Cardiovascular implications of inflammatory bowel disease: An updated review
Arshia Bhardwaj,Arshdeep Singh,Vandana Midha,Ajit Sood,Gurpreet Singh Wander,Bishav Mohan,Akash Batta
Abstract Emerging data highlights the heightened risk of atherosclerotic cardiovascular diseases (ASCVD) in patients with chronic inflammatory disorders,particularly those afflicted with inflammatory bowel disease (IBD).This review delves into the epidemiological connections between IBD and ASCVD,elucidating potential underlying mechanisms.Furthermore,it discusses the impact of current IBD treatments on cardiovascular risk.Additionally,the cardiovascular adverse effects of novel small molecule drugs used in moderate-to-severe IBD are investigated,drawing parallels with observations in patients with rheumatoid arthritis.This article aims to comprehensively evaluate the existing evidence supporting these associations.To achieve this,we conducted a meticulous search of PubMed,spanning from inception to August 2023,using a carefully selected set of keywords.The search encompassed topics related to IBD,such as Crohn’s disease and ulcerative colitis,as well as ASCVD,including coronary artery disease,cardiovascular disease,atrial fibrillation,heart failure,conduction abnormalities,heart blocks,and premature coronary artery disease.This review encompasses various types of literature,including retrospective and prospective cohort studies,clinical trials,meta-analyses,and relevant guidelines,with the objective of providing a comprehensive overview of this critical intersection of inflammatory bowel disease and cardiovascular health.
Key Words: Inflammatory bowel diseases;Cardiovascular disorders;Pericarditis;myocarditis;Thromboembolism;Chronic inflammation;Oxidative stress;Endothelial dysfunction
INTRODUCTION
Inflammatory bowel diseases (IBD),encompassing Crohn’s disease (CD) and ulcerative colitis (UC),are chronic inflammatory conditions affecting the gastrointestinal tract,characterized by a relapsing-remitting disease course.Extraintestinal symptoms may occur either concomitantly with or independently of luminal symptoms[1-4].An association has been established between cardiovascular disorders (CVD) and IBD,with a notably higher prevalence of CVD in IBD patients compared to the general population.The cardiovascular manifestations in IBD patients encompass pericarditis,myocarditis,venous and arterial thromboembolism,atherosclerotic CVD,heart failure,arrhythmias and conduction disorders,infective endocarditis,valvulopathy,and rarely,Takayasu arteritis[5-7].Potential mechanisms underlying CVD in IBD involve chronic inflammation,oxidative stress,altered platelet function,endothelial dysfunction,hypercoagulability,gut dysbiosis,and drug-related side effects[8].This review comprehensively synthesizes the latest evidence on the epidemiology,pathophysiological mechanisms,and cardiovascular manifestations in IBD.
PATHOPHYSIOLOGY OF CVD IN IBD
The intricate pathogenesis linking IBD and CVD remains an enigma,characterized by a complex interplay of diverse factors.Dysregulated immune responses,endothelial dysfunction,a pro-thrombotic state,accelerated atherosclerosis,and genetic polymorphisms collectively contribute to this intricate web connecting IBD with CVD.
Chronic low-grade inflammation,marked by alterations in both innate and adaptive immunity,plays a pivotal role in the pathogenesis of atherosclerotic CVD[9].In IBD,the stimulation of inflammatory T cell pathways,mediated by T helper (Th) 17 and Th1 responses fosters a pro-inflammatory milieu,leading to increased production of cytokines,including interleukin (IL)-1b,IL-6,IL-23,tumor necrosis factor (TNF),and interferon-gamma.Elevated expression of Tolllike receptors (TLR)-2 and TLR-4 further contributes to the pro-inflammatory state by amplifying IL-6 and IL-12 production[10,11].These pro-inflammatory cytokines,viaoxidative stress,provoke inflammation,tissue damage,and proliferation of endothelial and mesenchymal cells,synergistically contributing to the pathogenesis of CVD[12].Furthermore,TNF,IL-1,IL-6,vascular endothelial growth factor (VEGF),and reactive oxidative species promote endothelial dysfunction by increasing the expression of cell adhesion molecules like ICAM-1,MCP-1,E selectin and intensify endothelial cell apoptosis,micro-and macrovascular dysfunction,tissue remodelling,angiogenesis,lymphangiogenesis,and fibrosis[13-15].C-reactive protein (CRP),a marker of inflammation,is elevated in IBD and contributes to atherogenesis,correlating with increased CVD risk.Elevated CRP levels,especially exceeding 5 mg/L,serve as predictors of cardiovascular events.Notably,CRP levels rise with IBD disease activity,heightening cardiovascular risk during active disease[16,17].
Gut microbial dysbiosis,an important risk factor for IBD development,is also associated with CVD and increased thromboembolic event risk,particularly in younger age groups[12,18,19].Alterations in theFirmicutes/Bacteroidetesratio are linked to hypertension,while enrichment in Enterobacteriaceae,includingEscherichia coli,is observed in patients with IBD and CVD[20-23].Streptococcus spp.increase CVD risk,and opportunistic bacteria likeEnterobacterandOscillibacterare associated with ischemic stroke and transient ischemic attacks[24-26].
