Study on the Effect and Mechanism of Green Plum Citron Double Strain Fermentation Filtrate on Skin Barrier
Chen Zhixiong,CaoPing,Hong Ni,Zheng Zhongbo,Cui Yan
R&D Center,Huzhou Jahen Industrial Co.,Ltd.,China
Abstract To study the effect of Green plum citron double strain fermentation filtrate (DSF) on the proliferation rate of HaCaT keratinocytes by CCK8 assay.The effect of DSF on the mRNA transcription levels of filagolin(FLG),loricrin (LOR),and occludin 1 (CLD1) in HaCaT cells in sodium dodecyl sulfate (SLS) model wasinvestigated by qPCR.The effect of DSF on the content of TNF-α in the supernatant of HaCaT cells in the model was investigated by Elisa.The results showed that DSF had no toxicity to HaCaT cells,but it couldincrease the mRNA transcription levels of FLG,LOR and CLD1 in HaCaT cells in the model,and reduce the content of TNF-α in the supernatant of HaCaT cells in the model.DSF had a certain effect on skin care of strengthening skin barrier.
Key words green plum citron double strain fermentation filtrate;filaggrin;loricrin;claudin 1;TNF alpha
The skin is the largest organ on the surface of the human body.It is an important barrier between the human body and the environment,and the epidermal layer of the skin plays an important role in the skin's barrier function[1].With the development of microbiome,proteomics,and genomics,the regulatory mechanisms related to skin barrier have been further studied.Studies have shown that the expression levels of some important skin barrier related proteins in HaCaT cells play a key role in the maintenance of skin barrier function[2].FLGinteracts with keratin in the stratum corneum,causingits filaments to agglomerate into bundles and binding the keratinocytes into tight junctions[3].In addition,FLG forms a stable keratinized envelope with the action of transglutaminase,which prevents the loss of water in the epidermal layer and protects against the invasion of pathogenic microorganisms[4].Tan et al.[5]showed that oral administration of L-histidine significantly increased the expression level of FLG,thereby improving the skin barrier function of the explant.LOR is the most abundant protein in the cuticular mantle,accounting for about 80% of its total amount,and it is an important terminal differentiation protein[6].In addition,LOR has been shown to cross-link with SPRRs and form dimers under the catalysis of transglutaminase 3,and then cross-link with the outer cell scaffold under the catalysis of transglutaminase 1.Therefore,LOR plays a key role in the formation of tough keratinized mantle and is an important protein in the maintenance of skin barrier[7,8].Chen et al.[9]found that extracts from the genus macroptera could increase the expression of LOR in the skin,thereby improving the skin barrier function.CLD1 is a transmembrane protein belonging to the Claudins family.Claudins can form a tight junction structure with ZO-1,which plays animportant role in the skin barrier[10].The change in the expression level of CLD1 in epidermal cells will affect the permeability of epidermal cells to water andions,and also affect the adhesion force between cells,and ultimately affect the skin barrier function[11].Yu et al.[12]showed that the decreased expression level of CLD1 in skin keratinocytes affects the skin barrier function and ultimately aggravates atopic dermatitis.
1 Experimental part
1.1 Main materials and reagents
DSF,provided by the R&D Center of Huzhou Jiaheng Industrial Co.,LTD.;HaCaT cells,ShanghaiFuheng Biological Technology Co.,LTD.;Phosphate buffer salt solution,CCK8 kit,RPMI1640 culture medium (including double antibody),Beijing Leibao Biotechnology Co.,LTD.;Fetal bovine serum,Zhejiang Tianhang Biotechnology Co.,LTD.;TNF-α assay kit,Nanjing Jiancheng Bioengineering Institute,China;Primer,Jinsi Rui Biotechnology Co.,LTD.;RNAiso Plus Kit,PrimeScript ™ RT reagent Kit,TB Green qPCR,Baori Doctor Biological Technology Co.,LTD.
