Construction of CuNiAl-LDHs electrocatalyst with rich-Cu+ and —OH for highly selective reduction of CO2 to methanol
Gaiqin Miao, Lifei Liu, Xia An, Xu Wu
College of Chemistry, Taiyuan University of Technology, Taiyuan 030024, China
Keywords:Electrocatalysis CO2 reduction Methanol CuNiAl layered-double hydroxides Hydroxyl group
ABSTRACT In this work,a high-performance CuNiAl-LDHs catalyst was innovatively synthesized for electrochemical carbon dioxide reduction (CO2RR) of methanol (CH3OH) through the modulated synthesis of Cu-based layered double hydroxide(LDHs).It was found that the optimal CuNiAl-LDHs has superior CH3OH selectivity compared to CuAl-LDHs and CuMgAl-LDHs, with the Faraday efficiency (FE) of 76.4% for CH3OH generation at -1.2 V.And their FE and current density (4.8 mA·cm-2) remained stable during up to 24 h of electrolysis.Meanwhile, this study confirms the significant performance advantages of CuNiAl-LDHs over their derived composite oxides.Series characterization further proves that the excellent catalytic performance of CuNiAl-LDHs is importantly associated with their richness in Cu+ and hydroxyl group (—OH).The research expands the application fields of LDHs compounds.Meanwhile, the series of discoveries provide a new insight for the preparation of CH3OH by constructing CO2RR.
1.Introduction
Excessive carbon dioxide levels cause a serious energy crisis and environmental problems [1-3].Therefore, it is urgent to achieve the transformation and reduction of CO2.Compared to other conversion technologies, CO2RR is a prospective technology for converting CO2into valuable chemicals under relatively mild conditions [4-6].In recent years, the main products of CO2RR are carbon monoxide or formate[7,8].Meanwhile,CH3OH is a valuable chemical fuel with high energy density [5,9].However, sixelectron/proton coupling steps are required in the electrochemical reduction of CO2to CH3OH [10-12], which makes the reduction kinetically slow and more challenging.Therefore, it is important to design a suitable catalyst for CO2RR.
Cu-based oxides have been widely studied because of their excellent CO2RR performance [12-18].Le et al.[18] prepared cuprous oxide films by electrodeposition, which could convert CO2reduction to CH3OH in 0.5 mol·L-1KHCO3at 38% FE.It was confirmed that Cu+may have a crucial role in enhancing the CH3-OH selectivity.Furthermore,Malik et al.[13]synthesized a copperbased electrocatalyst of multi-walled carbon nanotubes(MWCNTs)containing Cu2O,which shows FECH3OHof 38%for the electrochemical reduction of CO2to prepare CH3OH.The bond between Cu2O and MWCNTs facilitated the stability of the catalyst.Xian Yang et al.[14] further proposed a novel MOF-derived Cu@Cu2O metal oxide multiphase electrocatalyst for the preparation of CH3OH by CO2reduction.It was found that in addition to the cooperative effect of Cu0and Cu+that effectively enhanced the adsorption of CO2, —OH adsorbed the surface of the catalyst promoted the further hydrogenation of CO*for the synthesis of CH3OH with 45%FE.Mostafa et al.[19] also speculated that the hydroxyl group may be a useful functional group for catalyzing CO2RR.
Recently, Iwase et al.[20] synthesized CuAl-LDHs at different pH for CO2RR, which concluded that the LDHs lamella size is a key parameter in factor in deciding the CO2RR activity.LDHs is known to be a class of layered hydroxides.Their interlaye ions are exchangeable, can coordinate with different metal ions and contain structural hydroxyl groups[21,22].Based on the characteristic properties revealed by Cu-based oxide CO2RR catalysts,it may be possible to construct high-performance CO2RR catalysts with modulated synthesis of LDHs.
Inspired by this, here the functional additives Mg and Ni were introduced into the CuAl-LDHs laminate.Meanwhile, CuNiAl-LDHs were calcined at different temperatures.In an attempt to construct high-performance CO2RR catalysts,the essential reasons for the difference in catalytic performance of LDHs are clearly revealed through effective conformational correlations.
2.Experimental
2.1.Materials
In this study, copper nitrate trihydrate (99.0%), aluminum nitrate nine-hydrate (99.0%), magnesium nitrate hexahydrate(99.0%)and nickel nitrate six-hydrate(99.0%)were purchased from Aladdin Chemical Co., Ltd.(USA).Anhydrous sodium carbonate(99.8%) was purchased from Tianjin Comio Chemical Reagent Co.,Ltd.(China).Sodium hydroxide (96.0%) and ethanol (99.7%) were provided by Aladdin Chemical Co., Ltd.Nafion (5%, mass) was obtained from Alfa Aesar Chemical Co., Ltd.(UK).Nafion 115 proton exchange membrane was purchased from Tianjin Gaoshi Rui Lian Photoelectric Technology Co., Ltd.(China).In addition, Ultrapure water was from the Taiyuan Nokwanpin Chemical Technology Co., Ltd.High-purity nitrogen (99.999%), high-purity Argon(99.999%), high-purity helium (99.999%), and carbon dioxide(99.999%) from Taiyuan Anxuhongyun Technology Development Co., Ltd.(China) were used during all experiments.
