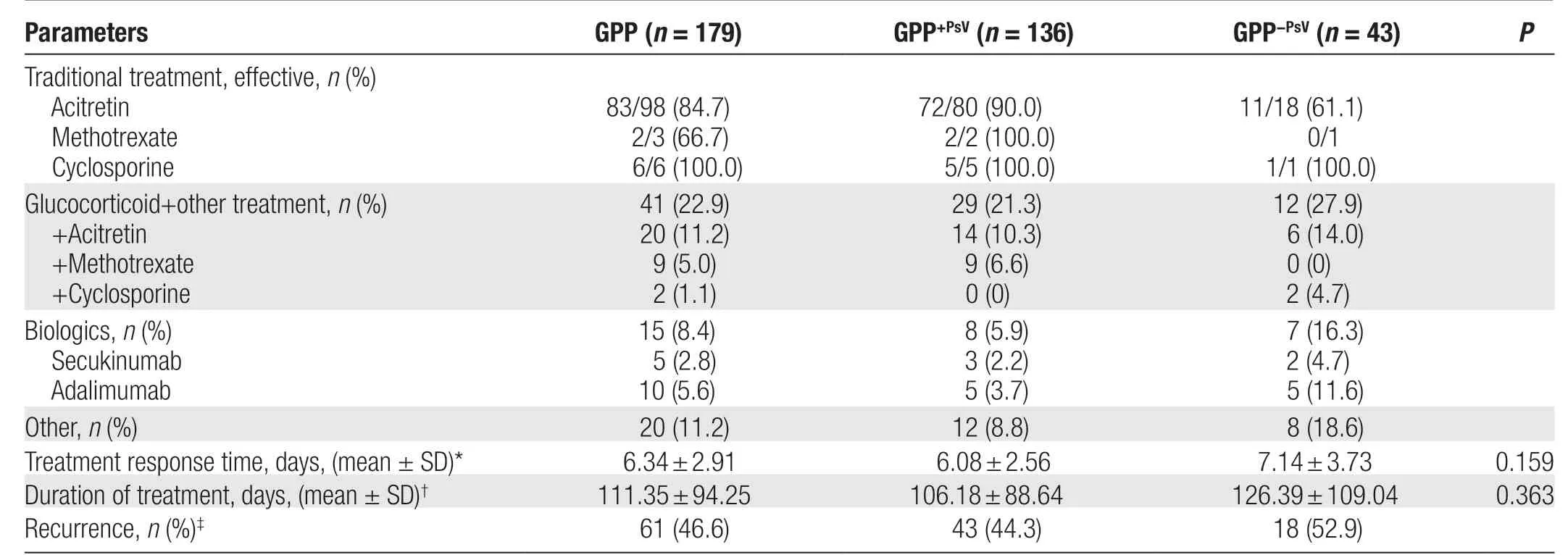
Table 2 Treatments of patients with GPP.
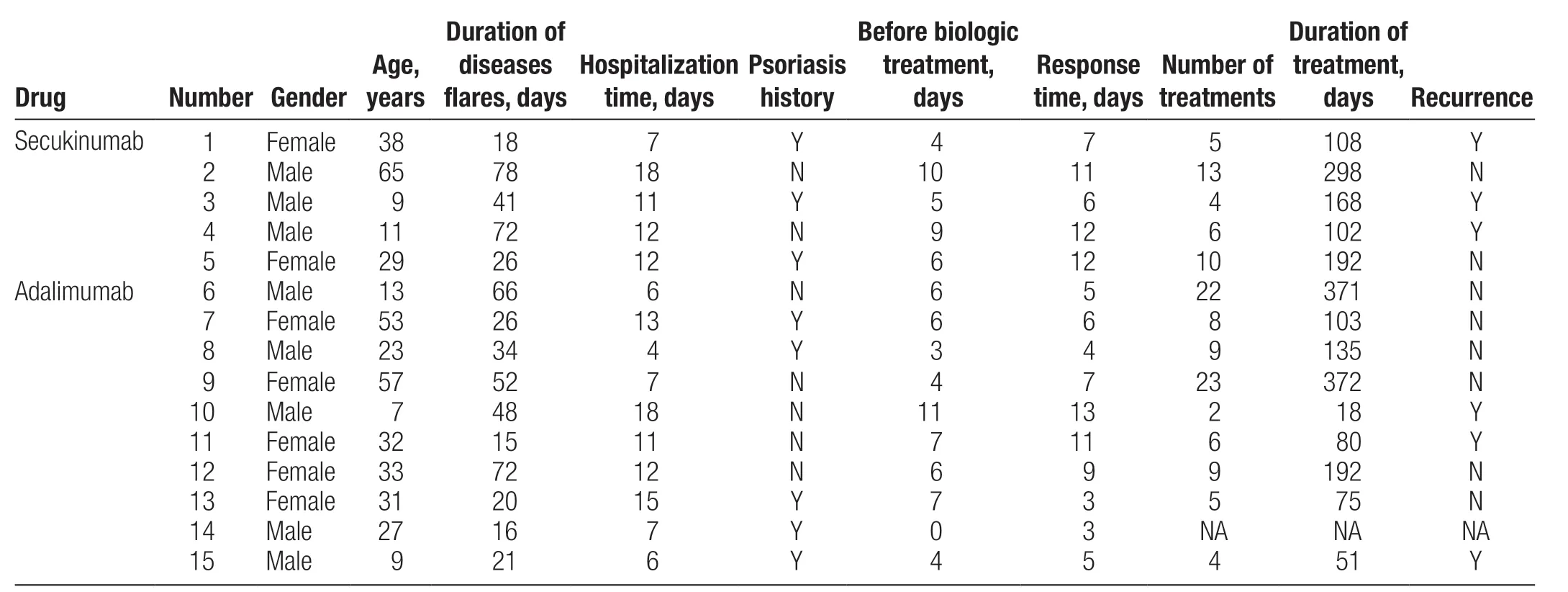
Table 3 Treatment details of GPP patients with poor response or unable to tolerate immunosuppressants.
