In situ growth of cobalt on ultrathin Ti3C2Tx as an efficient cocatalyst of g-C3N4 for enhanced photocatalytic CO2 reduction
Tongming Su, Jundong Meng, Ya Xiao, Liuyun Chen, Hongbing Ji,2, Zuzeng Qin
1 School of Chemistry and Chemical Engineering, Guangxi Key Laboratory of Petrochemical Resource Processing and Process Intensification Technology, Guangxi University,Nanning 530004, China
2 Fine Chemical Industry Research Institute, School of Chemistry, Sun Yat-sen University, Guangzhou 510275, China
Keywords:g-C3N4 MXene Cobalt Photocatalytic CO2 reduction
ABSTRACT Photocatalytic CO2 reduction to valuable product exhibit promising prospect for solving the energy crisis and the greenhouse effect.Herein, Co-Ti3C2Tx/g-C3N4 (Co-TC/CN) composite with enhanced photocatalytic performance for converting CO2 to CO and CH4 was constructed by electrostatic self-assembly method.The close contact interface between Co-Ti3C2Tx and g-C3N4 nanosheets can be used as fast transport channels of photogenerated electrons and effectively promote the separation of photogenerated electrons and holes, and the interface between the Co and Ti3C2Tx might be the active sites for CO2 adsorption and activation.The optimized Co-Ti3C2Tx/g-C3N4 composite exhibited the highest photocatalytic performance with the CO and CH4 production of 55.04 μmol·g-1 and 2.29 μmol·g-1, respectively,which were 7.5 times and 5.8 times than those of g-C3N4.Furthermore, the stability of g-C3N4 was improved after coupling with Co-Ti3C2Tx.
1.Introduction
With the development of modern society, the rapid growth of population and the continuous improvement of industrialization have brought about a series of problems.Among them,the energy crisis and the greenhouse effect attract the most attention.It is a potential strategy to solve these problems by using inexhaustible solar energy to drive the photocatalytic CO2reduction into CO,CH4,CH3OH,C2H4,and other high value-added product[1-6].Since the discovery of semiconductor materials that can convert solar energy into chemical energy [7,8], various kinds of catalysts have emerged.Among them, g-C3N4has attracted wide attention due to its low cost, non-toxicity, high physicochemical stability, and appropriate band gap [9-11].However, the photocatalytic performance of g-C3N4is adversely affected by the rapid recombination of photogenerated electrons and holes and the lack of sufficient active sites for CO2adsorption and activation.Therefore, the photocatalytic performance of g-C3N4has been improved by various strategies in recent years,such as,the regulation of surface defects[12-14], metal doping [15], heterojunction construction [16,17],nonmetallic doping [18,19], morphology regulation [20], functional group modification [21], co-catalyst modification [22,23],etc.Among them,the modification of g-C3N4with cocatalyst is considered as an effective means to improve the separation efficiency of photogenerated charge carriers and increase the active site of photocatalyst [24].In this way, it is important to select a suitable cocatalyst to improve the catalytic activity of g-C3N4.
Compared with other cocatalysts, MXenes, as a new 2D material, has attracted wide attention due to its advantages of good electrical conductivity, a large number of catalytic active sites,adjustable band gap, etc.[25-28].MXenes usually obtained by etching the A element out of the MAX phase(M,A,and X are early transition metals, main group IIIA or IVA elements, and carbon and/or nitrogen atoms, respectively), which have layered structures and surface termination groups (—O, —F, —OH) [29].Many studies have found that MXenes can be used as cocatalyst of g-C3N4, Bi2WO6, ZnIn2S4, and other semiconductor materials for enhancing their photocatalytic performance [30-32].
Among the family of MXenes, Ti3C2TxMXene have abundant terminal functional groups and good metal conductivity, which guarantees the rapid transfer of charge carriers and provides active sites for photocatalytic reaction.For example, when Ti3C2Txwas used as the cocatalyst of g-C3N4, a close contact interface was formed between the two-dimensional Ti3C2Txand g-C3N4, which promote the transfer of photogenerated charge carriers and prevent the recombination of photogenerated electrons and holes[33].In addition, g-C3N4/Ti3C2Txcatalyst was also prepared by Li et al.[34] and used for photocatalytic CO2reduction, and the CH4yield reached 2.117 μmol·g-1·h-1, which is 2.4 times higher than that of g-C3N4.Song et al.[35] constructed the Ti3C2Tx/g-C3N4Schottky junctions through electrostatic self-assembly strategy.In this system,the electrons were transferred from g-C3N4to Ti3C2-Tx, and the optimized catalyst of 20% Ti3C2Tx/Vc-CN showed excellent CO production of 20.54 μmol·g-1·h-1.In summary, the above studies prove that Ti3C2Txcan be used as an efficient cocatalyst for improving the photocatalytic performance of g-C3N4.However,the photocatalytic efficiency of CO2reduction over g-C3N4still low and need to be further improved with a more efficient cocatalyst.
According to the previous studies, the presence of metal nanoparticles on the catalyst surface can not only improve the electron transfer efficiency, but also enhance the photocatalytic reduction ability [36-39].For example, large Au nanoparticles loaded on g-C3N4and BiOBr can act as the bridge and the active sites for photocatalytic reactions, while small Au nanoparticles exhibited surface plasmon resonance effects[40].In general,abundant defects can be generated on the surface of MXene,which can be used as the substrate for immobilizing the metal active sites[41,42].For example,Min et al.[43]found that Ti3C2Txnanosheets can be act as a good support for Pt nanoparticles.In this system,the electrons can quickly transfer from Ti3C2Txnanosheets to the Pt nanoparticles due to the high work function (5.65 eV) of Pt,which is beneficial for enhancing the photocatalytic performance.Therefore, the combination of MXene metal-based cocatalysts and photocatalysts can be regarded as a promising strategy for enhancing the photocatalytic performance.
In this work,the Co-Ti3C2Tx/g-C3N4photocatalyst was prepared by electrostatic self-assembly strategy.The photocatalytic performance of the Co-Ti3C2Tx/g-C3N4photocatalyst was tested by photocatalytic CO2reduction under visible light irradiation.In addition, the microstructure and photochemical properties of the Co-Ti3C2Tx/g-C3N4composite photocatalyst was investigated by various characterization.Moreover, the photocatalytic CO2reduction mechanism over Co-Ti3C2Tx/g-C3N4photocatalyst was proposed.
2.Experimental
2.1.Materials
Ti3AlC2(38 μm,≥99%)was purchased from Foshan XinXi Technology Co., Ltd (China).Hydrofluoric acid (HF, 40% (mass)) was purchased from Shanghai Macklin Co., Ltd (China).Cobaltous nitrate hexahydrate, dicyandiamide, lithium fluoride, and Nafion solution were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd (China).Cobaltous nitrate hexahydrate was purchased from Jinshan Chemical Reagent Co., Ltd (China).Tetraethoxysilane, ammonia aqueous solution, sodium sulphate,ammonium bicarbonate, and anhydrous ethanol were purchased from Xilong Scientific Co., Ltd (China).Potassium borohydride was purchased from Cologne Chemicals Co., Ltd (China).Hydrochloric acid was purchased from Kelong Chemical Co., Ltd(China).Deionized (DI) water was used in all experiments.