2.2.Preparation of copper-based hydrotalcite
2.2.1.Synthesis of CuAl-LDHs,CuMgAl-LDHs and CuNiAl-LDHs catalyst
CuNiAl-LDHs was prepared by co-precipitation method, where n(Cu2+):n(Ni2+):n(Al3+)=3:1:1.Solution A was prepared by mixing Cu2+(0.12 mol·L-1), Ni2+(0.04 mol·L-1) and Al3+(0.04 mol·L-1)aqueous solution of 50 ml.In addition, solution B was prepared by mixing NaOH(0.30 mol·L-1)and Na2CO3(0.10 mol·L-1)aqueous solution of 50 ml.Solution A and B were placed in a dropping funnel and dripped simultaneously into a flask containing deionized water.The hybrid solution was hydrothermally treated at 65 °C for 10 h.The solution was then filtered, washed and dried at 80 °C to produce CuNiAl-LDHs.
For the preparation of CuAl-LDHs and CuMgAl-LDHs, the method is same with that for CuNiAl-LDHs.
2.2.2.Synthesis of L-T catalyst
A certain amount of CuNiAl-LDHs was set in a quartz crucible,placed in the center of a muffle furnace and calcined at 500 °C for 5 h with a heating rate of 2 °C·min-1.The L-500 °C catalyst was acquired after cooling.For further comparison, the CuNiAl-LDHs catalyst was calcinated at different temperatures to obtain a series of catalysts, denoted L-T (where T = 300 °C or 500 °C).
2.3.Electrode preparation
The working electrode was prepared by drop painting method.600 ml of ethanol were mixed with 4 mg of catalyst, 100 ml of 0.05% (mass) Nafion solution, and sonicated for 30 min to create a homogenous suspension.Then, the above liquid was uniformly dropped onto a 2 cm × 2 cm carbon paper and dried under an infrared lamp to make a working electrode.
2.4.Material characterization
X-ray powder diffraction (XRD)was performed with the Danton DX-2700 instrument.Using a Cu Kα radiation source (λ = 0.154184 nm,40 kV and 30 mA)at 8(°)·min-1from 5°-90°.Fourier transform infrared spectroscopy (FT-IR) was gained by Bruker TENSOR II FTIR, Germany, in the range of 4000-500 cm-1.Prior to the experiments, catalyst and KBr particles were pressed into transparent sheets at a ratio of 1:100.The N2adsorption/desorption isotherms were measured on the automatic gas adsorption analyzer ASAP-2460 from Micromeritics, America.The specific surface area, pore volume and pore size distribution of the catalysts were determined using the Brunauer-Emmett-Teller (BET)and Barrett-Joyner-Halenda (BJH) methods.The catalyst were pre-dried for 1 h before testing and then degassed at 150 °C under vacuum for 4 h to remove impurities and adsorbed water from the surface.The morphology and surface atomic concentration of CuNiAl-LDHs precursor were examined by a scanning electron microscope(SEM,SU8010,Japan)and Energy Dispersive Spectrometer (EDS).
Thermogravimetry(TG)was used to study the thermal stability of CuNiAl-LDHs on a simultaneous thermal analyzer STA449F3 from Netzsch,Germany.CO2programmed temperature desorption(CO2-TPD) experiments were performed in a VDSORB-91i fully automated chemisorbed to estimate the amount of base and alkaline strength of the catalyst surface.Carbon dioxide sorption isotherms were obtained with an automated volumetric adsorber(Micromeritics, ASAP2020, USA).The elemental composition of the catalysts was tested by X-ray photoelectron spectroscopy(XPS), which was performed on a Thermo Fisher ESCALAB 250xi system with Al Kα radiation (hν = 1486.6 eV).
2.5.Electrochemical measurements
All electrochemical measurements were performed using a Princeton electrochemical workstation in a hermetic H-cell that has a Nafion 115 proton exchange membrane-separated two compartments.Electrochemical CO2reduction is performed by a three-electrode system.The working electrode is positioned in the cathode chamber, an Ag/AgCl reference electrode and a platinum wire is positioned in the anode chamber as a counter electrode.The electrolyte in both sides was 60 ml of 0.1 mol·L-1 KHCO3solution.Before electrochemical testing, the cathode solution was bubbled with carbon dioxide or nitrogen for 30 min.It is worth noting that all potentials in this study were calibrated to reversible hydrogen electrodes (ERHE= EAg/AgCl+ 0.197 + 0.059 pH), and the electrolyte is 0.1 mol·L-1KHCO3saturated with CO2at pH 6.8.All potential values in this research are referenced to the reversible hydrogen electrode unless otherwise stated.
Atmospheric pressure and room temperature were used for all electrochemical reactions and measurements.Linear scanning voltammetry (LSV) was performed in CO2-saturated or N2-saturated 0.1 mol·L-1KHCO3at a scan rate of 50 mV·s-1from 0.6-1.4 V.Electrochemical impedance spectroscopy(EIS) was performed from 10-1to 106Hz at -0.9 V (versus Ag/AgCl) with an amplitude of 10 mV.