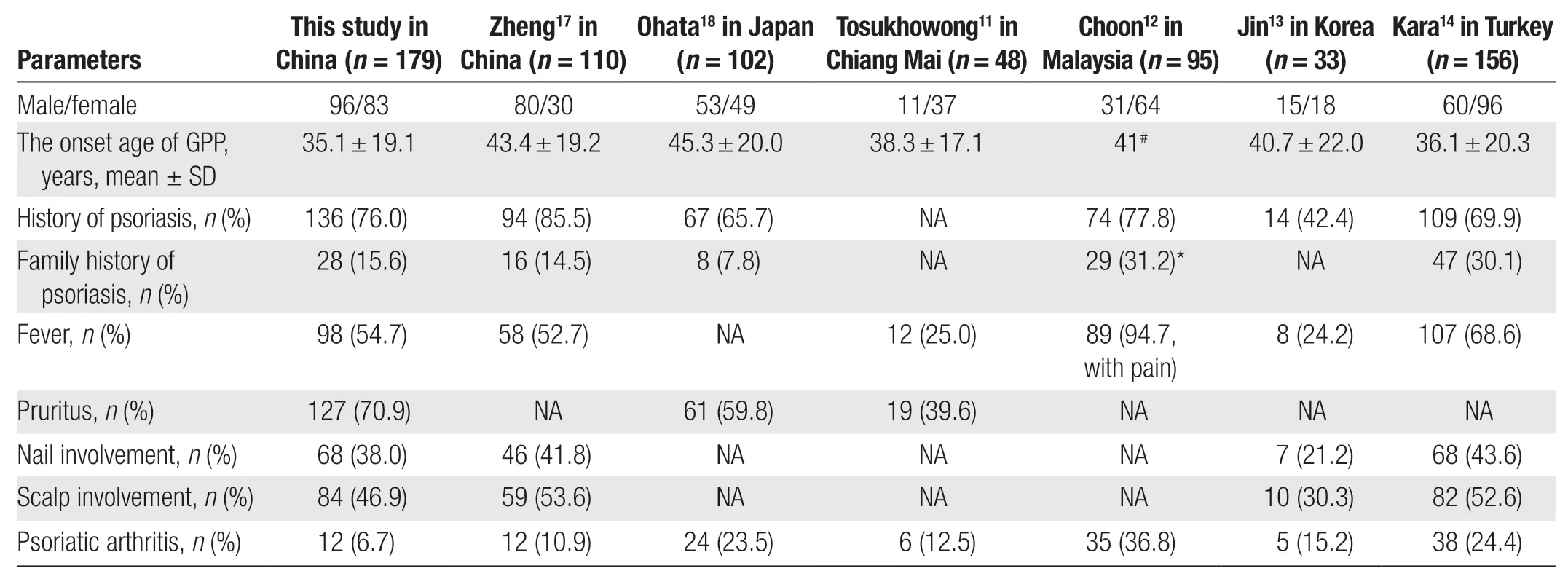
Table 4 Comparison of clinical features of the patients with GPP between current study and previous studies.
Suspected drug-induced GPP often needs to be differentiated from acute generalized eruptive pustulosis,which usually has an acute onset and improves with the discontinuation of allergenic drugs; patients do not have a history of psoriasis. The diagnosis is supported by histopathological findings such as vacuolar interface dermatitis, eosinophilia, and keratinocyte apoptosis.22-23
Fever is a common symptom of GPP, with 54.7% of patients developing a fever during the acute stage in our study, and 25.0%-68.6% in other studies11-12,14,17.