Gut dysbiosis can also increase gut permeability,leading to elevated absorption of lipopolysaccharide (LPS) from the intestines.The LPS,in turn,heightens pro-inflammatory cytokine secretion,exacerbating atherosclerosis,inducing macrophage activation,vascular endothelitis,and increasing CRP[27].Gut bacterial metabolites,such as indole and phenyl derivatives,also exacerbate atherosclerosis and lead to hypertension[26].Additionally,the gut bacteria-derived metabolite,Trimethylamine-N-oxide (TMAO),contributes to atherogenesis and hypertension,serving as a predictor of coronary artery disease.TMAO promotes platelet responsiveness,thrombosis,and cardiovascular risk through the expression of pro-inflammatory cytokines,ox-low density lipoprotein (LDL) deposition,and cardiac mitochondrial dysfunction[25,28,29].The drugs used for treatment in IBD,through various mechanisms,are also associated with cardiovascular side effects and are discussed in the subsequent sections[30].The pathogenesis of CVD in IBD is outlined in Figure 1.
CARDIOVASCULAR MANIFESTATIONS AND ITS MANAGEMENT IN IBD
Cardiovascular manifestations in IBD may be infrequent,yet they carry significant clinical implications when left unaddressed.We discuss the common CVD seen in patients with IBD.
Pericarditis and myocarditis
Pericarditis and myocarditis account for 70% and 10% of cardiac extra-intestinal manifestations (EIMs) respectively.Patients with IBD are at a greater risk of developing myopericarditis as compared to the general population[31,32].Notably,pericarditis displays a higher incidence in males with UC[30-32].On the other hand,myocarditis,constituting around 10% of cardiovascular EIMs in IBD,is more prevalent in patients diagnosed with CD[33].
Pathogenesis
It is difficult to determine whether the complications are secondary to the systemic disease or therapy related adverse events.Two possible mechanisms that are responsible for pericarditis and myocarditis in patients with IBD include immune mediated,secondary to the exposure of autoantigens,and cardiotoxicity associated with aminosalicylates and its derivatives[34-36].
Experimental models suggest that exposure to autoantigens produced during an acute flare of IBD,viainflammatory cytokines and an activated immune response,can lead to direct cytotoxicity of the cardiac myocytes[37].This process may involve both the myocardium and pericardium and lead to myopericarditis.Continued inflammation and remodeling may result in chronic myocarditis which may cause valvular abnormalities (viapapillary muscle fibroses and dysfunction),chamber dilation resulting in systolic dysfunction and decreased ejection fraction or arrhythmias[38,39].
Pericarditis almost exclusively occurs as a drug induced adverse event,in particular with 5-amino salicylic acid (ASA)derivatives such as sulfasalazine,mesalamine,and balsalazide[40-42].The underlying mechanisms responsible for pericarditis associated with mesalamine include IgE-mediated allergic reactions,direct cardiac toxicity,cell-mediated hypersensitivity,or a humoral antibody response against 5-ASA derivatives[43].
Clinical features and diagnosis
Patients with 5-ASA induced pericarditis usually develop symptoms within two weeks of initiation of therapy[44].Myopericarditis may present as acute coronary syndrome,new onset or decompensated heart failure,arrhythmias,cardiogenic shock or sudden death[45].The electrocardiogram may show ST segment and T wave changes or conduction disorders.Leucocytosis,elevated levels of erythrocyte sedimentation rate,CRP and cardiac biomarkers such as troponin,creatine kinase-MB,B-type natriuretic peptide and N-terminal pro-brain natriuretic peptide may be present[30,46].Echocardiographic features of myocarditis such as left ventricular dysfunction,anomalies of parietal kinetics,low ejection fraction,or pericardial effusion may be present.The cardiovascular magnetic resonance (CMR) imaging in a patient with myocarditis may reveal myocardial (regional or global) oedema,myocardial hyperaemia,and focal fibrosis or necrosis with non-coronary artery distribution[46].The endomyocardial biopsy is the gold standard but is seldom performed in view of its invasive nature and availability of non-invasive CMR.The endomyocardial biopsy is however indicated in patients where CMR is not feasible or in life threatening conditions to establish the diagnosis and aetiology of myocarditis[39].Histologically,two forms of IBD-associated myocarditis are known: The acute/chronic lymphocytic myocarditis and the giant cell myocarditis.Giant cell myocarditis is associated with a poor prognosis[47].
Management
The two major goals of treatment are optimal care of heart failure and arrhythmias,regardless of etiology and diseasespecific therapy.Patients with fulminant myocarditis and hemodynamic instability should be shifted to intensive care units (ICU) with facilities of advanced cardiopulmonary support such as mechanical ventilation and extracorporeal membrane oxygenation[36,48].
Discontinuation of the causative drug remains the mainstay of treatment for pericarditis and resolution occurs within 2 wk.For inflammatory myocarditis associated with IBD,immune-suppressive treatment should always be considered especially in the presence of ventricular systolic dysfunction and severe arrhythmias[49-52].The commonly used immune suppressive agents for treatment of inflammatory myocarditis are corticosteroids,azathioprine,cyclosporine,or immunoglobulins[53].Interestingly,these agents are also used for treatment of IBD and therefore no specific alteration in therapy may be required in majority of the patients.The current guidelines also discourage patients with myocarditis from participating in competitive and leisure sports[54].