Throughout the reaction, CO2(20 ml·min-1) was fed continuously and the gaseous products were analyzed every 30 min by gas chromatography (GC, Agilent 7890A) equipped with a thermal conductivity detector and a flame ionization detector.The carrier gas for the chromatography chamber was highpurity helium (99.999%) and high-purity nitrogen (99.999%).GC for online gas analysis detected mainly H2and very limited amounts of C2H4.All experiments were performed under constant potential conditions for 2 h.After the reaction was completed, 150 μl of electrolyte was collected from the cathode tank positioned at the outlet of the electrochemical cell,extracted with 150 μl of ethyl acetate, and the liquid product was analyzed by gas chromatography (GC, Agilent 7890A)[23].The performance of the method was evaluated by the Faraday efficiency (FE) of CH3OH (i.e., the selectivity of the reaction to produce CH3OH).Assuming that six electrons were required for each CH3OH molecule, the calculation was based on the following formula:
F stands for the Faraday constant(F = 96485 C·mol-1),n stands for the number of moles produced,and q refers to the total charge applied throughout the process.z is the number of theoretical charges exchanged to produce the desired product.
3.Results and Discussion
3.1.XRD results
Fig.1(a) shows the XRD pattern of CuAl-LDHs, CuMgAl-LDHs and CuNiAl-LDHs synthesized by the co-precipitation method,respectively.In Fig.1(a), the reflection peaks located at 11.8°,24.3°, 35.4°, 38.8°, 46.6° and 60.1° can be indexed to the (0 0 3),(0 0 6), (0 1 2), (0 1 5), (0 1 8) and (1 1 0) planes of the LDHs(PDF # 37-0630), indicating the successful preparation of the LDHs.The XRD patterns of CuNiAl-LDHs, L-300 °C and L-500 °C are shown in Fig.1(b).With increased temperature, the peaks observed at 2θ = 32.69°, 35.73°, 38.93°, 49.50°, 58.67° and 68.50°correspond to the (1 1 0), (1 1 1), (1 1 1), (1 1 2), (2 0 2) and(2 2 0) crystal planes of CuO (PDF # 74-1021).The peak at 2θ = 61.52° corresponds to the (2 2 0) crystal plane of Cu2O (PDF# 65-3288), the peaks at 43.29° and 75.44°correspond to the(0 1 2) and (1 1 3) crystal planes of NiO, respectively (PDF #44-1159).In addition,a faint peak was found on the(0 0 3)crystal plane at L-300 °C, while the peak of LDHs has completely disappeared at L-500°C.This may be related to the incomplete collapse of the LDHs laminate at 300 °C.It is noted, the characteristic diffraction peaks of Al2O3are not detected in the XRD patterns of all catalysts, which may be related to its amorphous state.

Fig.1.XRD patterns of (a) CuAl-LDHs, CuMgAl-LDHs and CuNiAl-LDHs.(b) CuNiAl-LDHs, L-300 °C and L-500 °C.
3.2.TG results
As shown in Fig.2, the thermogravimetric analysis (TG) curve indicates two weight loss processes in CuNiAl-LDHs.The first is heat absorption at a lower temperature stage (100-200 °C), corresponding to the loss of water adsorbed on the surface and in the interlayer,a reversible step that does not cause laminate collapse;the second is heat absorption at higher temperature stage (200-400 °C), where the structural hydroxyl groups and anions on the laminate are lost and the laminate gradually collapses [24].The weight loss peaks in the first and second phases are consistent with the thermal decomposition behavior of conventional classes of hydrotalcite.In addition, no new weight loss peaks appear above 500 °C, which proves that the CuNiAl-LDHs have been completely decomposed at this temperature.Therefore, 300°C and 500°C are choosed for calcination.

Fig.2.TG pattern of CuNiAl-LDHs.
3.3.FT-IR results
The FT-IR spectra of CuAl-LDHs, CuMgAl-LDHs and CuNiAl-LDHs are presented in Fig.3(a).All catalyst show a broad peak at 3436 cm-1, which can be attributed to laminate hydroxyl and interlayer water hydroxyl stretching vibrations [25], and the absorption peak near 1660 cm-1is due to bending vibrations of—OH in crystalline water [26].The broad peak at 1390 cm-1is associated with asymmetric stretching of interlayer COvibration[27,28].The absorption peaks at 832 cm-1and 605 cm-1may be ascribed to the metal-oxygen bond M—O on the hydrocalcite laminate [26,27,29,30].FT-IR results further confirm the successful synthesis of CuAl-LDHs, CuMgAl-LDHs and CuNiAl-LDHs catalysts.Fig.3(b) shows the FT-IR spectra of CuNiAl-LDHs, L-300 °C and L-500 °C.The characteristic peaks of CuNiAl-LDHs during 1200-1700 cm-1disappears after calcining.

Fig.3.FT-IR spectra of (a) CuAl-LDHs, CuMgAl-LDHs and CuNiAl-LDHs.(b) CuNiAl-LDHs, L-300 °C and L-500 °C.
3.4.SEM analysis
SEM tests were performed to study the morphology of the catalyst.In Fig.4(a)-(c), CuAl-LDHs, CuMgAl-LDHs and CuNiAl-LDHs show lamellae of different sizes and interlocking, which is consistent with the lamellar structure of hydrotalcite[31].Specifically,as presented in Fig.4(a), the lamellar structure of CuAl-LDHs is thicker, and the high surface energy and strong hydrophilicity of the lamellae lead to the close packing between the lamellae,which indicates the easy agglomeration property of CuAl-LDHs.After the introduction of Mg and Ni elements,the lamellae of CuMgAl-LDHs and CuNiAl-LDHs precursors became smaller and thinner to different degrees.Thus, the introduction of the third element on the CuAl-LDHs laminate can effectively improve the dispersion of the hydrotalcite-like lamellae, reduce agglomeration, and improve the buildup problem of CuAl-LDHs.In Fig.4(d) and (e), after calcinating in air at 300°C and 500°C,the partial collapse occurred at L-300 °C and the lamellar structure completely disappeared at L-500 °C.This may be ascribed to the collapse of the lamellar structure of CuNiAl-LDHs in the process of pyrolysis.It is obtained from EDS mapping that the molar ratio of Cu/Ni/Al in CuNiAl-LDHs is about 3:1:1.