1.2 Main instruments and equipment
CO2incubator bluepard,purchased from Shanghai Yiheng Scientific Instrument Co.,LTD.;Multifunctional microplate reader MULTISKAN,purchased from Thermo Fisher Technology Co.,LTD.;Inverted biological microscope,purchased from Olympus China Co.,LTD.;Centrifuge TDL-60C,purchased from Shanghai Anting Scientific Instrument Factory;Bio-rad CFX Connect,Bole Life Medical Products Co.,LTD.
1.3 Experimental methods
1.3.1 Cell culture and grouping
HaCaT cells were cultured in RPMI1640 medium (containing 1% cyanin-streptomycin and 1% fetal bovine serum) at 37 °C with 5% CO2.When the cell growth reached about 80%,the cells were digested with 0.25% trypsin and counted with a hand-held automatic cell counter.Finally,a certain concentration of cell suspension was prepared for subculture.In the skin barrier injury experiment,HaCaT cells were divided into three groups: blank control group,skin barrier injury model group,and sample experimental group,which were cultured in six-well plates at 5×104cells/mL.Cells in the blank control group did not receive any treatment (no SLS and samples),cells in the skin barrier injury model group were treated with SLS at a final concentration of 80 μg/mL for 4 h,and cells in the sample experimental group were treated with DSF at a certain concentration for 24 h,then DSF was removed and then washed with sterile PBS for 3 times.Finally,SLS at a final concentration of 80 μg/mL was added to the cells for 4 h[13].
1.3.2 Determination of the effect of DSF on cell proliferation activity
CCK-8 assay has the advantages of good repeatability and low cytotoxicity.The fourth to fifth passage of HaCaT cells in logarithmic growth phase were seeded in a 96-well plate at a cell density of 4×104cells/mL.The cells were cultured at 37 ℃ with 5% CO2for about 12 h,and then 10% of the culture medium volume of DSF was added.After 24 h of treatment with the same sample,the cells were cultured with the same concentration of DSF.Then 10% CCK-8 reagent was added and incubated in the cell incubator for about 2 h.Finally,the absorbance at 450 nm was measured on a microplate reader,and each concentration was repeated 5 times.Cell proliferation rate was calculated using the following formula[14]:
1.3.3 The mRNA expression levels of FLG,LOR and CLD1 were detected by fluorescence quantitative PCR
The mRNA expression levels of FLG,LOR,and CLD1 were determined in the pre-cell culture according to method 1.3.1.Total RNA was extracted from each well using RNAiso and then reversed into cDNA using PrimeScript ™ RT reagent Kit.After adding the SYBR reagent,gene primers and gene template,the real-time fluorescence quantitative PCR reaction was performed.The reaction procedure was a two-step method.The specific procedures were as follows: the predenaturation at 95 ℃ for 30 s;‚The PCR reaction was performed 40 cycles at 95 °C for 5 s and 60 °C for 30 s.Among them,the primer sequences are shown in Table 1.