2.2.Synthesis of protonated g-C3N4 nanosheets
The g-C3N4nanosheets were fabricated by thermal polymerization of dicyandiamide with SiO2spheres as the template according to our previous report[32].The preparation method of SiO2sphere can be found in the Supporting Information.For the synthesis of g-C3N4nanosheets,3.0 g SiO2microspheres and 6.0 g dicyandiamide were added to 60 mL distilled water.After that,the suspension was stirred and kept at 30 °C for 2 h, and then the temperature was raised to 60 °C until the water was completely evaporated to obtain the white solid.Whereafter, the white solid was calcined at 550 °C for 4 h with a heating rate of 2 °C·min-1to obtain the g-C3N4/SiO2mixture.Subsequently, the g-C3N4/SiO2mixture was immersed in 18 mL HF (40%) for 12 h to remove SiO2, and then the g-C3N4was washed with DI water and ethanol several times until the pH equal to 6.Then, the g-C3N4was dried at 60 °C for 12 h to obtain the g-C3N4nanosheets.To prepare the protonated g-C3N4,0.2 g g-C3N4was dispersed in 50 mL 0.5 mol·L-1HCl aqueous solution and treated by ultrasonic for 0.5 h.Whereafter, the protonated g-C3N4was collected and washed with DI water to remove HCl until the pH equal to 4, and then the protonated g-C3N4was dried by vacuum freeze drying.
2.3.Synthesis of Co-Ti3C2Tx nanosheets
The Co-Ti3C2Txnanosheets were prepared by chemical reduction of cobalt (Co) on the surface of Ti3C2Txwith NaBH4as the reductant.The preparation method of Ti3C2Txnanosheets was described in the Supporting Information.To synthesize the Co-Ti3C2Txnanosheets, 50 mg Ti3C2Txnanosheets was dispersed in 50 mL DI water and sonicated under Ar atmosphere for 10 min.After that, 0.5 mL Co (NO3)2·6H2O solution (the concentration Co2+of was 0.5 mg·mL-1)was slowly added to the Ti3C2Txsuspension, and then 1 mL 1.0 mol·L-1NH3·H2O solution was added and magnetically stirred for 10 min.Subsequently, 6 mg KBH4was slowly added to the above mixed solution and magnetically stirred for 1 h at 300 r·min-1.Finally,the Co-Ti3C2Txpowder with Co content of 0.5%(mass)was obtained by washing with DI water,filtering and vacuum freeze-drying.By changing the amount of Co(NO3)2·6H2O solution, Co-Ti3C2Txwith different Co contents (0.5%(mass), 1% (mass), 2% (mass), 3% (mass)) was synthesized, and was labeled as yCo-Ti3C2Tx(y = 0.5, 1, 2, 3).
2.4.Synthesis of Co-Ti3C2Tx/g-C3N4 composite
The Co-Ti3C2Tx/g-C3N4(Co-TC/CN) composite was prepared via the electrostatic self-assembly strategy.First, 6 mg Co-Ti3C2Txwas dispersed in 10 mL DI water by ultrasonic for 1 h at 5 °C to form the Co-Ti3C2Txsuspension.And the protonated g-C3N4was dispersed into 50 mL DI water to obtain the g-C3N4suspension.Subsequently, the Co-Ti3C2Txsuspension was added to the g-C3N4suspension dropwise and stirred for 0.5 h at 300 r·min-1.After that,the suspension was stood for 1 h,then the precipitate was collected by centrifugation and dried at 60 °C for 12 h.By changing the Co-Ti3C2Txwith different Co content (0.5% (mass), 1% (mass),2% (mass), 3% (mass)), the 0.5Co-Ti3C2Tx/g-C3N4, 1Co-Ti3C2Tx/g-C3N4,2Co-Ti3C2Tx/g-C3N4,and 3Co-Ti3C2Tx/g-C3N4composites with different Co content were obtained,and was labeled as yCo-TC/CN(y=0.5,1,2,3).For comparison,the Ti3C2Tx/g-C3N4composite was prepared, and the preparation method was described in the Supplementary Information.
2.5.Photocatalytic CO2 reduction
Photocatalytic reduction of CO2was carried out in a sealed quartz reactor (Beijing China Education Au-light Co., Ltd.).A 300 W xenon lamp (CEL-HXF300, Beijing China Education Aulight Co., Ltd.) with a 400 nm cut-off filter was used as the light source, and the light intensity was measured to be 200 mW·cm-2by an optical power meter (CENP2000, Beijing China Education Au-light Co., Ltd.).The spectrum of the 300 W xenon lamp with or without the 400 nm cut-off filter was measured by a fiber optic spectrometer (AULTT-P4000, Beijing China Education Au-light Co.,Ltd.) and was shown in Fig.S1 (see Supplementary Material).The temperature of the reactor was controlled at 25 °C by circulating cooling water, and the photocatalytic CO2reduction system was shown in Fig.S2.In detail,40 mg photocatalyst and 2 mL DI water were evenly dispersed at the bottom of the reactor and dried at 60 °C for 8 h.After that, moist CO2gas was fed into the reactor for 30 min at a rate of 40 mL·min-1before light irradiation.The gaseous product was quantitatively analyzed every hour using a gas chromatograph(SHIMADZU GC-2030)equipped with a barrier discharge ionization detector.For the cyclic experiment,the photocatalyst was dried under vacuum for 8 h after each cyclic reaction,and then moist CO2was introduced into the reactor for 30 min,other conditions remained unchanged.
2.6.Catalyst characterization
The characterization methods and photoelectrochemical measurements are supplemented in the Supporting Information.
3.Results and Discussion
The Co-TC/CN was successfully prepared by electrostatic selfassembly strategy (Fig.1).Initially, the Al layer of Ti3AlC2was etched by HCl/LiF solution to form the multilayer Ti3C2Tx.The few-layer Ti3C2Txnanosheets were prepared by exfoliating the multilayer Ti3C2Txwith ultrasonic treatment.The surface of the few-layer Ti3C2Txnanosheets was determined to be negative charge with a Zeta potential of -38.9 mV (Table S1).In addition,the Co-Ti3C2Txwas synthesized by chemical reduced of Co on the surface of the Ti3C2Txwith NaBH4as the reductant.Notably, the surface of the Co-Ti3C2Txnanosheets with different Co contents(0.5% (mass), 1% (mass), 2% (mass), and 3% (mass)) still exhibited negative charge with the zeta potential of -38.8, -35.7, -34.7,and -33.0 mV, respectively.Besides, g-C3N4nanosheets were synthesized by thermal condensation of dicyandiamide with SiO2sphere as the template.After protonation in HCl solution for 30 min, the surface of the g-C3N4was positively charged with a zeta potential of 29.2 mV.Finally, the Co-TC/CN composites were synthesized by electrostatic self-assembly strategy.

Fig.1.Schematic illustration of the synthesis of Co-Ti3C2Tx/g-C3N4 composite.
The crystal structure and composition of the photocatalysts were analyzed by XRD and FT-IR spectra.As shown in the XRD patterns of Ti3AlC2and Ti3C2Tx(Fig.S3),after Ti3AlC2is etched by HCl/LiF solution,the main diffraction peak(1 0 4)of Ti3AlC2was disappeared, indicating the successful removal of Al layer and the successful preparation of Ti3C2Tx[44,45].Moreover, the diffraction peak of the Ti3C2Tx(0 0 2)plane can be clearly observed at 6.2°,further demonstrating the successful synthesis of Ti3C2Tx.From the XRD patterns of Ti3C2Txand yCo-Ti3C2Tx(Fig.2(a)), the diffraction peaks of yCo-Ti3C2Txare similar to those of Ti3C2Tx,indicating that the presence of Co on the Ti3C2Txdid not change the crystal structure of Ti3C2Tx.Notably,a weak Co diffraction peak located at 44.6°can be observed in the XRD pattern of yCo-Ti3C2Tx, which corresponding to the (1 1 1) plane of Co [46].These results indicated that the Co was successfully loaded on the surface of Ti3C2Txto obtain the yCo-Ti3C2Txcocatalyst.