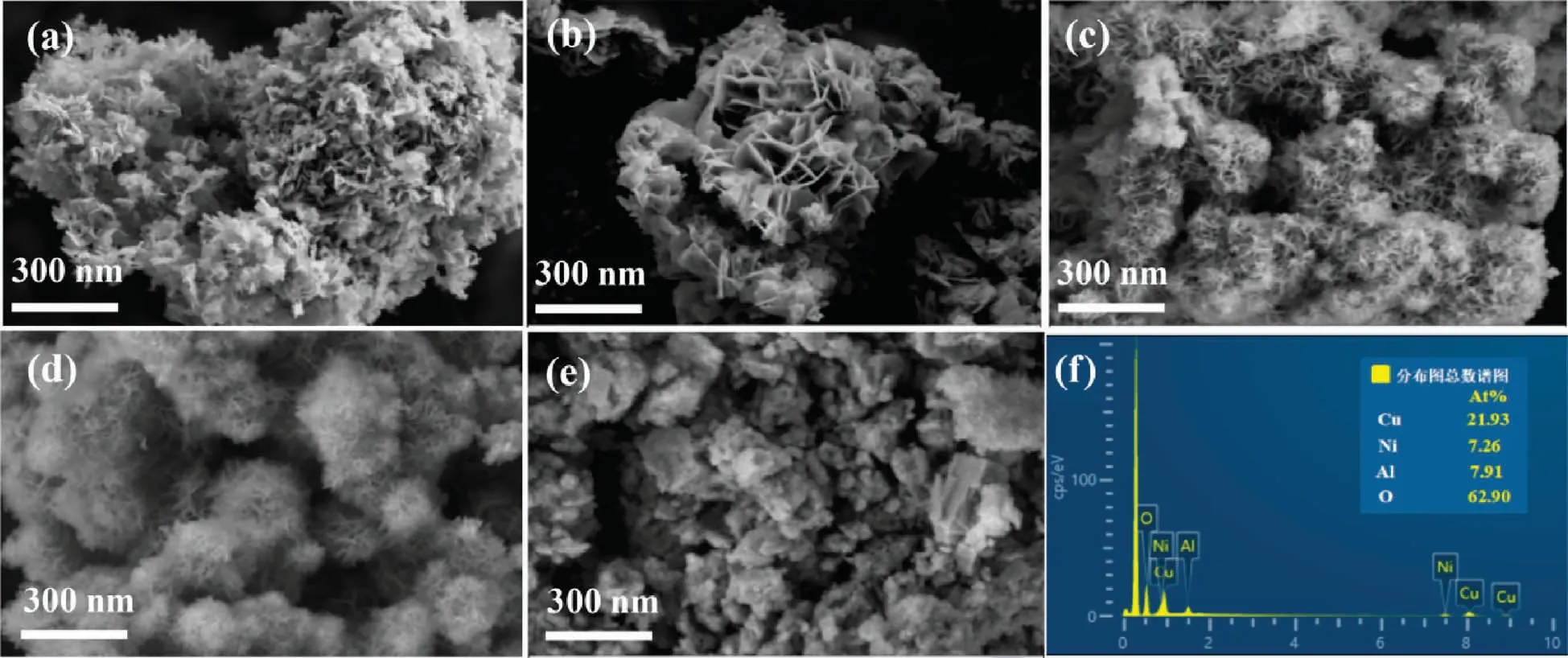
Fig.4.SEM images of (a) CuAl-LDHs, (b) CuMgAl-LDHs, (c) CuNiAl-LDHs, (d) L-300 °C, (e) L-500 °C and (f) EDS mapping of CuNiAl-LDHs.
3.5.TEM and HR-TEM analysis
To get a better understanding of the morphology and structure of the catalysts, TEM characterization was carried out, and the results are shown in Fig.5.It can be observed from the figures that the shaded part of the agglomeration is significantly weakened after the addition of the co-functional component, which further confirms the improved dispersion of the catalysts, as shown in Fig.5(a), (c) and (e).Meanwhile, it can be seen from Fig.5(g) and(i) that a part of the lamellar structure of LDHs was retained after calcination at 300 °C, while the lamellar structure of LDHs had all collapsed after calcining at 500 °C, which is consistent with the results of XRD and SEM.The lattice stripes of the above samples were observed in HR-TEM images with a scale of 5 nm, as shown in Fig.5(b), (d), (f), (h) and (i).The (1 1 1) crystal plane of Cu2O and the(1 1 1)crystal plane of CuO are present in all five catalysts with a lattice spacing of 0.25 nm and 0.23 nm, respectively.In addition, the (0 1 2) crystal plane of NiO is present in CuNiAl-LDHs, (0 1 2) crystalline planes of NiO were present in L-300 °C and L-500°C with a lattice spacing of 0.21 nm.However,the above phases were not retrieved in the XRD spectra of LDHs, which may be due to their high dispersion on the catalyst surface.
