AlCl3 modified Pd/Al2O3 catalyst for enhanced anthraquinone hydrogenation
Qinqin Yuan, Jingyue Liang, Wei Li, Jinli Zhang, Cuili Guo
School of Chemical Engineering and Technology, Tianjin University, Tianjin 300350, China
Keywords:AlCl3 Pd-based catalyst H2O2 Weak acid Hydrogenation
ABSTRACT Anthraquinone hydrogenation to produce H2O2 is an economically interesting reaction with great industrial importance.Here, we report a series of Pd/xAl catalysts with different AlCl3 contents by a conventional stepwise impregnation method.The optimal Pd/1.0Al catalyst exhibits a higher performance toward anthraquinone hydrogenation with 8.3 g·L-1 hydrogenation efficiency,99.5%selectivity and good stability, obviously superior to that of Pd/Al2O3 catalyst (5.2 g·L-1 and 97.2%).Detailed characterization demonstrates that AlCl3 can be grafted on the γ-Al2O3 support to obtain a modified support with abundant surface weak acid and Lewis acid, which can adsorb and activate anthraquinone.Meanwhile, its steric hindrance could isolate and disperse active metals to form more active sites.The synergies between metal sites and acid sites promotes the anthraquinone hydrogenation.Furthermore, the good stability after grafting AlCl3 could attribute to the enhanced metal-support interaction inhibiting metal particles agglomeration and leaching.
1.Introduction
Hydrogen peroxide(H2O2),a significant and valuable green product, can be widely applied in papermaking [1], disinfection [2],environmental protection [3], medicine [4], electronics [5] and so on.Currently, due to its low cost, renewable process and mature technology, the anthraquinone autoxidation is a more favorable route to produce H2O2[6,7].The worldwide H2O2production by this process reaches 98% [8,9].The production of H2O2by anthraquinone takes alkyl-anthraquinone derivatives (generally 2-ethyl-anthraquinone, denoted as EAQ) as raw materials.The process goes through four steps of hydrogenation, oxidation, extraction and purification.Among them, anthraquinone hydrogenation is a key step.It is a complex series-parallel reaction.Generally,EAQ is catalytically hydrogenated to generate 2-ethylanthradroquinone (EAQH2), and subsequent oxidized to obtain H2O2and regenerate EAQ for recycling.In addition, EAQH2would be further hydrogenated to generate 2-ethyl-5,6,7,8-tetrahydro-9,10-anthracenediol (H4EAQH2), H4EAQH2oxidation can also produce H2O2and H4EAQ for recycling.Here, EAQ and H4EAQ are called ‘‘active anthraquinone”.However, EAQH2and H4EAQH2would go through deep hydrogenation and form 2-ethylanthrone(EAN), 2-ethyl-octahydro-9,10-anthraquinone (H8EAQH2) and other byproducts.These byproducts are called ‘‘degradation products” because they are incapable of producing H2O2[10,11].The presence of degradation products would reduce activity and selectivity of catalyst and shorten life [12,13].
Pd/Al2O3has been widely applied in EAQ hydrogenation industry,but suffers from poor metal dispersion and deactivation due to Pd species agglomeration and leaching [14].In recent years, many measures have been taken to enhance the performance of Pd/Al2O3, for instance, doping second metal [15-17] and modifying support.As a prominent component of catalyst, the support has an important influence on the performance of the catalyst.Liang et al.[8] found that the Al2O3support pretreated by H2was rich in coordination unsaturated pentacoordinate Al3+sites, which not only anchored active metals and reduced Pd leaching and agglomeration,but also increased Pd dispersion.Bai et al.[12]synthetized a highly dispersed 0.3%Pd@Al2O3by microemulsion method,which inhibited Pd species migration and aggregation.Miao et al.[18]studied the interfacial effect on Pd/MgAl-LDH catalyst.The activity was improved by the remarkable enhanced hydrogen spillover at the interface structure.In addition, it is well known that catalyst acidity also plays a critical role in EAQ hydrogenation,which could adsorb EAQ and participate in the reaction[19,20].Feng et al.[21]found that the addition of SiO2to the Al2O3support increased the weak acid sites and metal dispersion.Li et al.[19] found that the grafting of 3-aminopropyltriethoxysilane on Al2O3support increased the weak acid of support and improved Pd dispersion.Wang et al.[20] utilized ionic liquid (IL) to improve the performance of catalyst.The IL prevented Pd species aggregation and provided tunable surface acid sites and Lewis acid sites, and then offer more active sites.Li et al.[22] found that the doping of P on Pd/P-Al2O3catalyst could adjust the surface acid intensity,improve the Pd dispersion and enhance the metal-support interaction.These results indicate that designing a support with rich weak acid can promote the EAQ hydrogenation.