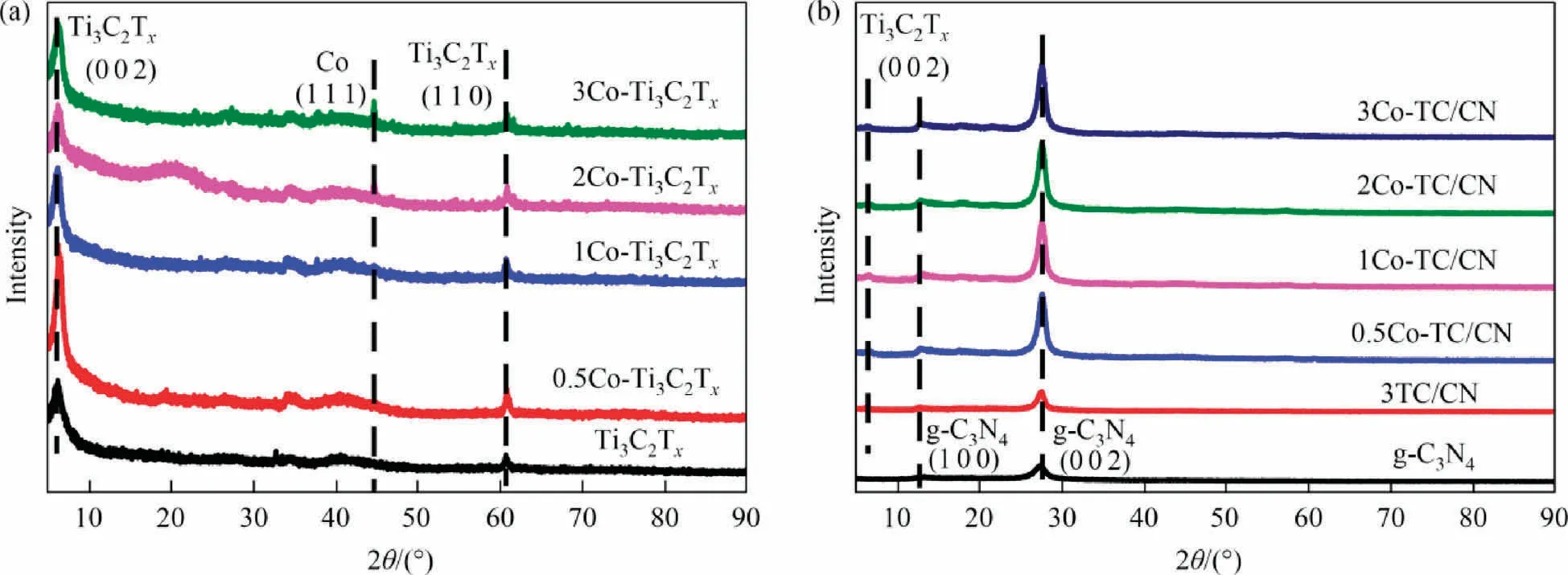
Fig.2.XRD patterns of Ti3C2Tx, yCo-Ti3C2Tx (a), g-C3N4, and yCo-TC/CN (b).
The XRD patterns of the g-C3N4, 3TC/CN, and yCo-TC/CN samples are shown in Fig.2(b).The XRD pattern of the yCo-TC/CN sample shows two characteristic diffraction peaks at 13.0° and 27.7°,corresponding to the (1 0 0) and (0 0 2) crystal planes of g-C3N4,respectively [47].It shows that the presence of Co-Ti3C2Txexhibited no obvious effect on the structure of g-C3N4.In addition, due to the low content of yCo-Ti3C2Txin the yCo-TC/CN composites,only a weak diffraction peak of the Co-Ti3C2Tx(0 0 2)crystal plane was observed in the XRD pattern of the yCo-TC/CN composites.These results indicate that Co-Ti3C2Txwas successfully combined with g-C3N4.In order to determine the Co content in Co-Ti3C2Tx,EDS was used to measure the Co content in the yCo-Ti3C2Txcocatalyst(Table S2),and the mass fraction of Co in 0.5Co-Ti3C2Tx,1Co-Ti3C2Tx, 2Co-Ti3C2Tx, and 3Co-Ti3C2Txwas measured to be 0.40%,0.88%,1.79%,and 2.84%,respectively,which was close to their theoretical value.
The structure and surface functional groups of g-C3N4, 1Co-Ti3C2Tx, and yCo-TC/CN were studied by FT-IR spectra.As shown in Fig.S4, in the FT-IR spectra of yCo-TC/CN composites, the peaks at 810 cm-1,1207-1683 cm-1,and 3077-3300 cm-1correspond to the breathing mode of the heptazine or s-triazine ring, and the elongation or bending vibration of the aromatic carbon and nitrogen heterocyclic ring, and the stretching vibrations of N—H bond and O—H stretching,respectively[48-50].These results show that the structure of g-C3N4is stable after removing SiO2or after coupling with yCo-Ti3C2Tx.
The specific surface area and pore size distribution of g-C3N4,Ti3C2Tx, yCo-Ti3C2Tx, 3TC/CN, and yCo-TC/CN were determined by N2adsorption-desorption method.As shown in Fig.S5 and Table S3, g-C3N4and Ti3C2Txdisplay relatively high surface areas of 48.41 m2·g-1and 34.29 m2·g-1, respectively, which was due to their ultra-thin two-dimensional nanosheet structure.After the addition of Co, the specific surface area of yCo-Ti3C2Txdecreased due to the accumulation of yCo-Ti3C2Txnanosheets.However, after coupling with g-C3N4, the specific surface area of yCo-TC/CN increased compared to that of yCo-Ti3C2Tx, which might benefit from the high specific surface area of g-C3N4nanosheets.Although the addition of Co makes the yCo-TC/CN composite aggregate to a certain extent, from the perspective of structure, it can still maintain the nanosheet structure, which can provide abundant active sites for photocatalytic reaction.
The morphology and microstructure of SiO2, g-C3N4, Ti3C2Tx,yCo-Ti3C2Tx, and yCo-TC/CN composites were investigated by scanning electron microscopy (SEM).As shown in Fig.S6(A), the SiO2shows the nanosphere morphology.Therefore, with the SiO2as the template, the g-C3N4exhibited porous nanosheet morphology(Fig.3(a)).Notably, without the SiO2as the template, the g-C3N4shows the bulk morphology (Fig.S6(B)).It can be seen from Fig.S6(C) that the Ti3AlC2exhibits the lamellar structure, and after etching with LiF/HCl solution, few-layer Ti3C2Txwas obtained(Fig.S6(D)).Notably, the stacking of the Ti3C2Txnanosheets was restrained (Fig.3(b)) when NH4HCO3was used in the collection process of Ti3C2Tx(Fig.3(b)).However,as shown in the SEM images of yCo-Ti3C2Tx(Fig.3(c),(d),(e),and(f)),the Co-Ti3C2Txnanosheets stacked together when the Co content is 0.5% (mass) (Fig.3(c)),which lead to the decrease in the specific surface area.With the Co content increasing from 0.5% (mass) to 1% (mass), 1Co-Ti3C2Txstill maintains the nanosheet structure, and the aggregation of 1Co-Ti3C2Txis relieved to a certain extent (Fig.3(d)).Further increasing the Co content to 2% (mass) and 3% (mass), the nanosheet structure of Co-Ti3C2Txremained unchanged (Fig.3(e)and (f)).However, the aggregation of Co-Ti3C2Txnanosheet increased compared to that of 1Co-Ti3C2Tx.The above results show that the Co-Ti3C2Txnanosheets aggregated to a certain extent after loading with Co on the surface of Ti3C2Tx, but the ultrathin nanosheet structure can still be maintained, which is conducive to exposing more reactive sites for photocatalytic reaction.