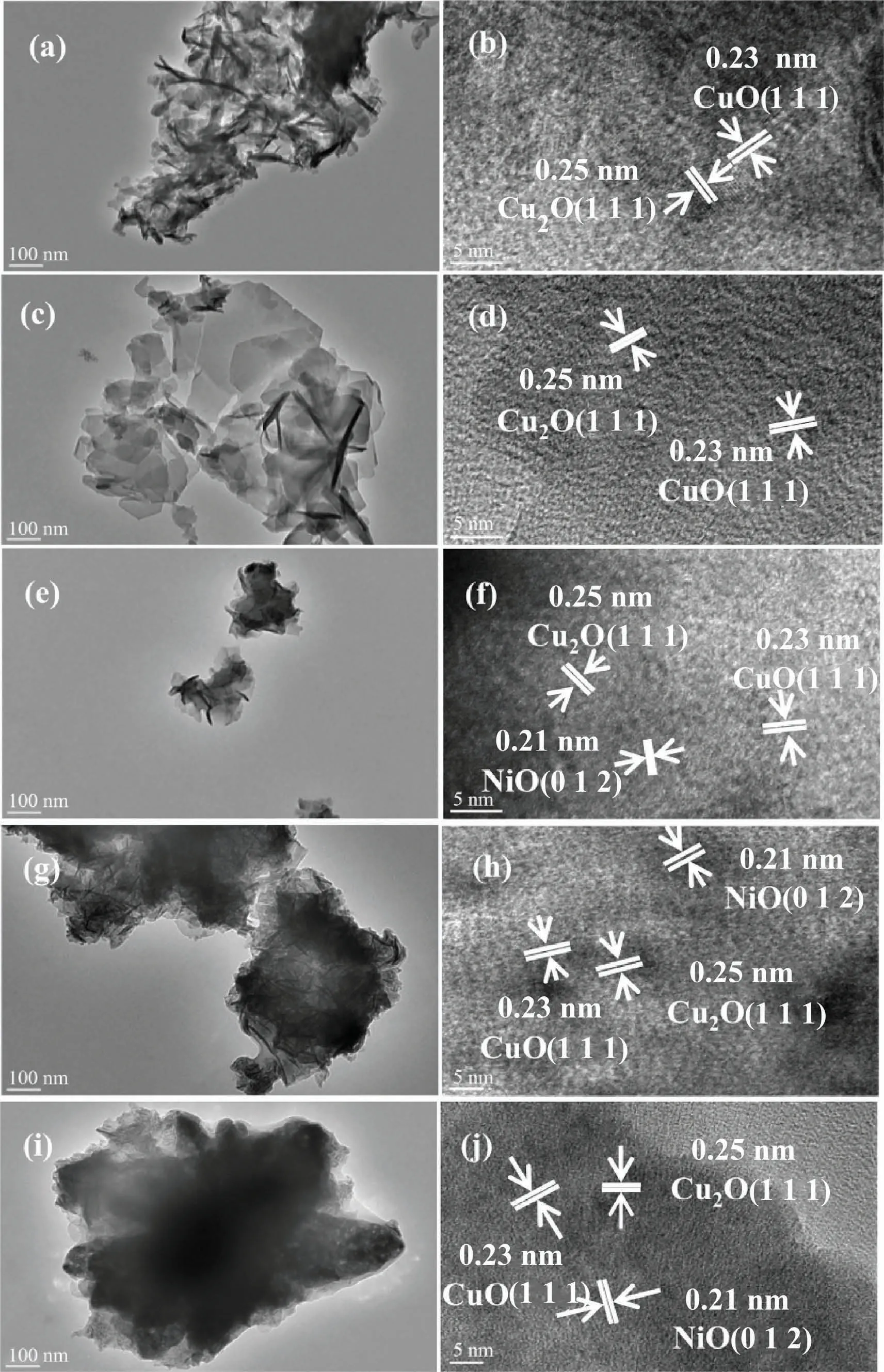
Fig.5.TEM and HR-TEM patterns of (a,b) CuAl-LDHs, (c,d) CuMgAl-LDHs, (e,f) CuNiAl-LDHs, (g,h) L-300 °C and (i,j) L-500 °C catalysts.
3.6.N2 adsorption-desorption analysis
The textural properties of catalysts were analyzed by N2adsorption-desorption measurements.In Fig.6,the isotherms of all catalysts have typed IV with H3-type hysteresis at high relative pressures,which implies that the catalysts have mesoporous properties [32,33].It can be seen from Table 1 that the specific surface area and pore volume of CuNiAl-LDHs are larger than the other catalysts, which is favorable to the diffusion of molecules [19].The specific surface area reduces with increased temperature.This is probably caused by the disruption of the structure of CuNiAl-LDHs during calcination, resulting in the blockage or fracture of a large number of pores.In general, the large specific surface area and good pore structure facilitate the exposure of active ingredients, thus promoting the CO2adsorption and activation.

Table 1 Data summary of N2 adsorption and desorption of the CuAl-LDHs, CuMgAl-LDHs,CuNiAl-LDHs, L-300 °C and L-500 °C

Fig.6.N2 adsorption-desorption isotherms of (a) CuAl-LDHs, CuMgAl-LDHs and CuNiAl-LDHs.(b) CuNiAl-LDHs, L-300 °C and L-500 °C.