Due to the favorable performance such as tunable acid properties and effective dispersion, AlCl3supported on Al2O3has been widely used as a promising support in many reactions such as alkylation, acylation, alkane isomerization, cracking and polymerization [23].Luo et al.[24] used AlCl3as a promoter to prepare Pt-Al/MCM-41 catalyst for tetralin hydrogenation.The grafting of AlCl3provided acid sites,increased metal dispersion and produced electron deficient Ptδ+species which improved activity and sulfurtolerance.Liang et al.[14]prepared AlCl3doped PdAl/AC-A catalyst for H2O2direct synthesis with high productivity.The addition of AlCl3not only increased the content of Pd2+, but also improved the dispersion of active metal.Researchers found that immobilized AlCl3catalysts had rich Lewis acid sites by chemical bonding,which showed high catalytic performance [23,25,26].Tsang et al.[27,28] prepared Al-doped carbon support for glucose isomerization to fructose, which promoted glucose isomerization through Lewis acid driven mechanism.
Herein, we attempt to design a series of AlCl3modified Pd/xAl catalysts for anthraquinone hydrogenation by producing more surface weak acid and Lewis acid sites.Simultaneously,the steric hindrance of AlCl3could isolate and disperse active metals.A combination investigation based on HR-TEM, in situ CO-FTIR, XPS,NH3-TPD and Py-FTIR reveals the influence of AlCl3on catalyst acidity and metal dispersion.This work provides a promising approach for EAQ hydrogenation.
2.Materials and Methods
2.1.Materials
Pseudoboehmite was supplied by Tianjin Chemical Research and Design Institute.Sodium tetrachloropalladate (Na2PdCl4,97%) was supplied by Saen Co., Ltd.(China).2-ethylanthraquinone (EAQ, 98%), tris(2-ethylhexyl) phosphate(TOP, 99%) and 1,2,4-trimethylbenzene (TMB, 98%) were supplied by TCI Co., Ltd.(China).Anhydrous aluminum chloride (AlCl3)was purchased from Aladdin Co., Ltd.(USA).Absolute ethanol(AR) was purchased from Tianjin Jiangtian Co., Ltd.(China).All chemicals and solvents were used directly.
2.2.Catalyst preparation
Calcining pseudoboehmite at 600 °C for 4 h to obtain γ-Al2O3support.The Pd/xAl catalysts were prepared by stepwise impregnation technique.First, a specific amount of AlCl3was dissolved in 40 ml absolute ethanol under the ultrasound.Then, 1.2 g of γ-Al2O3support was added and stirred at 45°C for 1.5 h.After filtering and washing with absolute ethanol, the mixture was dried at 60°C for 12 h.The AlCl3modified support was obtained by calcining the dried sample at 500 °C for 4 h, which is named as xAl(where x refers to the mass percentage of AlCl3).After that,0.0083 g of Na2PdCl4was dispersed in 40 ml absolute ethanol,1 g of modified support xAl was added, stirred at 45 °C for 1.5 h.After filtering and washing,the slurry was dried at 60°C overnight and then calcined at 500°C for 4 h.Finally,the Pd/xAl catalysts was obtained by reducing above sample at 500°C for 2 h under 10%H2/Ar.Theoretical mass percentage of Pd was all fixed at 0.3%, and x was 0.5, 1.0, 1.5 and 2.0, respectively.As a contrast, conventional Pd/Al2O3catalyst was prepared by the same steps without adding AlCl3.