Fig.3.SEM images of g-C3N4 nanosheets (a), few-layer Ti3C2Tx (b), 0.5Co-Ti3C2Tx (c), 1Co-Ti3C2Tx (d), 2Co-Ti3C2Tx (e), and 3Co-Ti3C2Tx (f).
The morphology of 1Co-Ti3C2Txnanosheets was further investigated by transmission electron microscopy (TEM).As shown in Fig.4(b),the ultrathin nanosheet structure can be clearly observed from the TEM image of 1Co-Ti3C2Tx.In addition, from the highresolution transmission electron microscopy (HRTEM) images of 1Co-Ti3C2Txnanosheets (Fig.4(a), (c)), a lattice fringe spacing of 0.26 nm corresponding to (1 1 0) crystal plane of Ti3C2Txcan be obviously observed [51], and the lattice fringe spacing of 0.2 nm can be ascribed to the (1 1 1) crystal plane of Co [52].Moreover,the corresponding EDS elemental (Ti, C, Co) mapping of 1Co-Ti3C2Tx(Fig.4(d), (e), (f), and (g)) indicated that the Co element are uniformly and highly dispersed on the surface of Ti3C2Tx.The above results further demonstrated the successful construction of the Co-Ti3C2Tx.
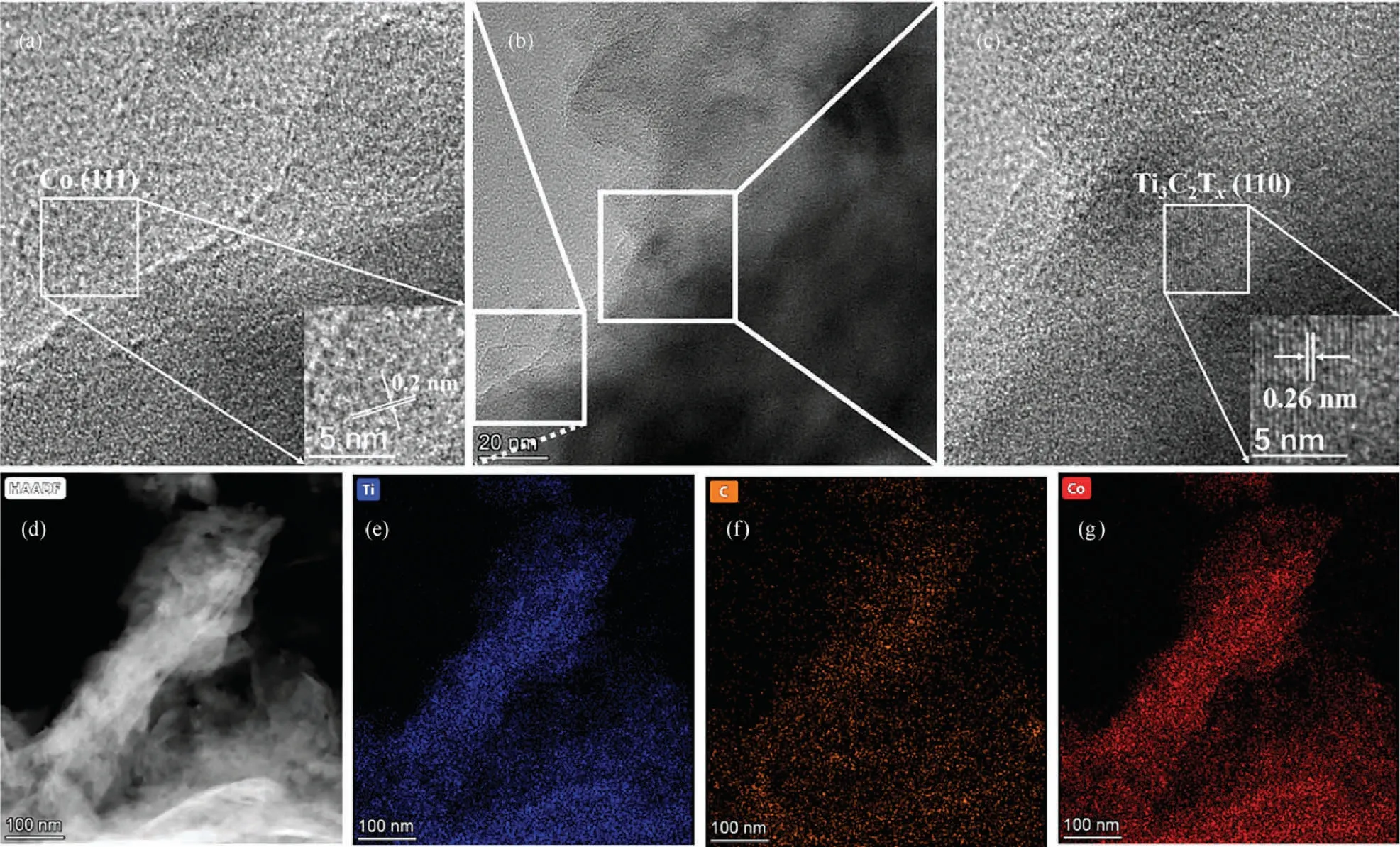
Fig.4.TEM and HRTEM images of 1Co-Ti3C2Tx ((a), (b), (c)), HAADF-TEM image (d) and the corresponding EDS elemental (Ti, C, Co) mappings of 1Co-Ti3C2Tx ((e), (f), (g)).
The morphology of the yCo-TC/CN sample was investigated by SEM.As shown in Fig.S7, the aggregation of nanosheets can be observed in the SEM images of the yCo-TC/CN sample.Due to the surface functional groups of yCo-Ti3C2Txwas dominated by O- and F-species, the surface of yCo-Ti3C2Txnanosheets was negatively charged, which was verified by the zeta potential of 0.5Co-Ti3C2Tx(-38.8 mV), 1Co-Ti3C2Tx(-35.7 mV), 2Co-Ti3C2Tx(-34.7 mV), and 3Co-Ti3C2Tx(-33.0 mV) (Table S1).In addition,the zeta potential of protonated g-C3N4was measured to be 29.2 mV, indicating that the surface of g-C3N4was positively charge.Therefore, when the yCo-Ti3C2Txwas in contact with g-C3N4, 2D/2D close contact interface can be formed between the Co-Ti3C2Txand g-C3N4,which result in the coupling of nanosheets.Notably, the formation of the 2D/2D Co-Ti3C2Tx/g-C3N4interface will effectively prevent the recombination of photogenerated electrons and holes,and thus promote the photocatalytic performance of the catalyst.