If pericarditis arises as an EIM,steroids are indicated after ruling out sepsis[55-57].Alternatively,indomethacin,aspirin and colchicine can be used.However,their use can exacerbate underlying IBD and caution is recommended.Pericardial effusion and tamponade can complicate pericarditis which can be managed with pericardiocentesis or pericardiectomy[30,58].

Figure 1 Factors implicated in development of cardiovascular disease in patients with inflammatory bowel disease. JAK: Janus kinase;ASA:Amino salicylic acid;TNF: Tumor necrosis factor;CVD: Cardiovascular diseases;IBD: Inflammatory bowel disease;VEGF: Vascular endothelial growth factor;IFN-γ:Interferon-gamma;CRP: C-reactive protein;S1P: Sphingosine-1-phosphate;IL: Interleukin.
VENOUS THROMBOEMBOLISM (VTE)
IBD patients are at an increased risk for VTE.Systematic reviews and meta-analyses report that patients with IBD are at a two-fold increased risk for VTE as compared to general population (RR=2.20;95%CI: 1.83-2.65)[59,60].The most common reported VTE events include deep vein thrombosis (DVT) and/or pulmonary embolism (PE).The involvement of portal,the superior mesenteric,the splenic,the internal jugular,and the cerebral veins has also been reported[61].The reported frequency is higher in patients with active IBD and directly proportional to the extent and severity of the disease in the absence of provoking factors[62].In a retrospective study,VTE was more common in patients with UC (pancolitis more than left sided colitis or proctitis).In CD,VTE is more frequent in patients with ileocolonic or colonic involvement than with ileal disease alone[62].
The risk of in-hospital and post-hospitalisation VTE in patients with IBD is increased with intestinal or non-intestinal surgery when compared to non-IBD patients (OR=2.03;95%CI: 1.52-2.70 and OR=4.45;95%CI: 1.72-11.49,respectively).The risk factors for VTE include emergency surgery,open procedure,longer operative time,ileostomy formation,anastomotic leak,ileus,diagnosis of UC (higher risk as compared with CD),age >65 years,and obesity[59,63,64].Patients with IBD are also at a significantly high risk of recurrent VTE (HR=2.5;95%CI: 1.4-4.2;P=0.001)[65].
Pregnant females with IBD (UC >CD,active disease) are also at two to three times increased risk of VTE during pregnancy and postpartum period (RR=2.13;95%CI: 1.66-2.73 and RR=2.61;95%CI: 1.84-3.69,respectively)[66,67].
Pathogenesis
The pathogenesis of VTE in IBD patients is multifactorial.The various mechanisms that contribute to thrombosis in IBD include genetic predisposition,inflammation,gut dysbiosis,spontaneous platelet aggregation,vascular thrombotic events secondary to flares,surgery,drug therapy and compounding risk factors such as pregnancy.Altered intestinal microbiota reduces mucus secretion and fibre fermentation that promotes inflammationviaendothelial damage[68].Genetic mutations in NOD2,ATG16L1,recombination activating gene 2,IL-10 receptor deficiency,and nuclear factor kappa beta essential modulator also lead to a pro-inflammatory state[36,69-71].Inflammatory cytokines such as TNF and IL-1 result in a prothrombotic state due to increased levels of thrombin (which initiates the coagulation cascade through tissue factor) and simultaneous suppression of antithrombotic factors (such as endothelial thrombin and protein C)[72,73].Another contributory mechanism is hyperhomocysteinemia secondary to inflammation induced malabsorption,and vitamin B and folate deficiency.Increased factor V thromboxane A2,arachidonic acid peroxidation product 8-isoprostaglandin F2,tissue factor and mRNA synthesis further promote platelet activation and inhibit thromboregulation[74,75].Estrogen based oral contraceptives and hormone replacement therapy promote production of coagulant factors leading to increased risk of VTE[76,77].There is increased production of fibrinogen and decreased production of protein S during pregnancy which increases the risk of VTE[78].
IBD drugs and impact on VTE
5-ASA:No VTE or related complications have been reported with 5-ASA.In vitro studies have shown that 5-ASA inhibits platelet activation by thrombin,and therefore could have a role in preventing VTE.However further studies are required to evaluate the beneficial effect of 5-ASA in reducing the VTE risk[79-81].
Corticosteroids:Corticosteroids are potent anti-inflammatory drugs that also exert an independent thrombogenic effect (viaincrease in the serum levels of the clotting factors and fibrinogen)[82].Systemic glucocorticoids and endogenous production of cortisol are associated with an increased risk of VTE.The risk of VTE is increased patients who are treated with corticosteroids,more so with higher doses [incidence risk ratio (IRR)=2.31,95%CI: 2.18-2.45][83,84].
Immunemodulators:There has been no reported risk of VTE with immunomodulatory drugs[85-87].Although,it has been hypothesized that thiopurines reduce VTE risk by decreasing platelet aggregation and inhibiting platelet-leucocyte aggregation in vitro,more studies are required to confirm this hypothesis[88].