2.3.Catalyst characterization
The texture properties of samples were tested by N2adsorption desorption at-196°C on a Quantachrome Autosorb iQ instrument.First, sample degassed at 150 °C for 4 h under vacuum.X-ray diffraction (XRD) was tested on a Smart lab using Cu Kα radiation(λ = 0.154 nm).The pattern was collected in the 2θ range of 15°-85°.Scanning electron microscopy (SEM) images was collected on a Apreo S LoVac, which has an acceleration voltage of 5.0 kV.Transmission electron microscopy (TEM) images and elemental mapping were carried out on a JEM-F200 with an accelerating voltage of 200 kV.H2-O2titration was performed to analysis the dispersion of Pd on a AutoChem II 2920 analyzer.After pretreating, Ar was purged to remove air and O2pulse was introduced to saturate the adsorption, Ar was purged again for 20 min, and then H2pulse was introduced to titrate the adsorbed oxygen.ICP was performed to analysis the content of Pd on an Agilent ICP-OES 730.In situ CO-DRIFTS was tested on Bruker Tensor FT-IR spectrometer.The catalyst was reduced at 500 °C for 2 h.10% CO/Ar was introduced for 1 h.The profile was recorded in the range of 2200-1700 cm-1.The valence state of Pd was analyzed by X-ray photoelectron spectroscopy(XPS)on ThermoFisher Science K-Alpha+with Al Kα radiation.NH3-TPD was performed on VDSorb-91i equipment.First, the sample was dried at 150 °C for 1 h and purged with 10%NH3/He until the adsorption was saturated.The profile was collected from 50°C to 800°C at 10°C·min-1under He flow.In situ pyridine-infrared (Py-IR) was recorded on Tensor 27 with a resolution of 4 cm-1.After being pretreated at 300 °C for 1 h in vacuum, the sample adsorbed pyridine at room temperature for 20 min, and heated to 150 °C, 200 °C and 300 °C for 30 min to collect spectra.Thermogravimetric analysis (TG)was carried out on Mettler Toledo TGA/DSC-2, the profile was recorded from 50 °C to 800 °C at 10 °C·min-1under N2flow.
2.4.Catalytic reaction
EAQ hydrogenation was carried out in a batch autoclave with 60°C and 0.3 MPa H2.The reaction conditions are the optimal conditions based on our previous research [8,22,29-31].The anthraquinone solution (120 g·L-1) was prepared with TOP and TMB as solvents (volume ratio 1:1).Generally, 0.6 g of catalyst and 60 ml of anthraquinone solution were added into the autoclave together,H2purged the air three times,and then temperature was raised to 60°C to start the reaction with an agitation speed of 1000 r·min-1.After 15 min, 2 ml of product was added into a separating funnel containing 2 ml of H3PO4(5 mol·L-1)and 20 ml of deionized water,then oxidizing it with O2for 30 min.After reaction, the solution was extracted and transferred to a conical flask with 5.0 ml of H2SO4(3 mol·L-1).The content of H2O2was calculated by titrating water layer with KMnO4solution.The hydrogenation efficiency(B,g·L-1) and H2O2yield (STY, g·g-1·h-1) are calculated as follows:
where CKMnO4is the concentration (mol·L-1) of KMnO4solution,VKMnO4is the volume (L) of consumed KMnO4solution, M is the molar mass of H2O2(g·mol-1), m is the Pd actual mass (g), V1and V are the working solution volume(L)of used for analysis and reaction and T is the reaction time (h).
In EAQ hydrogenation reaction,the contents of EAQ and H4EAQ were determined by liquid chromatograph with C18 separation column (Agilent HP1100 HPLC).The detector wavelength is 245 nm, and the mobile phase consisted of water and methanol(volume ratio 2:8).The selectivity of catalyst(S,%)is expressed as:
where nEAQand nH4EAQis the content of EAQ and H4EAQ in the anthraquinone solution after oxidation (mol·L-1), n0(EAQ)is the content of EAQ in the anthraquinone solution before hydrogenation(mol·L-1).
3.Results and Discussion
3.1.Catalytic performance
The catalytic performance of Pd/Al2O3and Pd/1.0Al catalysts was evaluated in an autoclave and the result is shown in Fig.1(a)and (b).Compared to Pd/Al2O3and Al/Al2O3catalyst, Pd/xAl catalysts exhibited remarkably hydrogenation efficiency as well as selectivity.With the increase of AlCl3,the hydrogenation efficiency and selectivity of Pd/xAl catalysts show a volcano trend.Pd/1.0Al catalyst displays the highest hydrogenation efficiency and active anthraquinone selectivity of 8.3 g·L-1and 99.46%, obviously superior to that of Pd/Al2O3(5.2 g·L-1and 97.2%)and Al/Al2O3(0.7 g·L-1and 94.8%).Moreover,the H2O2yield was calculated and shown in Table S1 in Supplementary Material.Pd/Al2O3catalyst shows a low H2O2yield (904.34 g·g-1·h-1), while the Pd/1.0Al has excellent H2O2yield (1276.92 g·g-1·h-1).Table S2 shows the performance comparison of different Pd-based catalysts in recent years,indicating that AlCl3modified Pd/xAl catalysts have a greater value.

Fig.1.(a) Hydrogenation efficiency and (b) selectivity of Pd/Al2O3, AlCl3/Al2O3 and Pd/xAl (x = 0.5,1.0,1.5,2.0) catalysts, (c) hydrogenation efficiency and (d) selectivity of Pd/Al2O3 and Pd/1.0Al catalysts in recycle run.(Reaction conditions: 0.6 g of catalyst, 60 ml of anthraquinone solution, 60 °C, 0.3 MPa H2, reaction time = 15 min).