The morphology and microstructure of 1Co-TC/CN were further studied by TEM and HRTEM.From the TEM images of 1Co-TC/CN(Fig.5(a),(b),(c),(d)),it can be observed that the 1Co-TC/CN composite exhibited nanosheet shape, and the 1Co-Ti3C2Txnanosheet was closely contacted with g-C3N4nanosheet.In addition, as shown in Fig.5(d), a close contact interface was formed between the Co-Ti3C2Txand g-C3N4, and the lattice fringe spacing of 0.26 nm was attributed to the (1 1 0) plane of Ti3C2Tx[51], while the amorphous structure was ascribed to g-C3N4(Fig.5(e)).In addition, the lattice fringe spacing of 0.2 nm corresponding to (1 1 1)plane of Co can also be found in Fig.5(e) [52].It is worth noting that the Co clusters can exist not only on the surface of Ti3C2Tx,but also between Ti3C2Txand g-C3N4in the 1Co-TC/CN sample.The Co cluster on the surface of Ti3C2Txare expected to serve as active sites for CO2adsorption and activation, while the Co cluster between Ti3C2Txand g-C3N4can be acted as the electron transfer channel at the 2D/2D Ti3C2Tx/g-C3N4interface.Besides, the EDS elemental (Co, C, Ti, N) mapping of 1Co-TC/CN (Fig.5(g), (h), (i),(j)) shows that the Co-Ti3C2Txis evenly distributed in the 1Co-TC/CN composite.

Fig.5.TEM images((a),(b),(c),(d)),enlarged HRTEM images of the square area at D(e),HAADF-STEM image(f)and the corresponding EDS elemental(Co,C,Ti,N)mapping of 1Co-TC/CN ((g), (h), (i), (j)).
The chemical state of 1Co-Ti3C2Txand 1Co-TC/CN was investigated by X-ray photoelectron spectroscopy (XPS).Notably, the Ti3-C2Tx, g-C3N4, and 3TC/CN sample used in this work is the same batch as that of the Ti3C2Tx, g-C3N4, and 3TC/CN used in our previous work [32].Therefore, the XPS of these samples was not retested in this work, and the XPS data of the Ti3C2Tx, g-C3N4,and 3TC/CN samples in our previous work was used for comparison.As shown in the XPS survey spectra of these samples (Fig.S8(A)), Ti, C, O, and F elements can be observed in 1Co-Ti3C2Tx, however, no peak of Co can be found in the XPS survey spectra due to its low content.The peak of O and F element might be due to the presence of —OH and —F functional groups on the Ti3C2Txsurface.Notably, Ti, C, and N element can be seen in the XPS spectra of 1Co-TC/CN composites, indicating that Co-Ti3C2Txare successfully combined with g-C3N4.However, due to the low content of Co, Co element was not detected in XPS spectra of the 1Co-TC/CN composite.
In the high-resolution XPS spectra of C 1s in 1Co-TC/CN composite (Fig.6(a)), the binding energy located at 288.3 eV and 286.7 eV was ascribed to the C 1s of N—C=N and C—N in 1Co-TC/CN composite, respectively, which was higher than that of N—C = N (288.2 eV) and C—N (286.6 eV) in g-C3N4[32].Due to the Fermi level of g-C3N4is higher than that of Co-Ti3C2Tx, the electron can transfer from g-C3N4to Co-Ti3C2Txafter Co-Ti3C2Txwas combined with g-C3N4, which accounts for the increased binding energy of C 1s in g-C3N4[33].In addition, according to the highresolution XPS spectra of C 1s in 1Co-Ti3C2Tx(Fig.S8(B)), four characteristic peaks can be observed in 1Co-Ti3C2Tx.Notably, it was found that the binding energy corresponding to the C 1s of C—Ti(281.3 eV), C—C (284.8 eV), C—O (286.9 eV), and O—C = O(288.9 eV) in 1Co-Ti3C2Txwas lower than that of C 1s (C—Ti(282.1 eV), C—C (285.1 eV), C—O (287.2 eV), and O—C = O(289.1 eV)) in Ti3C2Tx[32], indicating that there was a strong interaction between Co and Ti3C2Tx, which resulted in the change of binding energy of C 1s.
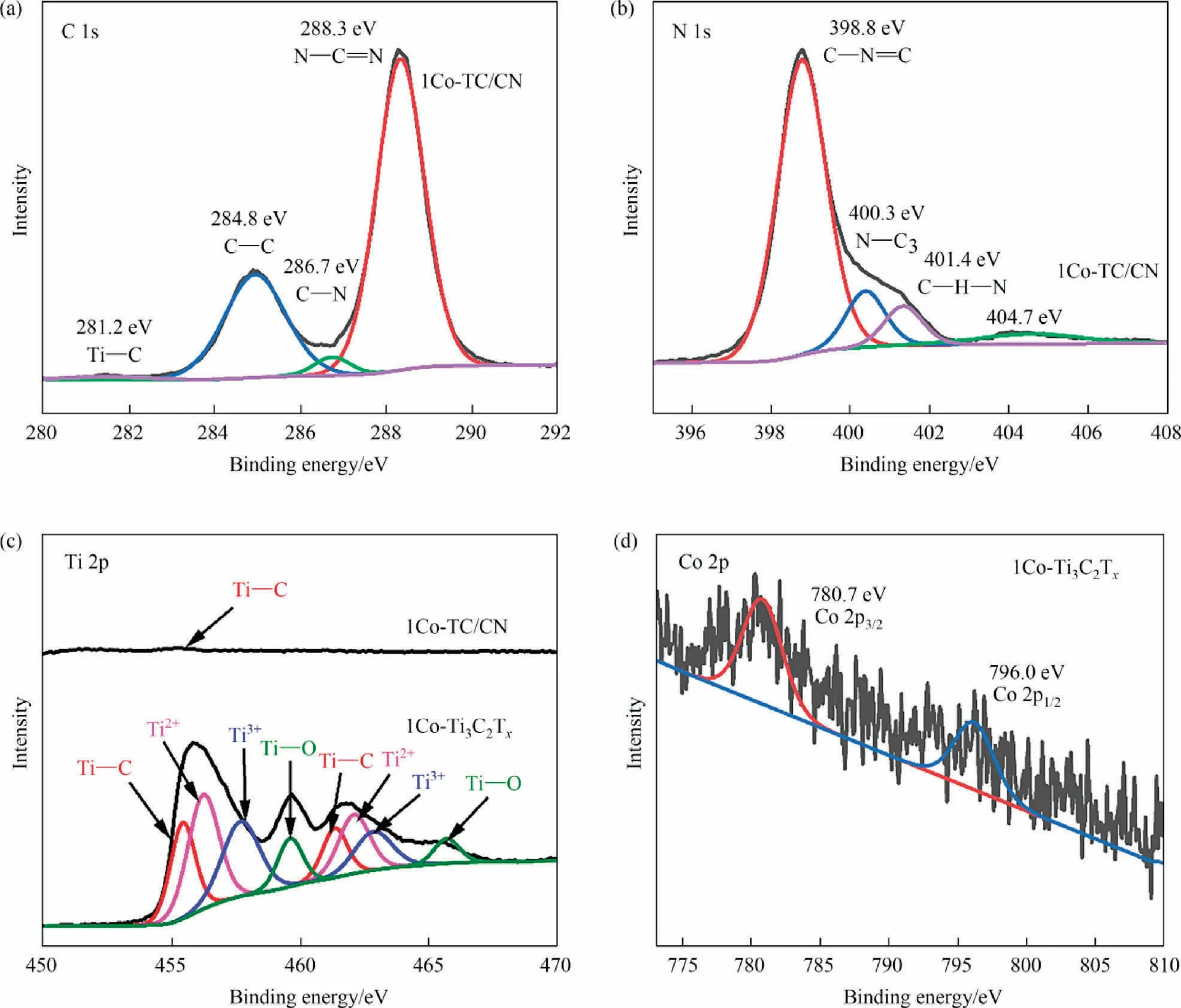
Fig.6.The high resolution XPS spectra of C 1s (a), N 1s (b) in 1Co-TC/CN, Ti 2p in 1Co-Ti3C2Tx and 1Co-TC/CN (c), and Co 2p of 1Co-Ti3C2Tx (d).