3.7.Electrochemical performance testing
3.7.1.Comparison of CO2 reduction activity in N2 and CO2 saturated electrolytes
The current density of the catalysts were studied by the LSV test as shown in Fig.7.The CuNiAl-LDHs catalyst displays the maximum current density in the potential scale from -0.8 to -1.4 V compared with CuAl-LDHs and CuMgAl-LDHs catalysts in Fig.7(a).Meanwhile, it is shown in Fig.7(b) that the CuNiAl-LDHs displays the maximum current density in the potential scale of -0.2 to-1.4 V with respect to the L-300°C and L-500°C.This shows that CuNiAl LDHs exhibit faster electron transfer and better CO2RR performance[34].The CO2-saturated electrolyte exhibits a larger current density in comparison to the Ar-saturated electrolyte,indicating that the catalyst has an activating effect on CO2during the electrochemical reaction.As presented in Fig.7(c), the current density of CuNiAl-LDHs saturated in CO2is apparently higher than that in Ar, demonstrating a high CO2RR catalytic activity [35].

Fig.7.LSV curves of(a)CuAl-LDHs,CuMgAl-LDHs and CuNiAl-LDHs.(b)CuNiAl-LDHs,L-300°C and L-500°C measured in CO2-saturated 0.1 mol·L-1 KHCO3.(c)CuNiAl-LDHs in CO2 and N2-saturated 0.1 mol·L-1 NaHCO3.
3.7.2.Selective product performance study and stability analysis
Fig.8(a) shows the FE of CH3OH catalyzed by CuAl-LDHs,CuMgAl-LDHs and CuNiAl-LDHs at different electrolytic potential.The FE of the catalysts show the same trend,as the potential shifts negatively from -0.8 V to-1.2 V,the FE increases gradually.However,as the potential continues to shift negatively,the FE begins to decrease.Namely, the FE of all catalysts reaches the maximum at-1.2 V, 76.4% (CuNiAl-LDHs), 45.2% (CuAl-LDHs) and 42.1%(CuMgAl-LDHs),respectively.Then the catalysts obtained at different calcinating temperatures were applied to CO2RR at -1.2 V for 2 h.As shown in Fig.8(b),the FE of the three catalysts also showed the same trend,reaching a maximum at-1.2 V.Meanwhile,the FE decreases gradually with increasing calcinating temperature, and this trend can be ascribed to the influence of the structure of LDHs.With increased temperature,the LDHs laminate collapses to different degrees,and the laminate collapses completely at 500°C.Its FE also reaches the lowest at 500 °C, which is 38.7%.It is consistent with the previous TG results and further proves that the LDHs structure is beneficial to the CO2RR performance.Fig.8(c) shows the partial current density of CuNiAl-LDHs at different voltages and the FE plots for different products.The products include CH3-OH, C2H4and H2, where the combined FE of CuNiAl-LDHs is about 100%and no other products are detected.Meanwhile, the trend of current density variation is consistent with the maximum value of-4.8 mA·cm-2.

Fig.8.(a) FE of electrolytic preparation of CH3OH from CuAl-LDHs, CuMgAl-LDHs and CuNiAl-LDHs at different voltages.(b) FE of electrolytic preparation of CH3OH from CuNiAl-LDHs,L-300°C and L-500°C at different voltages.(c)FE and current density of CH3OH,C2H4 and H2 for CuNiAl-LDHs at different electrolytic voltages.(d)Stability of CuNiAl-LDHs during CO2 electrochemical reduction.
To investigate the stability of CuNiAl-LDHs catalyst in the preparation of methanol from CO2RR,chrono-current test was performed.As shown in Fig.8(d), the current density does not decay obviously after 24 h of continuous reaction,and the FECH3OHmaintains steady at around 76.4%.The results show that CuNiAl-LDHs is highly stable in the electrocatalytic reduction process and is suitable for long-term electrocatalytic CO2RR.
3.7.3.Electrochemical surface area (ECSA) measurement and CO2 adsorption results
The CO2adsorption isotherms of all catalysts with different synergistic functional components were measured at 0 °C and atmospheric pressure (Fig.9(a)) to investigate the effect of catalysts on CO2adsorption performance.The CO2adsorption capacity of CuNiAl-LDHs catalysts could reach 17 mg·g-1that was 2.4 and 2.8 times as high as that of CuAl-LDHs (7 mg·g-1) and CuMgAl-LDHs (6 mg·g-1) catalysts, respectively.This indicates that the addition of the synergistic functional component Ni plays a key role in the adsorption of CO2.

Fig.9.(a)CO2 adsorption isotherms on the three catalysts materials at 0°C.(b)Corresponding current densities due to double-layer charging/discharging plotted against the potential scan rate.
The intrinsic activity of the catalyst for the effective reduction of CO2was studied by measuring the CV curves of different sweeps and calculating its ECSA according to the double-layer capacitance(Cdl) theory.In Fig.S6 in Supplementary Material, CV curves were plotted between 0.15 V-0.25 V (vs.Ag/AgCl) for different scan rates.As shown in Fig.9(b), the current density is linearly related to the scan rate.Among them, the Cdlof CuNiAl-LDHs (156 μF·cm-2) is much higher than that of CuAl-LDHs (112 μF·cm-2)and CuMgAl-LDHs (77 μF·cm-2).This indicates that the ECSA of CuNiAl-LDHs is the largest of all catalysts, providing more active sites for CO2RR [34,36].