Furthermore, we carried out six cycle runs for Pd/Al2O3and Pd/1.0Al catalysts under identical conditions as the first run.Fig.1(c) and (d) show the stability test results.After six cycles,Pd/1.0Al catalyst shows a slight loss in hydrogenation efficiency from 8.3 to 6.9 g·L-1, the selectivity remains above 99%.Pd/Al2O3catalyst decreases significantly from 5.2 to 2.1 g·L-1,and the selectivity remains above 92%.The decrease of activity after six runs may attribute to the loss and agglomeration of Pd species and deposition of byproducts.In conclusion, Pd/1.0Al catalyst shows superior catalytic performance and stability.
3.2.Structural characterization of catalysts
N2adsorption desorption was used to characterize the texture properties of samples and the result is shown in Fig.2(a) and (b).All supports and catalysts present an IV-type isotherm, indicating that samples have mesoporous structure,and have a H3-type hysteresis loop at P/P0>0.7.The pore diameter of all samples is about 10 nm.The detailed structure information is shown in Table 1.As the AlCl3addition increases from 0.5% (mass) to 1.5% (mass), the surface area and pore diameter of modified samples are slightly lower than γ-Al2O3and Pd/Al2O3, which is due to AlCl3and metal Pd occupying part of pore structure.The XRD patterns of samples are shown in Fig.2(c).The characteristic diffraction peaks at 19.34°, 31.86°, 37.6°, 39.5°, 45.8°, 60.7° and 67.0° corresponding to γ-Al2O3phase (JCPDS Card No.79-1558), which exists on all samples [32,33].It indicates that the crystal structure of γ-Al2O3does not change significantly after loading AlCl3and Pd.The diffraction peaks of AlCl3are invisible in the samples, which may be attributes to the interaction between AlCl3and γ-Al2O3.Moreover,the Pd species is also not observed,attributing to the low content and high dispersion.

Table 1 Texture properties of supports and catalysts

Fig.2.(a) N2 adsorption desorption isotherms, (b) pore size distributions curves and (c) XRD patterns of supports and catalysts.
Fig.3(a) and (b) display the SEM images of γ-Al2O3and Al/Al2O3.It is obvious that γ-Al2O3presents spherical particles with relatively smooth surface, however the surface of Al/Al2O3is rougher, indicating AlCl3is successfully loaded [24].In addition,Cl 2p XPS spectra of catalysts is collected in Fig.S1.There is an obvious peak of Cl on Al/Al2O3support.The element mapping diagram(Fig.3(c)-(g))directly reflects the well dispersion of Pd,Al,O and Cl elements on the catalyst surface.STEM-HAADF images are shown in Fig.4.Apparently, Pd species is more dispersed on Pd/xAl than Pd/Al2O3.The average Pd nanoparticles size of Pd/Al2O3catalyst is 2.16 nm, while that of Pd/0.5Al, Pd/1.0Al and Pd/1.5Al is 1.90 nm, 1.70 nm and 1.74 nm, respectively.The result shows that grafting of AlCl3can decrease the size of Pd nanoparticles and promote their dispersion, which is more favorable for EAQ hydrogenation.

Fig.3.SEM images of (a)γ-Al2O3 and (b) Al/Al2O3, (c) STEM image of Pd/1.0Al catalyst, (d)-(g) elemental mapping images of Pd (red), Al (blue), O (yellow), and Cl (green),respectively.

Fig.4.HAADF-STEM images and Size distribution of Pd nanoparticles (inset image) of (a) Pd/ Al2O3, (b) Pd/0.5Al, (c) Pd/1.0Al, (d) Pd/1.5Al.
The actual Pd loading of Pd/Al2O3, Pd/0.5Al, Pd/1.0Al and Pd/1.5Al catalysts measured by ICP are 0.23%, 0.27%, 0.26% and 0.26% (mass), respectively.The Pd dispersion was determined to be 84.46% for Pd/1.0Al and 71.96% for Pd/Al2O3by H2-O2titration.The result shows that the dispersion of Pd is enhanced after AlCl3grafted,which corresponds to the TEM results.This could attribute to the fact that AlCl3graft onto the γ-Al2O3support by chemical bonding with the hydroxyl groups on surface, and the steric hindrance of graft structure would separate the continuous Pd sites and prevent their sintering and agglomeration [24], which improves the dispersion of active metal.