As shown in the high-resolution XPS spectra of N 1s in 1Co-TC/CN (Fig.6(b)), the binding energy corresponding to N 1s of N—C = N(398.8 eV), N—C3(400.3 eV), and C—N—H (401.4 eV) in 1Co-TC/CN increased compared with that of N—C = N (398.3 eV), N—C3(399.8 eV) and C—N—H (400.9 eV) in g-C3N4[32].In addition,according to the high-resolution XPS spectra of Ti 2p in the 1Co-Ti3C2Txand 1Co-TC/CN sample(Fig.6(c)),the binding energy corresponding to Ti 2p of Ti—C (455.4 eV, 461.3 eV), Ti2+(456.2 eV,462.2 eV), Ti3+(457.7 eV, 463.3 eV), and Ti—O (459.7 eV,465.8 eV) in 1Co-Ti3C2Txis higher than that of Ti—C (455.3 eV,461.0 eV), Ti2+(456.1 eV, 461.9 eV), Ti3+(457.3 eV, 463.1 eV) and Ti—O (459.2 eV, 465.3 eV) in Ti3C2Tx[32].These results further confirmed that the electrons are transferred from g-C3N4to Co-Ti3C2Txin the 1Co-TC/CN composite[53].However,due to the content of Co-Ti3C2Txis 3%(mass)in 1Co-TC/CN,only a weak Ti 2p signal can be observed in the XPS spectra of 1Co-TC/CN.In the highresolution XPS spectra of Co 2p in 1Co-Ti3C2Tx(Fig.6(d)),the binding energy at 780.7 eV and 796.0 eV are attributed to the Co3+2P3/2and Co3+2P1/2orbitals of Co,respectively[52],confirming the successful loading of Co on the surface of Ti3C2Tx.However,due to the low Co content in 1Co-TC/CN,no obvious Co signal can be observed in the XPS spectra of the 1Co-TC/CN composite.These results indicate that the 1Co-TC/CN composite was successfully synthesized,and electrons can be transferred from g-C3N4to Co-Ti3C2Tx.
The optical absorption property of g-C3N4, Co-Ti3C2Tx, and yCo-TC/CN composite were investigated by UV-visible diffuse reflection spectroscopy.As shown in Fig.7(a), g-C3N4exhibited visible light absorption, while the 1Co-Ti3C2Txshowed strong absorption in the wavelength range of 300 nm-800 nm due to its dark color.In addition, according to the relationship between (F (R∞) hv)1/2and photon energy calculated by the Kubelka-Munk function(Fig.S9),the band gap of g-C3N4was determined to be 2.8 eV,indicating that g-C3N4has a suitable band gap for photocatalytic reaction under visible light.When g-C3N4was coupled with Ti3C2Txor Co-Ti3C2Tx, the optical absorption property of the yCo-TC/CN and 3TC/CN composite were greatly improved compared with that of g-C3N4due to the strong optical absorption of Co-Ti3C2Txand Ti3-C2Tx.It is worth noting that with the increase of Co content from 0.5% (mass) to 3% (mass), the light absorption intensity of the yCo-TC/CN composite decreased gradually, which might be due to the lighter color of Co-Ti3C2Txafter more metal Co was loaded on the surface of Ti3C2Tx.In general, the combination of Co-Ti3C2Txand g-C3N4improved the visible light absorption of g-C3N4, which will improve the utilization of solar energy and enhance the performance of photocatalytic CO2reduction reaction.
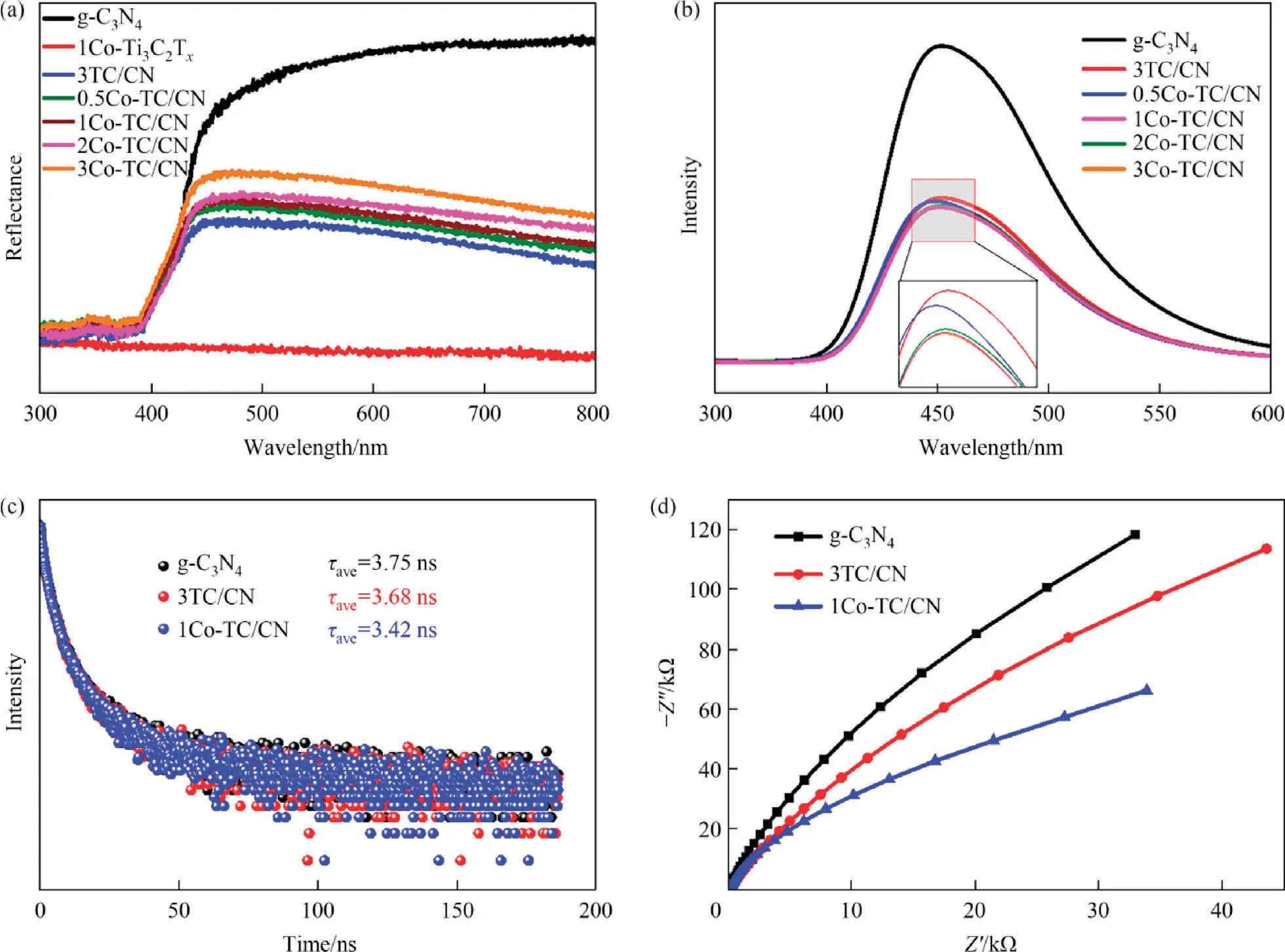
Fig.7.Ultraviolet-visible diffuse reflectance spectra of g-C3N4,1Co-Ti3C2Tx,3TC/CN,and yCo-TC/CN(a),steady-state PL spectra of g-C3N4,3TC/CN,and yCo-TC/CN(b),timeresolved photoluminescence (TRPL) spectra of g-C3N4, 3TC/CN, and 1Co-TC/CN (c), EIS Nyquist plots of g-C3N4, 3TC/CN, and 1Co-TC/CN (d).