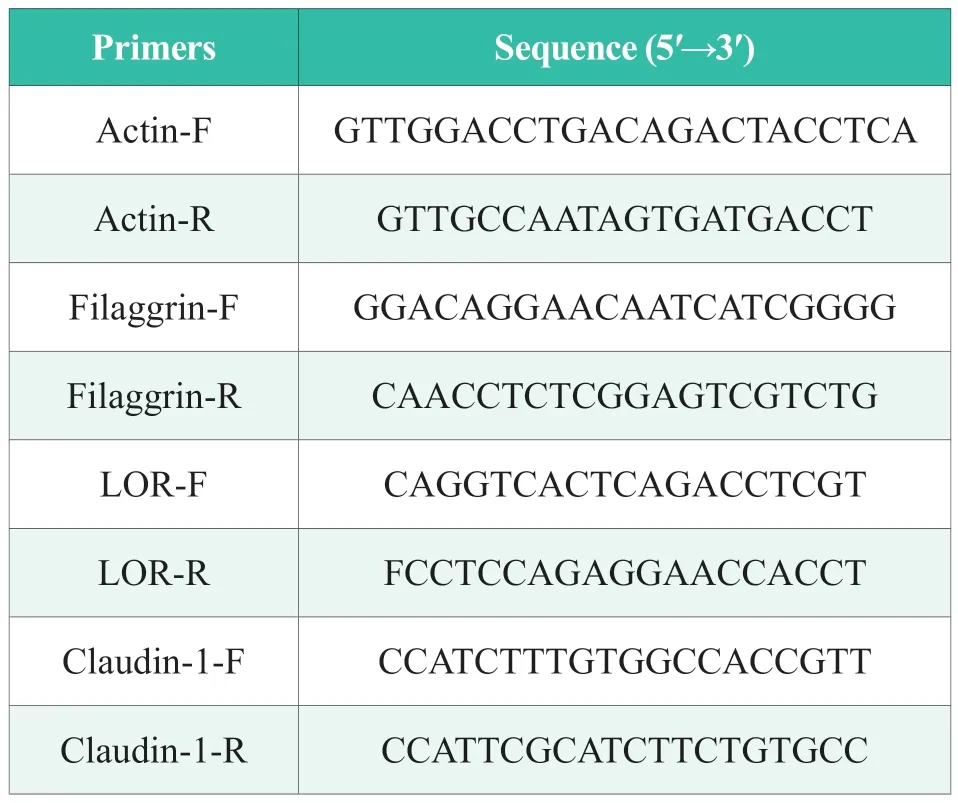
Table 1.Primer and sequence information of the amplification system
1.3.4 Determination of TNF-α content
After the cells were grouped and treated as described above,the cell supernatant in the six-well plate was collected and centrifuged at 10,000 r/min for 5 min.The test was further performed according to the instructions of the TNF-α content assay kit,and each concentration was repeated three times.
1.3.5 Statistical analysis
The experimental data were calculated by GraphPad Prism 9 software,and the results were expressed as mean±SEM.The homogeneity variance t-test was used for comparison under different experimental conditions,and then one-way ANOVO was used to complete the data analysis.Differences were considered statistically significant atp<0.05.
2 Results and discussion
2.1 Effect of DSF on the proliferation activity of HaCaT cells
After the HaCaT cells were treated with different concentrations of DSF for 24 h,the cell proliferation activity was tested using the CCK-8 kit,and the results are shown in Figure 1.It can be seen from the figure that the original concentration of DSF had no effect on the proliferation of HaCaT cells,while the filtrate diluted 10 times had a certain effect on the proliferation of HaCaT cells,and the proliferation rate was 109.76%±5.78%,which was statistically different from that of the blank control group (p<0.05).When DSF was diluted 100 times and 1,000 times,the proliferation of HacaT cells was not affected.Therefore,0.001 g/mL and 0.01 g/mL were selected as the experimental concentrations in the subsequent efficacy experiment.Based on the data of cell proliferation experiment,it is preliminarily concluded that DSF as a natural skin care active agent has certain safety.