3.3.The surface properties of catalysts
The surface geometric properties of catalysts were studies by in situ CO-DRIFTS measurement (Fig.5).The bands at 2170 cm-1and 2116 cm-1could be considered to the vibration of gaseous CO [34,35].The band at 2088 cm-1is attributed to linear adsorption of CO on highly dispersed low coordination Pd species [36-38].The band in the range of 1850-2000 cm-1is bridged and multi bonded CO on the continuous Pd sites [39].Table S3 shows that Pd/1.0Al catalyst has stronger CO adsorption bands and higher proportion of linear adsorption.Because of the relatively low adsorption enthalpy, products are easy to desorb from the low coordination Pd sites[18],and deep hydrogenation reactions often occur in continuous Pd sites.The increase of low coordination Pd sites in Pd/1.0Al catalyst improves the selectivity.Yang et al.[38]reported that the adsorption amount of CO was consistent with the dispersion of Pd.Therefore, the Pd/1.0Al catalyst has higher dispersion, which accords well with the H2-O2titration results.Furthermore,the linear CO band of Pd/1.0Al catalyst shifts slightly to higher wavenumber,indicating that the electronic density of Pd decreases [17,18], which implies an enhanced metal-support interaction.

Fig.5.CO-DRIFT spectra of Pd/Al2O3 and Pd/1.0Al.
XPS test(Fig.6)was employed to analyze the influence of AlCl3on the surface electronic structure.The peaks at 335.2 eV,336.9 eV,340.4 eV and 342.2 eV are assigned to 3d5/2and 3d3/2of Pd0andPd2+, respectively [17,40,41].With the increase of AlCl3, the Pd binding energy moves to higher value due to the decrease of Pd electron density.That is,electrons are transferred from Pd to modified support, it implies that enhanced metal-support interaction[20].This is beneficial to the desorption of the product and inhibits the deep hydrogenation[42].Furthermore,Table 2 lists the details of Pd 3d XPS.The content of Pd2+gradually increased with the increase of AlCl3.It implies that AlCl3can regulate the Pd species valence.Recently,many studies reported that catalyst with a moderate content of Pd2+exhibits the higher catalytic performance,Pd0and Pd2+play a synergistic effect [8,31,43].AlCl3is bonded to the support and forms a unique graft structure, which is conducive to disperse continuous Pd sites.Meanwhile, the special structure enhances the metal-support interaction, which is beneficial for the anchoring of active components.Therefore, the Pd/1.0Al catalyst shows superior performance.

Table 2 The relative contents of different valence Pd species

Fig.6.Pd 3d XPS spectra of (a) Pd/Al2O3, (b) Pd/0.5Al, (c) Pd/1.0Al, (d) Pd/1.5Al.
3.4.Acid properties of catalysts
To determine the acidity of catalysts, NH3-TPD and Py-FTIR measures were performed.The NH3-TPD result of catalysts was shown in Fig.7(a).The Pd/Al2O3catalyst shows an obvious weak acid desorption peak at 107 °C, another two broad peaks in the range of 200-450 °C and 500-750 °C, which are moderate and strong acid sites.In contrast,the Pd/xAl catalysts show two obvious weak acid desorption peaks at 118 °C and 227 °C, respectively.Table 3 lists the acid distributions result.The total acid content of Pd/xAl catalysts especially weak acid is remarkably improved,which attributed to the formation of Al-O-Al bond and acid centers by the interaction between AlCl3and Al2O3[14,23,24,26,28].However, the total acid content of Pd/xAl catalysts gradually decreases with the increase of AlCl3, which could be due to pores blockage and surface acid center coverage [44].