Biologics:TNF-α directly promotes endothelial dysfunction resulting in increased thrombus formation[89].Patients treated with anti-TNF-α agents are therefore likely to have a decreased risk of VTE (OR=0.267;95%CI: 0.106-0.674,P=0.005)[83].It has been demonstrated that clot lysis profile normalises and there is a reversal of clotting abnormalities in patients receiving infliximab,suggesting benefit to reduce the VTE risk[63,72,82,90].
The overall risk of VTE with vedolizumab is low[91,92].Pooled safety analysis from Phase 2/3 studies on ustekinumab reported no significant difference in VTE risk in patients treated with ustekinumab compared to placebo (0.75/100 person yearsvs0.34/100 person years,respectively)[93-95].
Janus kinase (JAK) inhibitors:A safety study done in patients older than 50 year with rheumatoid arthritis and more than one cardiovascular risk factor showed that VTE,DVT and PE was higher for tofacitinib when used in the dose of 10 mg twice daily[95].Similar to these observations,in the OCTAVE open study,with a follow-up of 7 years,10 mg tofacitinib group had 0.1% and 0.7% prevalence of DVT and PE,respectively [IR=0.06 (95%CI: 0.00-0.31) and 0.28(95%CI: 0.09-0.65)].There were no cases of DVT or PE in 5 mg tofacitinib group.Overall IR for tofacitinib was 0.06(95%CI: 0.00-0.31) and 0.28 (95%CI: 0.09-0.65),for DVT and PE,respectively.Majority of the patients with thromboembolic complications had one or more underlying risk factors for DVT,except one patient with no pre-existing risk factors[96].In IBD,therefore,tofacitinib appears to have an acceptable safety profile from VTE point of view,though The United States Food and Drug Administration has issued a black box warning recommending avoidance of JAK inhibitors in patients at risk of DVT,VTE and PE[97].These risk factors include history of recent surgery,trauma,stroke or myocardial infarction (MI) in previous 3 mo,age >50 years,morbid obesity,use of oral contraceptive pills,long flights and previous history of DVT,PE or acute thromboembolic event[98].In case,when no therapeutic alternatives are available,a close coordination with cardiologist is required.The 10 mg twice daily dose is restricted to a maximum of 3 mo (for induction of remission) with de-escalation to 5 mg twice daily as soon as possible[99].The randomized controlled trials (RCTs) of upadacitinib and filgotinib did not report a higher rate of VTE[100,101].
Management
All patients with IBD hospitalised for any cause,should receive a prophylactic dose of low-molecular-weight heparin(LMWH) or fondaparinux.LMWH is recommended over unfractionated heparin in critically ill patients[102].Thromboprophylaxis during hospitalisation reduces the risk of VTE in IBD after discharge by 54% and should be maintained during the inpatient period[103].Older age,Clostridioides difficileinfection in index admission,longer hospital stay (>7 d),ICU admission,previous VTE,and coronavirus disease are indications of extended prophylaxis (at least 2 mo after discharge)as these conditions are associated with increased the risk of post-discharge VTE[104].IBD patients treated in the outpatient settings with moderate to severe flare and a high risk profile for VTE may benefit from thromboprophylaxis until resolution[105].
Guidelines recommend that the treatment of VTE should follow the general antithrombotic therapy guidelines.Direct oral anticoagulants are first line drugs and should be used at a therapeutic dose in IBD,LMWH is an alternative.In case of unprovoked VTE,the duration of treatment is indefinite.For provoked VTE secondary to an identifiable risk factor,anticoagulation is continued for 3 mo beyond the resolution of the risk factor.It is essential to know that thromboprophylaxis does not increase the risk of further IBD-related gastrointestinal bleeding in patients with active disease.It is important to remember that controlling the disease activity is most critical to prevent the recurrence of VTE[102,105,106].The duration of anticoagulation is summarized in Figure 2.
ATHEROSCLEROTIC AND ATHEROTHROMBOTIC CARDIOVASCULAR DISEASE (ASCVD)
In addition to VTE,there is a moderate increase in the risk of arterial thrombotic events,such as acute myocardial infarction (MI),mesenteric ischemia,and stroke,in IBD,albeit lower than the VTE risk.Interestingly,this risk is comparable between UC and CD patients[14].The risk of ischemic heart disease (IHD) is slightly elevated in younger age groups and women,peaking within the first year of IBD diagnosis[12,13,107,108].
Pathogenesis
The pathogenesis involves a multifaceted interplay between inflammatory cytokines,endothelial dysfunction,smooth muscle proliferation mediated by VEGF,ICAM-1,MADCAM-1,E-selectin,reduced vasodilator nitric oxide,and NOD2 polymorphisms[106].

Figure 2 Duration of anticoagulation in inflammatory bowel disease patients with venous thromboembolism. IBD: Inflammatory bowel disease;VTE: Venous thromboembolism.
The incidence of CVD and cerebrovascular accidents is higher in females with IBD,and could be due to inherent differences in distribution of risk factors in males and females,including greater immune response and higher levels of CRP in females[109,110].The role of sex hormones in the development of ASCVD in IBD patients remains inconclusive.Moreover,younger IBD patients exhibit an increased relative risk of ASCVD,possibly stemming from earlier disease onset and a more severe disease course that results in prolonged exposure to chronic inflammation.Notably patients with IBD have similar prevalence traditional risk factors for coronary artery disease such as hypertension,diabetes,smoking,and obesity[111] (Figure 3).