3.8.CO2-TPD results
Fig.10 shows the CO2-TPD curves for several catalysts.The desorption temperature of CO2represents the strength of its basic center,and the area of the desorption peak reflects the amount of basic catalyst sites.It can be observed from the figure that weak Lewis basic sites and strong Lewis basic sites are evident in all catalyst.The weak basic sites (111-173 °C) can be ascribed to the adsorption of CO2by surface hydroxyl groups [37].The mutual position and area of the desorption peaks are also influenced by the different calcinating temperatures.Table S2 shows the desorption peak areas of the catalysts.It can be observed that the desorption peak area of CuNiAl-LDHs is the largest at the weak Lewis basic site,indicating the strong adsorption of CO2by its hydroxyl group.However, the total desorption peak area of CuNiAl-LDHs was slightly smaller than that of CuMgAl-LDHs,but the area of its weak basic sites was 2.5 times larger than that of CuMgAl-LDHs.Combined with the electrochemical performance test of both (Fig.8(a)), it is speculated that the adsorption of CO2by —OH promotes the performance of CO2RR.

Fig.10.The CO2-TPD profiles of (a) CuAl-LDHs, CuMgAl-LDHs and CuNiAl-LDHs.(b) CuNiAl-LDHs, L-300 °C and L-500 °C.
3.9.XPS results
The surface oxidation states of the metal and oxygen of the catalyst were explored by X-ray photoelectron spectroscopy(Fig.11),and the relevant atomic concentrations of the various valence elements were calculated from peak fitting regions (Table 2).In Fig.S3, the Cu 2p XPS spectra of all catalysts exhibit broad asymmetric peaks, and Cu 2p can be divided into two major split peaks(Cu 2p3/2and Cu 2p1/2) with binding energies located at 932.8 eV and 954.0 eV [32,38], respectively.When fitted to Cu 2p3/2, peaks with binding energies of 933.0 eV and 935.0 eV can be attributed to Cu2+, Cu+or Cu0, respectively [38,39].The binding energy of Cu+was reported to be similar to that of Cu0.Therefore, with the purpose of characterizing Cu0and Cu+, the Cu LMM X-induced Auger peak were also tested.As shown in Fig.11(a) and (c), the main peak is decomposed into three contributions near ~913.0 e V, ~915.6 eV, and ~ 917.7 eV, which are attributed to Cu+(~913.0 eV), Cu2+(~915.6 eV), and Cu0(~917.7 eV).The ratio of Cu+/(Cu2++Cu++Cu0) can be obtained by calculating the peak areas of each curve after splitting the peaks of the catalysts using Origin integration.The results show that the ratio of Cu+/(Cu2++Cu++Cu0)in CuNiAl-LDHs catalysts (46%) is higher than that of CuAl-LDHs(34%) and CuMgAl-LDHs (35%).It indicates that the introduction of auxiliary Ni does play a role in coordinating more Cu+.Previous studies have shown that Cu+has a strong adsorptive impact on CO*, making it easier to create the intermediate product CHO*,which then causes CO2to be reduced to CH3OH [14].Therefore,the proper Cu+content may be a reason for the excellent CO2RR performance of CuNiAl-LDHs.It is noteworthy that the XRD spectra of CuNiAl-LDHs do not retrieve the physical phase of Cu2O, while the peaks attributed to Cu+are observed in XPS with the highest Cu+content, which may be due to the high dispersion of Cu2O on its surface.In addition, the Ni 2p spectra of CuNiAl-LDHs, L-300 °C and L-500 °C (Fig.11(e)) show the characteristic peaks at 855.5 eV and 873.3 eV originate from Ni 2p3/2and Ni 2p1/2[40].Among them, the Ni 2p3/2of CuNiAl-LDHs catalyst has two peaks at 855.7 eV and 858.3 eV [41], the first peak is probably Ni3+and the other peak is Ni2+.The binding can be attributed to Ni cations or surface Ni(OH)2species [19].Table 2 shows that the ratio of Ni3+/(Ni2++Ni3+) gradually decreases with the increase of calcinating temperature, with a maximum of 65% for CuNiAl-LDHs.This indicates that the electrode surface is enriched with Ni3+metal ions, which facilitates the reduction of the energy barrier formed by CO*intermediates, improves the affinity between the hydroxyl groups and the catalyst, and ultimately increases the catalytic activity and greatly enhances the performance of CO2RR.

Table 2 The surface components obtained by XPS analysis

Fig.11.The XPS spectra of CuAl-LDHs,CuMgAl-LDHs and CuNiAl-LDHs(a)Cu LMM XAES,(b)O 1s.The XPS spectra of CuNiAl-LDHs,L-300°C and L-500°C.(c)Cu LMM XAES,(d) O 1s, (e) Ni 2p.