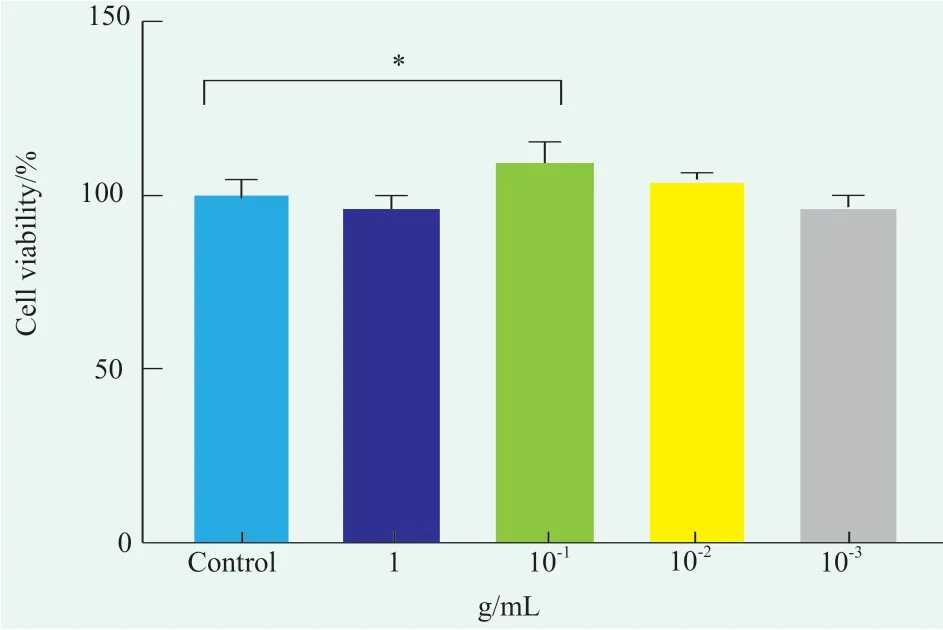
Figure 1. Effect of DSF on the proliferation of HaCaT cells
2.2 Effects of DSF on the number and growth morphology of SLS-induced HaCaT cells
HaCaT cells are located in the epidermal layer of skin and play an important role in skin barrier function.Figure 2 shows the morphology of HaCaT cells under an inverted fluorescence microscope(eyepiece 10x,objective 20x).Figure 2 shows that the cells in group a did not undergo any treatment.The normal growth pattern of HaCaT cells was adherent growth,with clear outline,flat polygonal shape and good refractive index.Cells in group b were treated with 80 μg/mL SLS for 6 h.At this time,most of the cells shed and produced debris,and most of the attached cells changed in morphology,the structure was incomplete,and vacuoles could be seen in the cells.The cells in group c were treated with 0.01 g/mL DSF for 4 h and then 80 μg/mL SLS for 6 h.At this time,although HaCaT cells did not completely grow,there was no significant differencein cell morphology from that in group a.The cellsin group d were treated with 0.001 g/mL DSF for 4 h followed by 80 μg/mL SLS for 6 h.At this time,the morphology and number of HaCaT cells did not reach the level of the 0.01 g/mL experimental group,but the number of adherent cells was more than that of the model group,and most of the cells had a relatively normal morphology.After 80 μg/mL SLS treatment,the number of adherent cells was significantly reduced and the morphology of HaCaT cells was abnormal.However,after 4 h of DSF pretreatment and 6 h of 80 μg/mL SLS treatment,the number of adherent cells increased and the morphology of HacaT cells became normal.These results indicated that SLS could induce the damage of HaCaT cells,and DSF could protect HacaT cells in a concentration-dependent manner.
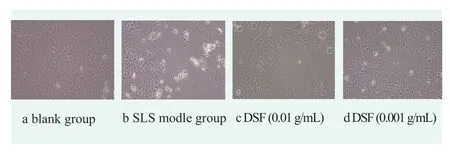
Figure 2. DSF sample group morphology of keratinocytes
2.3 Effect of DSF on the transcriptional levels of barrier-related proteins in SLS-induced HaCaT cells
SLS can destroy the barrier structure of skin epidermis,and the mRNA transcription levels of FLG,LOR and CLD1 play a key role in the skin barrier[15].In this study,the mRNA transcription levels in each group were measured by qPCR assay.It was found that the relative mRNA expression levels of FLG,LOR,and CLD1 in the SLS model group decreased to 0.40±0.01,0.37±0.03 and 0.48±0.01,respectively,which were statistically different from those in the blank control group (p<0.0001,p<0.0001,andp<0.0001),indicating that the SLS model was successfully established.It can significantly inhibit the expression of skin barrier related genes.After 4 h of DSF treatment at 0.001 g/mL followed by 6 h of SLS treatment at 80 μg/mL,the relative mRNA expression levels of FLG,LOR and CLD1 in HaCaT cells were 0.46±0.22,0.45±0.01 and 0.52±0.02,respectively.Compared with the model group,there were statistically significant differences(p<0.01,p<0.01,p<0.05).When treated with 0.01 g/mL DSF for 4 h,followed by 80 μg/mL SLS for 6 h,the relative mRNA expression levels of FLG,LOR and CLD1 in HaCaT were 0.64±0.03,0.57±0.03 and 0.88±0.03,respectively.Compared with the model group,there were statistically significant differences(p<0.000,1,p<0.000,1,p<0.000,1).In summary,SLS can significantly inhibit the mRNA expression of skin barrier related proteins,while DSF can significantly promote the mRNA expression of skin barrier related proteins in the model cells in a concentrationdependent manner,indicating that DSF has a certain effect on strengthening skin barrier at the level of gene transcription.