Table 3 Content and Distribution of acid sites on catalyst surface
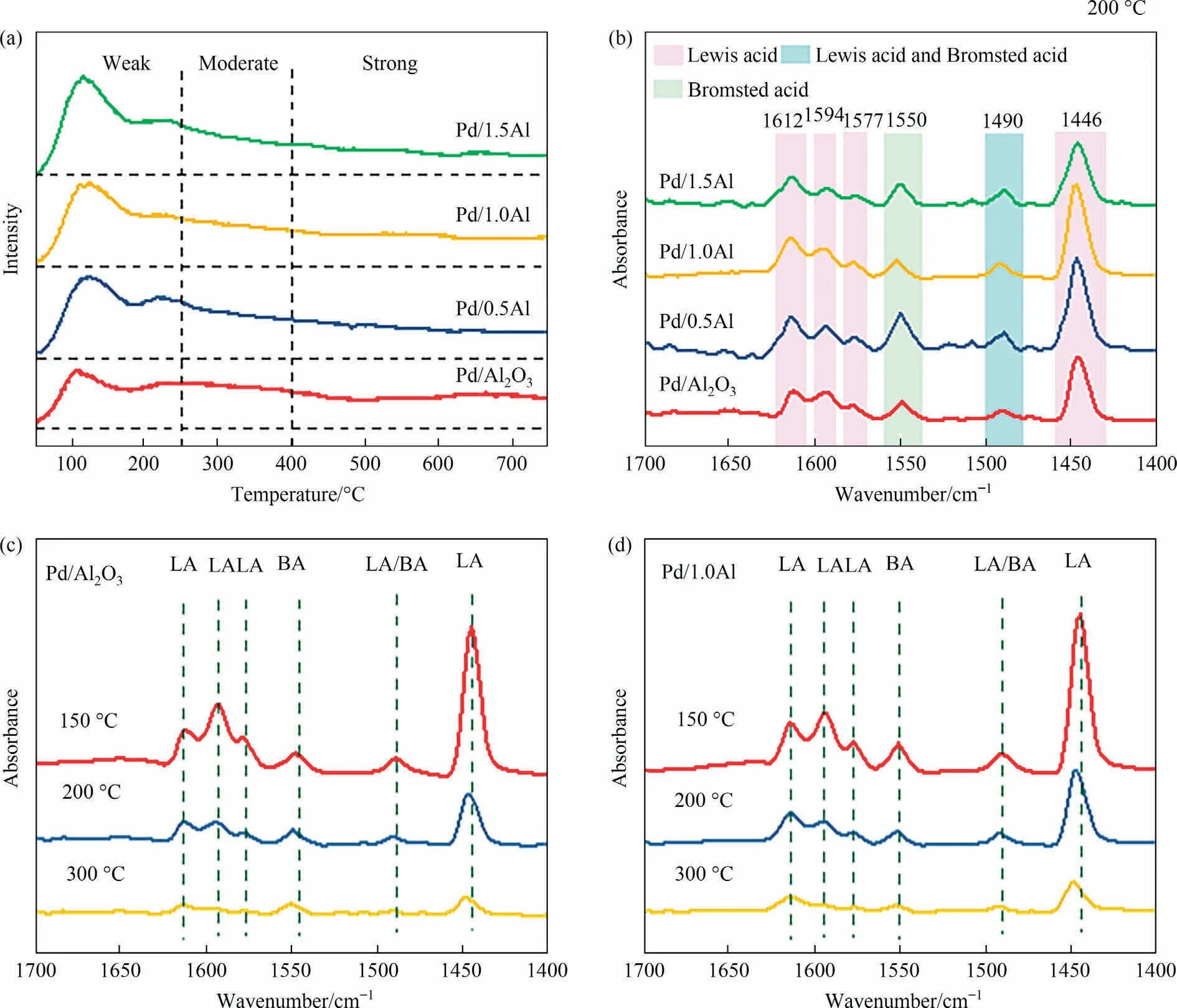
Fig.7.(a) NH3-TPD profiles, (b) Py-IR spectra of Pd/Al2O3 and Pd/xAl (x = 0.5, 1.0, 1.5) catalysts, in-situ Py-IR spectra of (c) Pd/Al2O3 and (d) Pd/1.0Al catalysts.
Fig.7(b) shows the Py-FTIR spectra of catalysts.The peaks at about 1446, 1577, 1594, 1612 cm-1are considered to be pyridine bonded to Lewis acid[45],the peak at about 1490 cm-1is attributed to Lewis and Brønsted acid[46],the peak at about 1550 cm-1is assigned to Brønsted acid[47].The result demonstrates that Pd/xAl catalysts contain Brønsted acid and Lewis acid, and Lewis acid is major acid centers.The Lewis acid content of Pd/xAl are significantly higher than Pd/Al2O3catalyst, and Pd/1.0Al catalyst holds the most Lewis acid sites,which enhanced the interaction between surface acidity and C=O on EAQ,and thus promotes hydrogenation reaction [20,28].Fig.7(c) and (d) shows the in-situ Py-FTIR results of Pd/Al2O3and Pd/1.0Al catalysts.With the desorption temperature increasing from 100°C to 300°C,the peak at 1446 cm-1declines sharply, indicating that Lewis acid is mainly weak and moderate acid.Based on previous studies, the weak acid sites on surface were favorable for the adsorption of EAQ,while the strong acid would lead to excessive hydrogenation due to strong adsorption,thus reduced the activity and selectivity of the catalyst[6,19-22,31,34].Therefore, the Pd/xAl catalysts provide unique adsorption sites for EAQ with moderate adsorption strength.At the same time, active metal promotes the activation of H2.The synergies between metal center and acid sites promotes the EAQ hydrogenation.