Despite the lack of conventional risk factors for coronary artery disease,both UC and CD are independently associated with an increased risk of acute MI[112].The IBD patients with CVD have been reported to have a higher level of high sensitive-CRP and fibrinogen,and greater prevalence of NOD-2 mutations[113].Paradoxically,IBD patients tend to have lower levels of total cholesterol and LDL-cholesterol,with unaffected high density lipoprotein (HDL)-cholesterol and triglyceride concentrations.In CD patients,ileal resection and ileoanal anastomosis are inversely correlated with plasma total cholesterol and LDL-cholesterol levels[114,115].
The overall level of serum lipids in IBD patients is negatively associated with disease severity[113,116].On the contrary,disease activity has been reported to be an independent risk factor for development of CVD[117-119].This quandary is explained on the basis of presence of a more pro-atherogenic lipid profile characterized by small dense LDLcholesterol particles and dysfunctional HDL-cholesterol in chronic inflammation associated with IBD[120].The extent and location of inflammation is also associated with CVD risk.Patients with colonic involvement,in both UC and CD,have a threefold higher risk of developing MI[112].
Subclinical atherosclerosis
The occurrence of subclinical atherosclerosis is more frequent in individuals with IBD.To identify subclinical atherosclerosis,various diagnostic measures are employed,including assessing arterial stiffness through pulse-wave velocity between the carotid and femoral arteries (carotid-femoral pulse wave velocity,calculated asΔdistance/Δtime),measuring carotid intima-media thickness,evaluating flow-mediated dilation of arteries,and determining the coronary artery calcium score[106].
IBD drugs and impact on ASCVD
5-ASA:As with nonsteroid anti-inflmmatory drugs such as aspirin,5-ASA shares anti-inflammatory,anti-platelet and antioxidant properties.Thus 5-ASAs may be associated with a decreased risk of IHD in patients with IBD[121,122].IBD patients using 5-ASA were reported to have a lower risk of IHD than non-users (IRR=1.16;95%CI: 1.06-1.26 and IRR=1.36;95%CI: 1.22-1.51P=0.02,respectively).In long-term users of 5-ASA,the risk of IHD was even lower (IRR=1.08;95%CI: 0.98-1.19)[103,123].
Corticosteroids:Corticosteroid users are at a higher risk of developing IHD compared to non-users[123-125].Corticosteroids predispose to risk factors such as hypertension,obesity,dyslipidemia and insulin resistance which may exacerbate IHD in IBD[126].However,a direct causal association cannot be established.
Thiopurines:Thiopurines are not associated with acute arterial events in IBD and the effect of methotrexate on IHD in IBD is unknown.However in a beneficial effect on arterial stiffness has been demonstrated in various other chronic inflammatory disorders[127,128].Thiopurines also decrease the production of transforming growth factor-beta and IL-10,which are responsible for endothelial dysfunction,and hence may have some protective role,though there is very limited data to make any conclusive recommendations at the moment[129,130].

Figure 3 Prevalence of risk factors for atherosclerotic cardiovascular disease in patients with inflammatory bowel disease.
Biologics:In vitrostudies on infliximab have suggested an atheroprotective effect in monocytes by increasing both ABCA1 and LXR gene expression and removing excess cholesterol and preventing foam cell formation[127,129,131].However,thein vivobiological mechanisms are very complex.TNF-α is proatherogenic.Contradictory results have been reported with regards to the effect of anti TNF-α agents on the lipid profile.While some studies report an increase the levels of HDL-cholesterol and apoprotein-A1,others report an increase in the small dense LDL-cholesterol and total cholesterol.Also,TNF-α inhibition increases abdominal fat,leading to increased risk of ASCVD.On the contrary,the anti TNF-α agents exert beneficial effect by improving insulin sensitivity,endothelial function,arterial stiffness and fibrinolysis[89,132,133].The anti-TNF-α agents may be associated with reduced risk of new-onset acute arterial events and prevent recurrence when used in patients with previous history of acute arterial events.Vedolizumab and ustekinumab have not reported any augmented risk of ASCVD[134,135].
JAK inhibitors:Small molecules tend to increase the risk of cardiovascular diseases by causing dyslipidaemia.Tofacitinib is associated with reversible changes in the lipid profile specifically total cholesterol,HDL-cholesterol and LDLcholesterol[136,137].Clinical trials in rheumatoid arthritis showed that tofacitinib is associated with higher rates of major adverse cardiovascular events (MACE).Older patients aged >50 years with at least one cardiovascular risk factor had higher risk of MACE (death from cardiovascular causes,nonfatal myocardial infarction,or nonfatal stroke) as compared with anti TNF agents (HR=1.33;95%CI: 0.91-1.94)[138].However,the same has not been shown in the clinical trials in IBD.In the OCTAVE trials,one patient with several risk factors had acute coronary syndrome,one died of dissecting aortic aneurysm and one patient with a history of cardiovascular disease had congestive heart failure.During the maintenance phase,one subject with several risk factors receiving tofacitinib 5 mg twice daily had an adjudicated MACE(myocardial ischaemia/ myocardial infarction),and one patient,also with multiple risk factors,receiving tofacitinib 10 mg twice daily had an adjudicated MACE (haemorrhagic stroke).The overall incidence rate for MACE in the OCTAVE trials was 0.16 (95%CI: 0.04-0.42).Multiple real life studies of tofacitinib also did not demonstrate increase in the risk for MACE compared to anti TNF agents[96,139,140].