Pruritus is often ignored in GPP. Jaworeckaet al.24stated that 92.9% (n= 212) of patients with psoriasis and 100% (n= 11) of patients with GPP experienced pruritus. In our study,70.9% of patients experienced pruritus, which is higher than the proportion (59.8%) in the study by Ohataet al.18.Pruritus was more common in the GPP-PsVgroup (76.7%)than in the GPP+PsVgroup (69.1%) in our study, which is consistent with the results reported by Ohataet al.18.
In our study, 38.0% of patients had nail involvement,with more patients in the GPP+PsVgroup affected; this is consistent with the results reported by Zhenget al.17(41.8%) and Kara Polatet al.14(43.6%). In our study,46.9% of patients had scalp involvement, with more patients in the GPP+PsVgroup affected; this is consistent with the findings of Zhenget al.17(53.6%) and Kara Polatet al.14(52.6%) and higher than the rate reported by Jinet al.13(30.3%). These findings suggest that nail and scalp involvement may be more closely associated with PsV. The rate of tongue involvement in GPP was 4.5%, with more patients in the GPP-PsVgroup affected; this rate is lower than that in the study by Kara Polatet al.14(14.1%). In a previous study, the development of a geographic tongue appeared to be associated with mutations in theIL-36RNgene or an imbalance in the expression of IL-36Ra and IL-36γ in patients with GPP.25Unfortunately, the patients with tongue involvement in our study did not undergo genetic testing forIL-36RN.
Acitretin is the most common treatment regimen in patients with GPP. Acitretin had an efficacy rate of 84.7%in our study, which was higher than the 71.4% efficacy rate reported by Zhenget al.17In our study, the efficacy rate was 66.7% for methotrexate and 100% for cyclosporine. Fujitaet al.8showed that the efficacy rate of etretinate was 87.1%, that of methotrexate was 84.9%, and that of cyclosporine was 87.6%. Because fewer patients were enrolled in our study, the efficacy rates of methotrexate and cyclosporine require further verification.
In our study, the mean response time to treatment was 6.34 ± 2.91 days (median, 6 days). However, this was based on a combination of treatments, making it impossible to accurately assess the response time of a single drug. The mean duration of the treatment regimen was 111.35 ± 94.25 days (median, 81 days). Kromeret al.26reported a median drug survival of 14 months for systemic treatment with GPP, which was significantly longer than that in the present study; this may have been related to the duration of follow-up. Unfortunately, the adverse effects of the drug were not well documented. Excluding patients with no record of follow-up, approximately 46.6% (n= 131) of patients presented with recurrence. Recurrence appears to be common in patients with GPP. Zhuet al.27reported that of 61 patients with GPP treated with maintenance doses of acitretin (10–30 mg/d) for 2 to 4 years of follow-up, 46 had no or mild recurrence (0–2 times/y), 5 had moderate recurrence(3–4 times/y), and 10 had severe recurrence (5 times/y).
Fifteen patients treated with secukinumab or adalimumab showed good responses. A phase III study performed by Moritaet al.28showed that adalimumab was effective and well-tolerated in Japanese patients with GPP.Nine among 10 patients developed one or more adverse events (AEs), most commonly nasopharyngitis, pruritus,or hypoalbuminemia; 3 patients developed serious AEs(bacterial enterocolitis and worsening of pustular psoriasis, chronic sinusitis, dehydration, cardiac failure, and renal failure), Other AEs including congenital heart failure, worsening or new onset of psoriasis, liver failure, or other liver events; and 7 patients developed infections.28The efficacy of secukinumab in GPP has also been widely reported.29-30A recent real-world study of Chinese children with GPP showed that secukinumab had an excellent therapeutic effect and lasted for up to 48 weeks.31In the phase III study by Imafukuet al., all patients (n= 12)developed AEs, the most common of which were nasopharyngitis, urticaria, diabetes mellitus, and arthralgia;additionally; 3 patients developed serious AEs, including Bowen disease and cellulitis, drug-induced liver injury,upper gastrointestinal hemorrhage, hypoglycemia, and abnormal hepatic function.29A systematic evaluation showed that the mean duration of treatment with biologic therapy against TNF-α (n= 55) and IL-17 (n=32) was 9.3 and 12.7 months, with 58.1% (n= 55) and 66.7% (n= 32) achieving a complete response and 27.9%(n= 55) and 23.3% (n= 50) achieving a partial response,respectively.32Because of data limitations, we were unable to accurately assess the treatment effects in our patients.Among the 15 patients treated with biologics, 6 experienced different degrees of relapse, the reasons for which included colds, taking herbal preparations, irregular use of biologics, or short duration of use.