Fig.11(b)and(d)show the XPS spectra of O 1s for the catalysts.The O 1s of the catalyst was synthesized by splitting into three peaks, and the peak with binding energy at 530.1-531.0 eV can be ascribed to the catalyst surface lattice oxygen O2-[42],denoted as Oα;binding energies at 531.6-532.3 eV can be attributed to chemisorbed oxygen O-, O2-, and O22-, denoted as Oβ; the binding energy of 532.3-533.5 eV corresponds to the hydroxyl oxygen in the adsorbed water [43], denoted as Oγ.The calculated results of the corresponding characteristic peak area ratios(i.e.Oγ/(Oα+Oβ+Oγ)) are presented in Table 2.The ratios of Cu+/(Cu2++Cu++Cu0) at L-300°C and L-500°C are comparable,but the ratio of the hydroxyl peak at L-300 °C (26%) is larger than that at L-500 °C (16%).Combined with the electrochemical performance tests of both (Fig.8(b))it is speculated that—OH may be a favorable group for CO2RR.
3.10.Kinetic analysis (Tafel slope and EIS analysis)
To investigate the efficiency of the catalysts in terms of charge transfer kinetics, the EIS results of all electrocatalysts were measured in a three-electrode system.In Fig.12(a) and Fig.12(b), a semi-circle is discovered in the low frequency area, which indicates that the electrode surface reaction is the rate-limiting step of the whole electrochemical reaction.The smallest semicircle of the CuNiAl-LDHs catalyst indicates its high charge transfer efficiency, high electron conductivity, and low resistance.It shows that the CuNiAl-LDHs promotes the accelerated oxidation transformation of Ni II hydroxide to Ni III hydroxide,reduce the impedance of the electron exchange reaction, and increase the conduction of LDHs.This makes its contribution to the charge transfer performance in the CO2RR process outstanding.

Fig.12.Nyquist impedance plots of(a)CuAl-LDHs,CuMgAl-LDHs and CuNiAl-LDHs.(b)CuNiAl-LDHs,L-300°C and L-500°C.Tafel slope plot of the(c)CuAl-LDHs,CuMgAl-LDHs and CuNiAl-LDHs.(d) CuNiAl-LDHs, L-300 °C and L-500 °C.
An essential way for analyzing the kinetics of electrode reactions is the Tafel plot.As presented in Fig.12(c)and(d),the corresponding Tafel slopes for CuAl-LDHs, CuMgAl-LDHs, CuNiAl-LDHs,L-300 °C and L-500 °C were 128 mV·dec-1, 132 mV·dec-1,108 mV·dec-1, 122 mV·dec-1and 124 mV·dec-1, respectively.The results are approaching the theoretical value of 118 mV·dec-1,demonstrating that the fromation of CO2-intermediates is the rate-determining step [44,45].In other words, CO2adsorbs on the electrode surface and gets an electron to transform into the adsorbed CO-2, which further gets an electron and combines with the hydrogen ion in the electrolyte to produce the CH3OH.In addition, the CuNiAl-LDHs catalyst has the lowest Tafel slope, indicating that the LDHs structure makes the formation of CO2-easier.Thus,CuNiAl-LDHs can be considered to have a faster reaction rate in CO2RR.At the same time, the lower Tafel slope also reveals its higher intrinsic catalytic activity.And the effect is consistent with the overall activity described by the LSV curve.
In this work, the pristine CuNiAl-LDHs interface has higher Cu+content and more abundant—OH groups compared to the calcined catalyst, which effectively enhances the CH3OH produced by CO2reduction.It was shown in several studies that the —OH groups readily adsorb H*.Meanwhile, CO*had stronger adsorption on Cu+[15].This research gives a new perspective for the synthesis of high-performance copper-based catalyst for CO2RR generation of CH3OH.
4.Conclusions
In summary, CuAl-LDHs, CuMgAl-LDHs and CuNiAl-LDHs were prepared by means of co-precipitation method, and CuNiAl-LDHs were calcined at at 300 °C and 500 °C and applied to the CO2RR reaction.It was found that the optimal CuNiAl-LDHs has superior CH3OH selectivity compared to CuAl-LDHs and CuMgAl-LDHs,with the(FE)of 76.4%for CH3OH generation at-1.2 V.And their FE and current density(4.8 mA·cm-2)remained stable during up to 24 h of electrolysis.Meanwhile,this study confirms the significant performance advantages of CuNiAl-LDHs over their derived composite oxides.Series characterization further confirms that the excellent catalytic performance of CuNiAl-LDHs is strongly associated with the coordinated interaction of Cu and Ni in the LDHs laminate to induce the proper Cu+content and the structural hydroxyl group(—OH)of LDHs.And Cu+has a highly adsorptive effect on CO*,perhaps facilitating the formation of the inter-product CHO*, which accelerates the reduction of CO2to CH3OH.
Data Availability
No data was used for the research described in the article.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51978436, 22272116).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.06.022.
 Chinese Journal of Chemical Engineering2023年12期
Chinese Journal of Chemical Engineering2023年12期
- Chinese Journal of Chemical Engineering的其它文章
- Intrinsic kinetics of catalytic hydrogenation of 2-nitro-4-acetylamino anisole to 2-amino-4-acetylamino anisole over Raney nickel catalyst
- Experiments and model development of p-nitrochlorobenzene and naphthalene purification in a continuous tower melting crystallizer
- α-Synuclein: A fusion chaperone significantly boosting the enzymatic performance of PET hydrolase
- Influence of water vapor on the separation of volatile organic compound/nitrogen mixture by polydimethylsiloxane membrane
- Mass transfer mechanism and relationship of gas-liquid annular flow in a microfluidic cross-junction device
- Enhanced photocatalytic activity of methylene blue using heterojunction Ag@TiO2 nanocomposite: Mechanistic and optimization study