In a meta-analysis assessing safety of JAK inhibitors in IBD and other immune-mediated inflammatory diseases evaluated MACE in 32765 patients on JAK inhibitors (17 tofacitinib;6 upadacitinib;4 baricitinib;3 filgotinib),the incidence rate of MACE was 0.67 per 100 patient-years[141].Real life safety data on upadacitinib and filgotinib are lacking,however in the registry trials,MACE were infrequent and no difference was reported compared to placebo[142,143].Though the risk of MACE appears low,JAK inhibitors should be used cautiously in patients over the age of 50 years with concomitant risk factors for CVD.
Management of ASCVD
Current risk assessment tools for predicting CVD,such as the Framingham risk score and the ASCVD risk calculator,lack validation for individuals with chronic inflammatory conditions,potentially leading to an underestimation of their CVD risk.European guidelines suggest incorporating a 1.5-fold multiplier when assessing the 10-year CVD risk in patients with rheumatoid arthritis.However,there remains an information void regarding whether a similar adjustment is warranted for patients with IBD.
Controlling inflammation in IBD is the key to reduce the risk of CVD[105].Adequate treatment of underlying IBD with the aim to achieve and maintain remission is important.Additionally,the patients should be screened for atherosclerotic risk factors such as obesity,smoking,hypertension,diabetes,dyslipidaemia and positive family history[144].Definitive role of statins in IBD is controversial,but statins in addition to the lipid lowering function have pleiotropic effects,including modulation of the immune system[145,146].IBD is not a contraindication to low-dose aspirin for primary and/or secondary prevention.
HEART FAILURE
The risk of heart failure is twice as higher in IBD than non IBD subjects when adjusted for traditional cardiovascular risk factors with the highest risk reported in females with UC[112].
Heart failure in individuals with IBD may manifest as either new-onset (de novo) or as a consequence of deteriorating health in those with pre-existing conditions.These underlying conditions,which predispose individuals to heart failure,encompass IHD,hypertension,dyslipidemia,diabetes,smoking,valvular heart disease,congenital heart disease,etc.The frequent triggers for decompensation leading to heart failure involve infections or inadequate adherence to prescribed treatment regimens.
Pathogenesis
Heart failure in IBD could be a consequence of the chronic inflammation or the drug therapy used.The compromised integrity of the intestinal barrier and ongoing intestinal inflammation are contributing factors to the development of heart failure.This can be attributed to the translocation of bacterial LPS,which triggers the production of TNF-α.Both LPS and TNF-α are implicated in inducing structural changes in the heart that progress to heart failure[147,148].Additionally,several other proposed mechanisms may contribute to the development of heart failure in these patients.These mechanisms include myocardial fibrosis due to altered collagen metabolism,impaired nitric oxide-mediated vasodilation,deficiencies in essential vitamins and trace elements,heart muscle atrophy resulting from prolonged corticosteroid use,total parenteral nutrition,myocarditis,endocarditis,and valvulopathy[30,149-151].
Anti-TNFs and heart failure
There have been case reports and studies of anti TNF induced heart failure[152,153].The biological effects of TNF-α are mediatedviatwo distinct cell surface receptors.TNFFR-1 is cardiotoxic and antagonising its action attenuates ventricular dysfunction and improves post MI survival whereas TNFR2 is cardioprotective and its inhibition upregulates TNFR1 and increases ventricular dysfunction and remodelling[154,155].The effects of TNF-α are concentration dependent and involve two pathways.In lower concentrations,survivor activating factor enhancement pathway is activated,while higher concentration leads to stimulation of death-promoting pathway functions[156,157].Chunget al[158] evaluated the effect of infliximab in patients with New York heart association (NYHA) Class III or IV heart failure with ejection fraction≤ 35% and found that patients in the 10 mg/kg infliximab group were more likely to die or be hospitalized for heart failure than patients in the placebo group or 5 mg/kg infliximab group (HR=2.84,95%CI: 1.01-7.97;P=0.043).
Prevention
Routine screening tests for cardiovascular diseases prior to the administration of biologics is not recommended.However,employment of an echocardiogram prior to initiation of anti TNF therapy to evaluate baseline cardiac function is vital[105,157].Although there are no specific guidelines for the use of anti TNF in heart failure,it is suggested to avoid anti TNF agents in patients with NYHA class III or IV disease and switching to an alternative non-TNF inhibitor in patients with patients who develop acute heart failure on anti TNFs[105,157,159,160].