Biologics targeting IL-36R have emerged based on the role of IL-36 in the pathogenesis of GPP.33Spesolimab is an anti-IL-36R monoclonal antibody, and the results of a phase I trial showed that the pustules cleared quickly in seven patients who received a single dose of 10 mg/kg, but all had mild to moderate adverse effects.34The authors suggested that spesolimab was effective regardless ofIL-36RNmutation.34Phase II clinical trials showed that the treatment group had a higher pustule clearance rate within 1 week than the placebo group, but the infection rate was higher.35Baumet al.36analyzed the sequencing results of blood and tissue samples from patients with GPP before and after spesolimab treatment and found that 987 genes in diseased skin decreased to near nondisease levels after 1 week of treatment in association with congenital and Th1/Th17-mediated inflammation and proinflammatory processes of keratinocyte activation. Whole-blood RNA sequencing showed a significant reduction in proinflammatory mediators of neutrophil activation after treatment.36Imsidolimab is also a humanized monoclonal antibody targeting IL-36R, and clinical trials have shown good efficacy and safety.10,37
Previous studies have demonstrated the role of IL-36 in GPP. Targeting IL-36R to antagonize downstream inflammatory pathways has great prospects for treating GPP,but longer and larger trials are needed to verify its efficacy and safety.
Cases of exacerbation of psoriasis or GPP after COVID-19 vaccination have been reported.38-40In our study, a 38-year-old woman with an approximately 10-year history of psoriasis presented with erythematous pustules on the trunk and extremities 11 days after the second injection of the COVID-19 vaccine; this was accompanied by a burning sensation on the skin and mild pruritus, but no fever, arthralgia, or other discomforts. Blood tests showed elevated leukocytes (11.28 × 109/L), elevated neutrophils(8.66 × 109/L), an elevated C-reactive protein concentration (13.8 mg/L), and a normal erythrocyte sedimentation rate; the patient also had fatty liver, hypertension,and hyperlipidemia. After hospitalization, she was treated with nutritional support, anti-infection therapy, and topical glucocorticoids followed by subcutaneous injection of 300 mg of secukinumab. She improved with this therapy, and after discharge, she continued treatment with 4 additional doses of secukinumab (300 mg each time). She developed a relapse of psoriasis-like lesions after about 8 months. Her therapy was therefore changed to adalimumab, which was effective.
Clinical trials have shown that the expression levels of IL-2, IL-12, TNF-α, and interferon (IFN)-γ are increased in patients after COVID-19 vaccine vaccination.41In 1 study, IFN was significantly increased in patients with GPP, and this increase was correlated with abnormal IL-36 signaling.42Plasmacytoid dendritic cells are the primary source of IFN-γ. These results suggest that COVID-19 vaccination (or infection) may lead to IFN-I-mediated immune responses through stimulation of plasmacytoid dendritic cells.43
This study revealed the clinical characteristics and treatments of patients with GPP in a single center in Northwest China and compared the clinical characteristics between GPP with psoriasis and GPP alone. These findings can help clinicians to better understand the disease characteristics of GPP and more effectively manage patients. However, this study still has some limitations.As a retrospective study, there may be partial deviation in the data because of the recall of medical history and other data. Therefore, the study results should be generalized with caution. Additionally, the treatment response time and effectiveness were based on a combination of treatments, making it impossible to accurately assess a single drug’s specific efficacy. Because of data limitations,further evaluation of the patients’ prognosis is impossible. Patients with GPP+PsVand GPP-PsVshow significant differences regarding age at onset, family history of psoriasis, predisposing factors, nail involvement, andresponse to treatment. Some differences in the clinical features of GPP are influenced by geography, race, and geneticbackground. Acitretin has good therapeutic efficacy, but recurrence should be noted. Biologics are increasingly becoming effective treatments, but their superiority and safety need further research.
Acknowledgments
The authors express their gratitude to the patients and the hospital involved in this study.
Source of funding
This study was supported by the Shaanxi Province Key R&D Program (No. S2019-YF-GXYB-0170).
- 国际皮肤性病学杂志的其它文章
- Efficacy and Safety of lxekizumab in Chinese Patients With Moderate-to-Severe Plaque Psoriasis: 60-Week Results From a Phase 3 Study
- Understanding the Pathogenesis of Generalized Pustular Psoriasis Based on Molecular Genetics and lmmunopathology
- Perspective on Melanoma in the Arab World:A Quantitative and Qualitative PubMed-Based Analysis of Research Output (2004–2019)
- Laboratory Safety of Dupilumab, and lts Effect on lnflammatory Biomarkers, in Chinese Adults With Moderate-to-Severe Atopic Dermatitis: An Analysis of a Randomized, Double-Blind Phase lll Study
- Sexual Behavior and Awareness of Sexually Transmitted Diseases Among Street-Based Female Sex Workers in the Florence Area, Central Italy
- Perceptions of Acne and Its Treatments Among Chinese College Students: A Cross-Sectional Survey