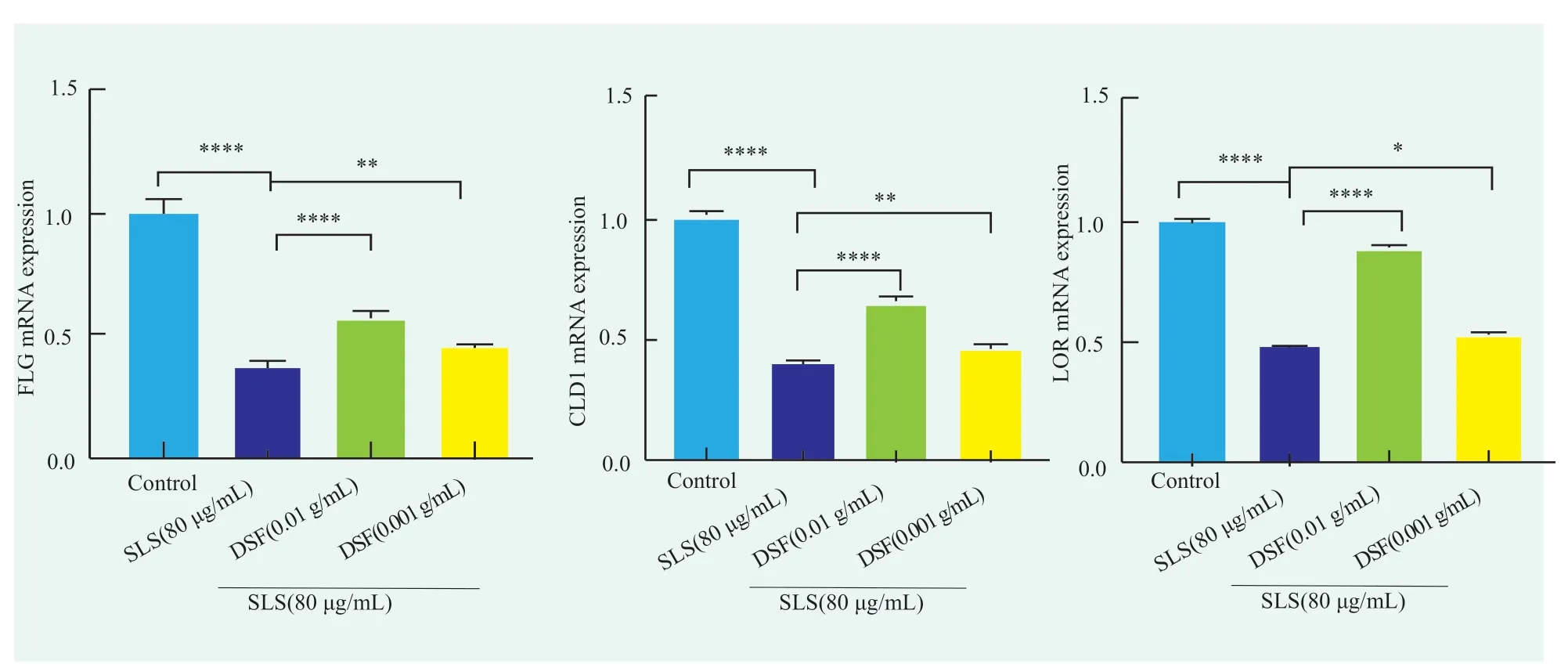
Figure 3. Effect of DSF on (a) FLG,(b) LOR and (c) CLD1 mRNA transcript levels in keratinocytes
2.4 Effects of DSF on SLS-induced release of TNF-α expression in HaCaT cells
SLS can destroy the skin barrier and induce the overexpression of pro-inflammatory factor TNF-α in HaCaT cells,which leads to inflammatory response and eventually cell apoptosis[13,16].In this study,the content of TNF-α in the supernatant of each group was detected by Elisa experiment.Figure 4 shows that the content of TNF-α in the SLS model group increased to(45.39±2.00) pg/mL,which was statistically different from that in the blank control group (p<0.000,1).After treatment with DSF at 0.001 g/mL for 4 h,and then with SLS at 80 μg/mL for 6 h,the TNF-α content in HaCaT cells decreased to (40.36±1.57) pg/mL,which was statistically different from that of the model group(p<0.01).After 4 h of treatment with 0.01 g/mL DSF and then 6 h of treatment with 80 μg/mL SLS,the content of TNF-α in HaCaT cells decreased to (30.24±2.40)pg/mL,which was statistically different from that of the model group (p<0.000,1).In conclusion,DSF can reduce the content of pro-inflammatory factor TNF-αin the SLS model,protect HaCaT cells,and indirectly maintain skin barrier.
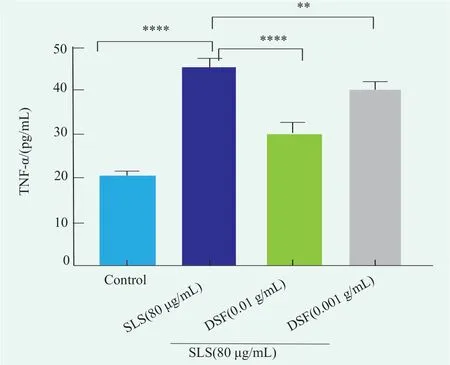
Figure 4. Effect of DSF on SLS-induced TNF-α expression in HaCaT cells
3 Conclusion