ARRHYTHMIAS AND CONDUCTION DISORDERS
As with other chronic inflammatory disorders,IBD carries a risk of major cardiac arrhythmias,which include atrial fibrillation,atrial flutter,ventricular tachycardia,and ventricular fibrillation.The risk of arrhythmias correlates with the disease activity[43,161].A large population-based cohort study found that risk of atrial fibrillation was increased in patients with IBD with a higher risk in CD and was particularly increased in younger patients with age <45 years[21].
Pathogenesis
Although the pathogenesis of arrhythmias is incompletely understood,chronic inflammation is hypothesized to predisposes to rhythm disorders and conduction abnormalities in patients with IBD[30,162].Also,patients on systemic steroids,immunomodulators or biologics had a higher risk,highlighting the role of moderate-to-severe active disease.Atrial electromechanical conduction delay,a predictor of atrial fibrillation,has been shown to be significantly prolonged in patients with IBD,especially those with active disease and longer disease duration.
IBD drugs and arrhythmias
Sphingosine-1-phosphate (S1P) receptor modulators (ozanimod) have been implicated in cardiac arrhythmias.S1PRMs have 5 G protein coupled receptor subtypes S1PR1 to S1PR5.The S1PR1,which is extensively expressed on cardiomyocytes and vascular endothelial cells,is the target of S1P modulators.In phase 3 RCT of ozanimod in UC,five cases of bradycardia were reported during the induction period and none during the maintenance period[163-165].In the TOUCHSTONE open label long term extension study of ozanimod 1 mg per day in patients with UC,no bradycardia nor evidence of atrioventricular (AV) block was reported at 44 wk[163,166].In the OASIS trial,a phase 2 induction trial of etrasimod which selectively target S1P1,S1P4,and S1P5,no such cardiac events were reported[167,168].In the ELEVATE UC study,5 patients receiving etrasimod reported bradycardia and 1 patient had first degree AV block that resolved without interventional treatment.The real-world studies,though scarce,did not report cardiac conduction abnormalities after 26 wk of treatment exposure of ozanimod.
Recommendations for patients with IBD to mitigate cardiovascular risk
The probability of experiencing cardiovascular events is inherently intertwined with the presence of systemic inflammation and the level of disease activity in individuals with IBD.It is imperative to adopt a proactive approach by conducting regular screenings and monitoring of cardiovascular risk factors for all IBD patients.Those identified as being at risk should adhere to established recommendations applicable to the general population.Collaborative efforts with cardiologists are vital in managing these risks effectively.Considering that these risk factors may evolve over time,especially with advancing age,routine screening and monitoring are indispensable for sustaining optimal cardiovascular well-being.It is of paramount importance to provide counseling and education to patients regarding their specific cardiovascular risks.Encouraging the adoption of healthy lifestyle modifications is crucial in this regard[169].A concise summary of practical guidance for managing CVD in individuals with IBD is presented in Table 1.
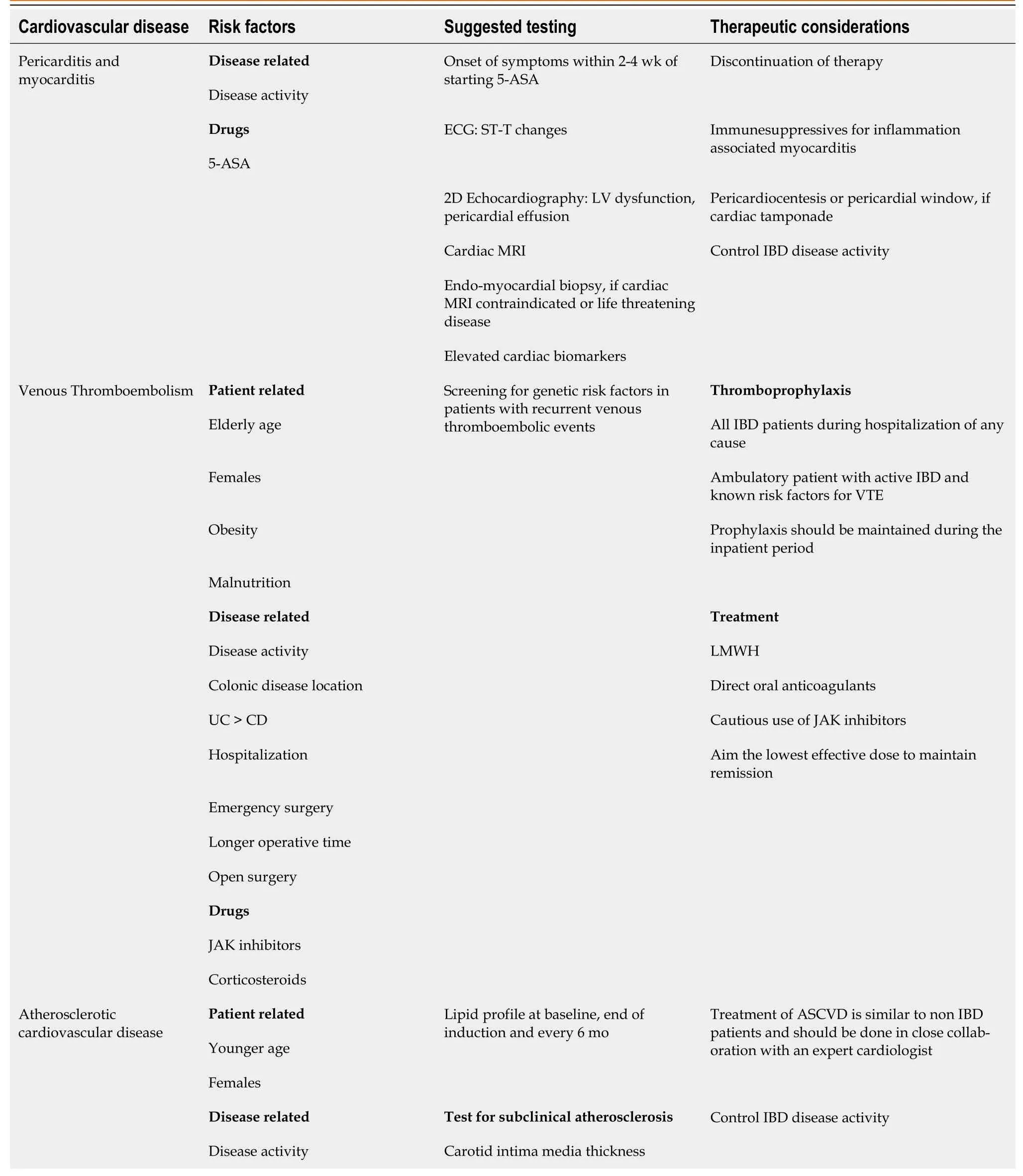
Table 1 Practical guide to management of cardiovascular diseases in inflammatory bowel disease

ASA: Amino salicylic acid;ECG: Electrocardiogram;LV: Left ventricular;MRI: Magnetic resonance imaging;IBD: Inflammatory bowel disease;CD:Crohn’s disease;UC: Ulcerative colitis;VTE: Venous thromboembolism;LMWH: Low-molecular-weight heparin;JAK: Janus kinase;ASCVD:Atherosclerotic cardiovascular diseases;CRP: C-reactive protein;TMT: Treadmill test;TNF: Tumor necrosis factor;NYHA: New York heart association;S1P: Sphingosine-1-phosphate.