In order to investigate the effect of Co-Ti3C2Txon the photogenerated charge carrier transfer and separation in g-C3N4, steadystate photoluminescence (PL) spectra, time-resolved photoluminescence (TRPL) spectra, and electrochemical impedance spectroscopy were carried out.As shown in the PL spectra of g-C3N4,3TC/CN,and yCo-TC/CN composites (Fig.7(b)), the addition of Ti3-C2Txor Co-Ti3C2Txsignificantly reduced the PL intensity of g-C3N4,indicating that the addition of Ti3C2Txor Co-Ti3C2Txgreatly promoted the charge carrier transfer and separation of photogenerated electrons and holes in g-C3N4.It is worth noting that the PL intensity of the yCo-TC/CN composite is lower than that of 3TC/CN,indicating that the addition of Co further improves the separation efficiency of photogenerated electrons and holes in yCo-TC/CN composite.The separation efficiency of photogenerated electrons and holes in g-C3N4, 3TC/CN, and 1Co-TC/CN photocatalyst was further analyzed by time-resolved photoluminescence(TRPL)spectra (Fig.7(c)).Obviously, the average fluorescence lifetime of the 1Co-TC/CN photocatalyst (3.42 ns) is shorter than that of g-C3N4(3.75 ns) and 3TC-CN (3.68 ns), indicating that the Co-Ti3C2Txcocatalyst can promote the transfer of photogenerated electrons from g-C3N4to Co-Ti3C2Txand inhibit the recombination of photogenerated electrons and holes.
The charge transfer efficiency and charge transfer resistance of the photocatalysts were also analyzed by photoelectrochemical measurements.As shown in Fig.7(d), the arc radius of the 3TC/CN composite is smaller than that of g-C3N4, indicating that the addition of Ti3C2Txcan reduce the resistance of charge transfer.In addition, the arc radius of 1Co-TC/CN is smaller than that of the 3TC/CN composite, indicating that the separation efficiency of photogenerated electrons and holes on the 1Co-TC/CN photocatalyst was further improved with Co-Ti3C2Txas the cocatalyst.The above results indicate that Co-Ti3C2Txcan be used as an efficient cocatalyst for reducing the charge transfer resistance and improving the separation efficiency of photogenerated electrons and holes in g-C3N4, which will significantly enhance the photocatalytic CO2reduction.
The photocatalytic performance of the photocatalysts was evaluated by photocatalytic CO2reduction under visible light irradiation (>400 nm).As shown in Fig.8 (a) and (b), the production of CO and CH4over g-C3N4was 7.29 μmol·g-1and 0.39 μmol·g-1,respectively, under visible light irradiation for 4 h.In addition,the production of CO and CH4over 3TC/CN reached 27.11 μmol·g-1and 1.88 μmol·g-1, respectively, which is 3.7 times and 4.8 times higher than that of g-C3N4.Notably, it is well known that Ti3C2Txexhibit a highly efficient photothermal effect.Therefore,to exclude the photothermal effect on the photocatalytic CO2reduction, control experiment using Ti3C2Txas the catalyst were carried out.The results show that no gaseous products (CO or CH4) were detected during the photocatalytic CO2reduction reaction with Ti3C2Txas the catalyst, indicating that the reduction of CO2cannot be driven by the photothermal effect of Ti3C2Tx.
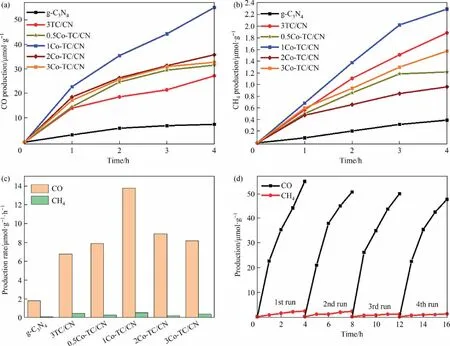
Fig.8.Photocatalytic CO2 reduction to CO (a) and CH4 (b) over g-C3N4, 3TC/CN, and yCo-TC/CN, photocatalytic CO2 reduction rate over g-C3N4, 3TC/CN, and yCo-TC/CN (c),stability of photocatalytic CO2 reduction over 1Co-TC/CN (d).
When yCo-Ti3C2Txwas used as the cocatalyst of g-C3N4, the photocatalytic CO2reduction efficiency of yCo-TC/CN was greatly increased, and the 1Co-TC/CN showed the best photocatalytic CO2reduction performance, and the production of CO and CH4reached 55.04 μmol·g-1and 2.29 μmol·g-1, respectively, which were 7.5 times and 5.8 times higher than that of g-C3N4.However,when the Co content in Co-Ti3C2Txexceeds 1% (mass), the photocatalytic CO2reduction efficiency of the yCo-TC/CN composite decreased gradually.When 3Co-Ti3C2Txwas used as the cocatalyst of g-C3N4, the production of CO and CH4decreased to 32.68 μmol·g-1and 1.57 μmol·g-1, respectively.The decreased performance might be due to the fact that the excessive Co was aggregated on the surface of Ti3C2Txand covered some of the active sites.As shown in Fig.8(c), the CO production rate of 1Co-TC/CN(13.76 μmol·g-1·h-1) is 7.5 times and 2.0 times higher than that of g-C3N4(1.82 μmol·g-1·h-1) and 3TC/CN (6.78 μmol·g-1·h-1),respectively.
The above results indicated that the photocatalytic CO2reduction performance was obviously enhanced with the addition of Co.In the 1Co-TC/CN photocatalyst, Co can act as the fast channel for the transfer of photogenerated charge carriers when Co is located at the interface between g-C3N4and Ti3C2Tx.At the interface, photogenerated electrons can first transfer from g-C3N4to Co, and then further transfer from Co to Ti3C2Tx, thus enhanced the separation of photogenerated electrons and holes.This conclusion was confirmed by the steady-state PL spectra, time-resolved photoluminescence (TRPL) spectra in Fig.7(b) and (c).In addition,the resistance of charge carrier transfer was greatly reduced after the addition of Co to the TC/CN according to the EIS Nyquist plots in Fig.7(d), which further demonstrated that the Co was acted as the fast channel for photogenerated charge carrier transfer.Moreover,as shown in Fig.S10,the selectivity of CO was increased from 93.5%to 96%by the addition of Co,which indicating that the Co or the interface between the Co and Ti3C2Txmight be the active sites for the adsorption and activation of CO2, thus affect the selectivity of photocatalytic CO2reduction.However,the overlarge Co cluster located between the g-C3N4and Ti3C2Txinterface will increase the transfer distance of the photogenerated electrons, which is not conducive to the rapid transfer and separation of photogenerated charge carriers.These results indicate that the synergistic effect of Co and Ti3C2Txon the photocatalytic CO2reduction performance of g-C3N4is superior to that of Ti3C2Tx.Moreover, as shown in Fig.S10, the selectivity of CO for the g-C3N4, 3TC/CN, and 1Cu-TC/CN sample was calculated to be 94.9%,93.5%,and 96.0%,respectively.In addition, the selectivity of CH4can only reach 5.1%, 6.5%,and 4.0%, respectively.