The proliferation rate of HaCaT cells treated with different concentrations of DSF was more than 90%,indicating that DSF has certain safety as a skin care active.An in vitro skin barrier injury model was made by SLS.The cells in the model group showed abnormal morphology and decreased number of adherent cells,while the cells in the sample group were normal both in number and morphology.qPCR showed that the mRNA transcription levels of FLG,LOR and CLD1 in HaCaT cells in the model group were significantly lower than those in the blank group,while the mRNA transcription levels of FLG,LOR and CLD1 in the experimental group pretreated with DSF were significantly higher than those in the model group.Finally,Elisa showed that the content of TNF-αin the supernatant of HaCaT cells in the model group was significantly lower than that in the blank group,and the content of TNF-α in the experimental group pretreated with DSF was significantly lower than thatin the model group,indicating that DSF could protect HaCaT cells by reducing the content of TNF-α in cells.Thus,the skin barrier is indirectly maintained.
 China Detergent & Cosmetics2023年4期
China Detergent & Cosmetics2023年4期
- China Detergent & Cosmetics的其它文章
- The Haircare Efficacy Research of Prinsepia Utilis Oil
- Study on the Antibacterial and Deodorization Effect of 4,4-Dichloro-2-Hydroxydiphenyl Ether in Laundry Gel Beads
- In Vivo Study of a Natural Whitening Agent
- In Vitro Study on the Soothing and Oil Control Efficacy of P-Hydroxyacetophenone
- The 3rd Summit Forum on Amino Acid Surfactant was Held
- The 2023 (12th) National Sulfonation/Ethoxylation Technology and Market Seminar was Held