Future directions
The pathophysiology of CVD in IBD needs further elaboration.The current knowledge gaps include the following:Immunological mechanisms at play in both the development of IBD and the formation of atherosclerosis,prevalence of cardiometabolic risk factors,risk stratification and identification of IBD patients at the highest risk for cardiovascular complications,allowing for more targeted preventive measures,role of pre-emptive screening for subclinical atherosclerosis and its cost effectiveness,long term outcomes in patients with CVD and IBD,and effective strategies for monitoring cardiovascular risk factors in IBD patients,and how often should such monitoring occur.
CONCLUSION
The prevalence of cardiovascular manifestations in patients with IBD,though rare,is higher when compared to the general population.The CVD in IBD represent a complex and multifaceted relationship between chronic gastrointestinal inflammation and cardiovascular health.The inflammatory cytokines,immune responses and chronic systemic inflammation associated with disease activity contributes to the development and progression of CVD.Individual patient factors,such as age,gender,pre-existing cardiovascular conditions,and genetics,also play a significant role in determining the cardiovascular impact.The spectrum of CVD in IBD is wide.Additionally,the cardiovascular effects of drugs used in IBD are multifaceted and depend on various factors,including the specific medicines involved and individual patient characteristics.
In individuals with IBD who are at an elevated risk of cardiovascular issues,there is a need to shift the focus of care from a reactive approach to a proactive one,emphasizing preventive measures for cardiovascular management.To minimize cardiovascular risk a multidisciplinary approach involving gastroenterologists and cardiologists is often necessary.This will ensure that IBD treatment is optimized while minimizing cardiovascular risk.
FOOTNOTES
Author contributions:Bhardwaj A writing the article;Singh A writing the article,analysis and interpretation,critical revision of the article;Midha V critical revision of the article,supervision;Sood A critical revision of the article,supervision;Wander GS critical revision of the article,supervision;Mohan B critical revision of the article,supervision;Batta A conception and design,critical revision of the article,final approval of the article.
Conflict-of-interest statement:Authors declare no conflict of interests for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:India
ORCID number:Arshia Bhardwaj 0000-0001-5820-7758;Arshdeep Singh 0000-0001-7163-0454;Vandana Midha 0000-0003-0192-3969;Ajit Sood 0000-0001-6961-6389;Gurpreet Singh Wander 0000-0002-4596-4247;Bishav Mohan 0000-0002-4337-3603;Akash Batta 0000-0002-7606-5826.
S-Editor:Qu XL
L-Editor:A
P-Editor:Yuan YY
 World Journal of Cardiology2023年11期
World Journal of Cardiology2023年11期
- World Journal of Cardiology的其它文章
- Novel predictors of permanent pacemaker implantation following transcatheter aortic valve replacement
- Clinical impact of portal vein pulsatility on the prognosis of hospitalized patients with acute heart failure
- Hypertrophic cardiomyopathy secondary to deficiency in lysosomeassociated membrane protein-2: A case report
- Down syndrome child with multiple heart diseases: A case report
- Acute myocardial infarction in myeloproliferative neoplasms