3.5.Deactivation analysis
To explore the reason of deactivation, the recycled catalysts were tested by N2adsorption desorption, ICP, TEM and TG.Fig.S2 and Table 4 shows the N2adsorption desorption results of recycled Pd/Al2O3and Pd/1.0Al catalysts.The pore diameter and volume of catalysts after cycling decreased slightly, which could attribute to the deposition of byproducts.Fig.8 shows the STEMHADDF images of spent Pd/Al2O3and Pd/1.0Al.After six cycles,the Pd/Al2O3catalyst shows partial metal aggregation,and the size increased from 2.16 to 2.94 nm.However,no obvious metal aggregation is observed on Pd/1.0Al catalyst.Table 4 shows the ICP-OES result of catalysts after six runs.After six cycles, the Pd content of Pd/Al2O3decreased from 0.23% (mass) to 0.16% (mass), while the Pd content of Pd/1.0Al catalyst maintains 0.26% (mass), consistent with fresh catalyst.These results show that introduction of AlCl3can prevent the loss and sintering of Pd particle.TG analysis results are shown in the Fig.S2.The main mass loss of unused catalysts is adsorbed water [8].For recycled catalysts, the mass loss at 200-500 °C is attributed to the combustion of carbonaceous deposits[42].The deposition amount of byproducts is calculated by mass loss of catalysts before and after cycling.The spend Pd/Al2O3and Pd/1.0Al catalysts have almost the same amount of byproduct deposition.Correlating with these characterization results, the Pd/Al2O3catalyst deactivation are assigned to the loss and agglomeration of Pd species and deposition of byproducts.However, that of Pd/1.0Al catalyst could attribute to the deposition of byproducts.The good recyclability of Pd/1.0Al catalyst could be explained by the enhanced metal-support interaction inhibiting the agglomeration and leaching of Pd species during reaction.

Table 4 Structural characteristics of the recycled catalysts
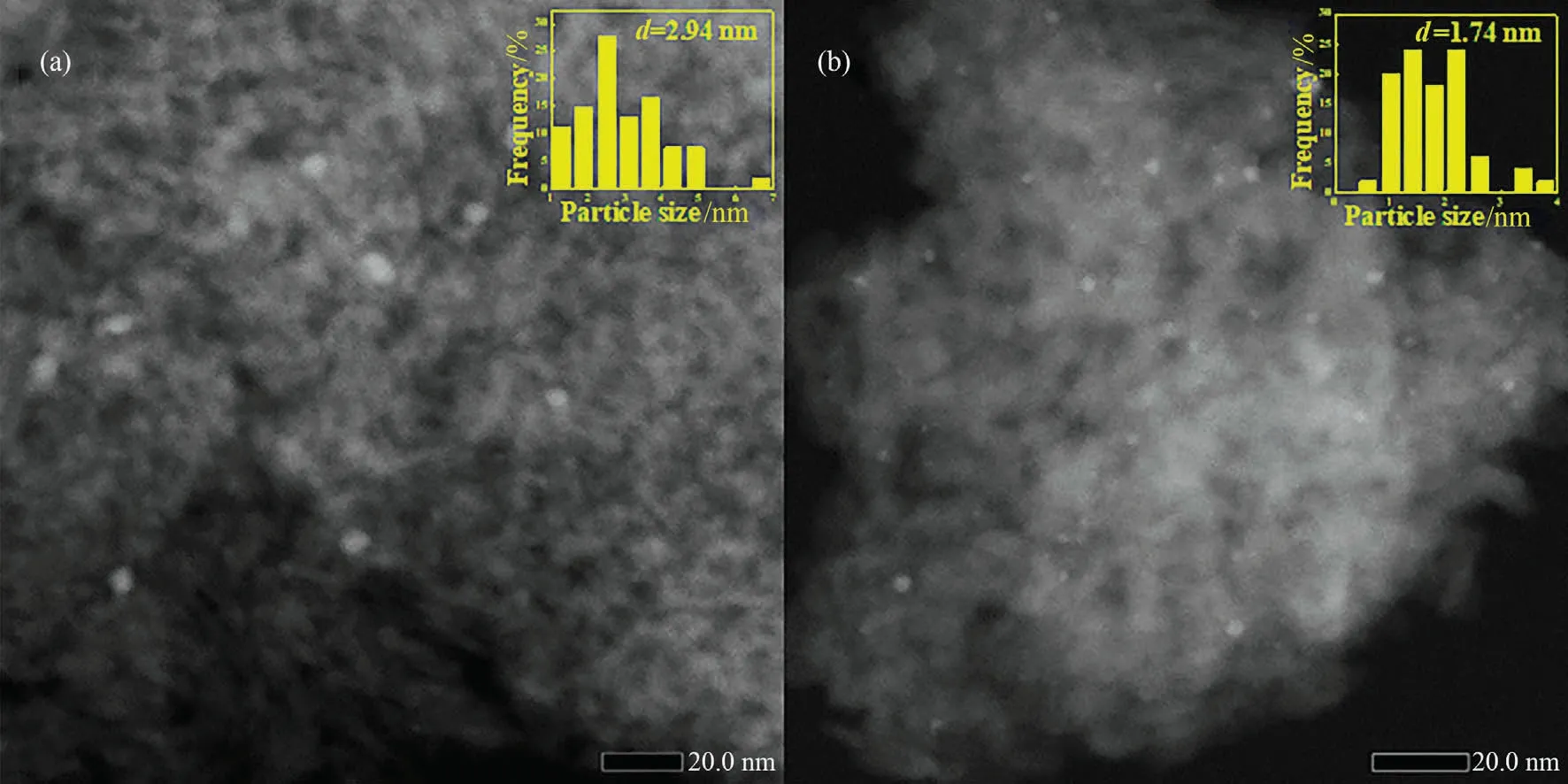
Fig.8.HAADF-STEM images and Size distribution of Pd nanoparticles (inset image) of (a) Pd/Al2O3-spent and (b) Pd/1.0Al-spent.
3.6.Regeneration of catalyst
The regeneration ability of catalyst is an important indicator for evaluating the quality of catalyst.The deactivation of Pd/1.0Al catalyst could attribute to the deposition of byproducts.The catalyst can be effectively regenerated by calcining to remove byproducts[42].TG result indicated that the mass loss of catalyst at 200-500°C is attributed to combustion of byproduct.To solve this problem,the Pd/1.0Al catalyst after six cycles was calcined at 500°C for 4 h, and then reduced again at 500 °C.The performance of regenerated Pd/1.0Al catalyst was evaluated.Table 5 shows that the hydrogenation efficiency of regenerated Pd/1.0Al catalyst was 6.6 g·L-1, which was slightly lower than that of the fresh catalyst.This indicates that the byproducts can be removed by calcination,but the performance of the regenerated catalyst was lower than that of the fresh catalyst.The regeneration ability of Pd/1.0Al catalyst needs further improvement.