In order to confirm the C source in the CO and CH4products,the following controlled experiments were carried out:(1)experiment with 1Co-TC/CN,CO2,and H2O,without light irradiation;(2)experiment with CO2, H2O, and light irradiation, without photocatalyst;
(3)experiment with 1Co-TC/CN,H2O,and light irradiation,without CO2; (4) experiment with 1Co-TC/CN, CO2, and light irradiation,without H2O.For these four controlled experiments, no CO or CH4can be detected, indicating that CO and CH4were generated by photocatalytic CO2reduction reaction over 1Co-TC/CN composite, and the H2O play an important role for photocatalytic CO2reduction to CO and CH4.
To investigate the stability of the 1Co-TC/CN composite for the photocatalytic CO2reduction reaction,four cycle experiments with 1Co-TC/CN as the photocatalyst was carried out.The reactor was filled with a gas mixture of CO2and H2O under the same conditions after each cyclic experiment,and the results are shown in Fig.8(d).Notably, for each cycle, the production of CH4and CO decreased after 1 h irradiation, indicating that there is an induction period in the early stage of photocatalytic CO2reduction reaction over 1Co-TC/CN composite.This phenomenon can also be observed in other photocatalytic systems[54].In the photocatalytic CO2reduction process,moist CO2gas was fed into the reactor for 30 min at a rate of 40 mL·min-1to achieve the saturation adsorption of CO2before light irradiation,which was conducive to the photocatalytic CO2reduction reaction.However,after reaction for 1 h,most of the adsorbed CO2was reduced to CO,and the adsorption of CO2cannot reach the point of saturation due to the low concentration of CO2in the reactor.Therefore,the production of CO was higher in the first hour and then decrease after the first hour.
The CO production decreased only by 13%after the fourth cycle compared with that of the first cycle, indicating the good stability of 1Co-TC/CN composite during the photocatalytic reaction.To further study the stability of the 1Co-TC/CN composite,the 1Co-TC/CN sample was characterized by XRD, SEM, TEM, and XPS before and after the reaction.After the fourth cycle, no obvious change can be observed in the XRD patterns of 1Co-TC/CN (Fig.S11(A)), indicating that the crystal structure of the catalyst did not change after reaction.Moreover,no significant change can be seen for the morphology of 1Co-TC/CN according to the SEM and TEM images after reaction (Fig.S11(B), Fig.S12).In addition, the HRTEM images of 1Co-TC/CN (Fig.S12(C)) showed that the interface between Co-Ti3C2Txand g-C3N4can also be seen after reaction,and the EDS elemental (C, N, Ti, Co) mapping of 1Co-TC/CN after reaction demonstrated that Co, Ti, C, and N were still highly dispersed on 1Co-TC/CN(Fig.S12(D)).Furthermore,XPS spectra showed that the chemical composition of 1Co-TC/CN did not show obvious change after reaction(Fig.S13).The above results demonstrated the high stability of 1Co-TC/CN in the photocatalytic CO2reduction reaction.
Combining the photocatalytic experiments and the characterization results, the reaction mechanism of photocatalytic CO2reduction over the Co-Ti3C2Tx/g-C3N4composite was proposed,and the results are shown in Fig.9.When Co is located at the interface between g-C3N4and Ti3C2Tx, photogenerated electrons can firstly transfer from the conduction band (CB) of g-C3N4to Co,and then further transfer from Co to Ti3C2Tx.Moreover, the electrons can directly move from the CB of g-C3N4to Ti3C2Txwhen g-C3N4directly contact with Ti3C2Tx.Therefore, there are two paths of charge transfer in the Co-TC/CN ternary photocatalyst, and the addition of Co enhanced the separation of photogenerated electrons and holes.Therefore, Co can be used as a charge transfer bridge between g-C3N4and Ti3C2Tx.In addition, except for the Co locate at the interface between g-C3N4and Ti3C2Tx, the other Co can be distributed on the surface of Ti3C2Tx.Notably, the interface between the Co and Ti3C2Txmight be act as the active sites for the adsorption and activation of CO2to enhance photocatalytic CO2reduction.In summary, the photogenerated electrons gathered on Co-Ti3C2Txcocatalyst can be used for efficient photocatalytic CO2reduction, and the holes in the VB of g-C3N4can oxidize H2O to O2.

Fig.9.The proposed mechanism of photocatalytic CO2 reduction over the Co-TC/CN composite.
4.Conclusions
In summary, the Co-Ti3C2Tx/g-C3N4composite photocatalyst was successfully synthesized by the electrostatic self-assembly method.The close interface between the Co-Ti3C2Txand g-C3N4nanosheet is beneficial to the transfer of photogenerated charge carriers and prevents the recombination of photogenerated electrons and holes.Both the Ti3C2Txand Co cluster can be used as fast transport channels for photogenerated electrons, which promotes the separation of photogenerated electrons and holes.Moreover,the interface between Co and Ti3C2Txmight be the active site for photocatalytic CO2reduction.The synergistic effect of Co clusters and Ti3C2Txgreatly improves the efficiency of the photocatalytic CO2reduction reaction over g-C3N4.The 1Co-Ti3C2Tx/g-C3N4composite showed the best photocatalytic CO2reduction performance,with the production of CO and CH4reached 55.04 μmol·g-1and 2.29 μmol·g-1after reaction for 4 h, which was 7.5 times and 5.8 times than that of g-C3N4, respectively.This work revealed the effect of Co-Ti3C2Txon the enhanced photocatalytic performance of g-C3N4, and promoted the application of MXene-metal-based cocatalyst in the field of photocatalysis.
Data Availability
Data will be made available on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22208065), Guangxi Natural Science Foundation(2022GXNSFBA035483, 2020GXNSFDA297007), Opening Project of Guangxi Key Laboratory of Petrochemical Resource Processing and Process Intensification Technology (2021K009, 2020K002),and Special funding for ‘Guangxi Bagui Scholars’.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.06.018.
 Chinese Journal of Chemical Engineering2023年12期
Chinese Journal of Chemical Engineering2023年12期
- Chinese Journal of Chemical Engineering的其它文章
- Intrinsic kinetics of catalytic hydrogenation of 2-nitro-4-acetylamino anisole to 2-amino-4-acetylamino anisole over Raney nickel catalyst
- Experiments and model development of p-nitrochlorobenzene and naphthalene purification in a continuous tower melting crystallizer
- α-Synuclein: A fusion chaperone significantly boosting the enzymatic performance of PET hydrolase
- Influence of water vapor on the separation of volatile organic compound/nitrogen mixture by polydimethylsiloxane membrane
- Mass transfer mechanism and relationship of gas-liquid annular flow in a microfluidic cross-junction device
- Enhanced photocatalytic activity of methylene blue using heterojunction Ag@TiO2 nanocomposite: Mechanistic and optimization study