Table 5 Hydrogenation efficiency of regenerated Pd/1.0Al catalyst
4.Conclusions
Here, we prepared a series of efficient and stable Pd/xAl catalysts with different AlCl3contents by a typical stepwise impregnation technique.With the increase of AlCl3, the hydrogenation efficiency and selectivity of Pd/xAl catalysts show a volcano trend.The optimal Pd/1.0Al catalyst with 1% (mass) AlCl3exhibits a higher performance with 8.3 g·L-1hydrogenation efficiency,99.5% selectivity and good stability, obviously superior to that of Pd/Al2O3catalyst (5.2 g·L-1and 97.2%).AlCl3acts as a promoter to create more surface weak acid and Lewis acid,which can adsorb and activate anthraquinone molecules as well as participate in hydrogenation reaction.Besides that, AlCl3can be grafted on the γ-Al2O3surface and its steric hindrance could separate active metals and prevent their sintering and agglomeration,which improves the metal dispersion and forms more active sites.The enhanced hydrogenation efficiency and selectivity are mainly attributed to synergistic effects between highly dispersed active sites and abundant weak acid sites.This work provides a way for H2O2producing from anthraquinone hydrogenation employing cheap AlCl3modified Pd/xAl catalysts and has an important industrial application prospect.
Data Availability
No data was used for the research described in the article.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21776215 and 21621004).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.06.021.
 Chinese Journal of Chemical Engineering2023年12期
Chinese Journal of Chemical Engineering2023年12期
- Chinese Journal of Chemical Engineering的其它文章
- Intrinsic kinetics of catalytic hydrogenation of 2-nitro-4-acetylamino anisole to 2-amino-4-acetylamino anisole over Raney nickel catalyst
- Experiments and model development of p-nitrochlorobenzene and naphthalene purification in a continuous tower melting crystallizer
- α-Synuclein: A fusion chaperone significantly boosting the enzymatic performance of PET hydrolase
- Influence of water vapor on the separation of volatile organic compound/nitrogen mixture by polydimethylsiloxane membrane
- Mass transfer mechanism and relationship of gas-liquid annular flow in a microfluidic cross-junction device
- Enhanced photocatalytic activity of methylene blue using heterojunction Ag@TiO2 nanocomposite: Mechanistic and optimization study
