Enzymatic preparation of mono-and diacylglycerols: A review
Jiwei Zheng ,Yudong Ling ,Jixi Li ,Shuping Lin ,Qingyue Zhng ,Knghu Zuo ,Nnjing Zhong,*,Xueing Xu,*
a School of Food Science,Guangdong Pharmaceutical University,Zhongshan 528458,China
b College of Food Science and Technology,Henan University of Technology,Zhengzhou 450001,China
c Wilmar (Shanghai) Biotechnology Research and Development Center Ltd.,Pudong New District,Shanghai 200137,China
Keywords:Monoacylglycerol Diacylglycerol Enzymatic production Esterification Glycerolysis Tert-butanol Ionic liquids Patent evaluation
ABSTRACT Monoacylglycerols(MAGs)and diacylglycerols(DAGs)are partial glycerides widely used in food industry.They are safe and non-toxic food emulsifiers,especially for MAGs.MAGs account for approximately 75% of the total emulsifiers in food industry worldwide.DAGs are recognized as functional cooking oils,they can suppress body fat accumulation and postprandial serum triacylglycerols(TAGs) level.The traditional production of MAGs and DAGs is based on the chemical method,which requires high reaction temperature usually up to 200–260 ◦C.Such high temperature is not suitable for oil containing heat sensitive polyunsaturated fatty acids.Enzymatic approach has been received increasing attentions.Enzymatic production of partial glycerides to replace chemical processes has been in industry,particularly for DAGs production as the products have been claimed as a functional and nutritional oil.Enzyme technology for the processing of oils and fats has been moved to industry step by step and case by case during the last 20 years.More and more applications are particularly moving into bulky oils and fats processing.At the same time,the cost of enzymes as a commercial product is reducing steadily.This review summarized the recent 15 years advances on the the enzymatic preparation of MAGs and DAGs.The critical process parameters under different reaction routes were presented and emphasized.The reaction media not only increased the homogeneity of the reaction system,but also shifted the reaction equilibrium towards the target product generation,and this part was stated in detail.In addition,the patent evaluation was included,and the application of MAGs and DAGs was covered.
1.Introduction
Monoacylglycerols(MAGs)and diacylglycerols(DAGs)are nonionic molecules with both hydrophilic and hydrophobic portions.They occur in edible oils and fats in minor amounts.Based on the position of the ester linkage that covalently bonded to glycerol,MAGs can be divided into 1(3)-MAGs and 2-MAGs isomers,and DAGs consist of 1,2(2,3)-DAGs and 1,3-DAGs isomers (Fig.1).For the MAGs,1(3)-MAGs are the predominant isomers,they account for 92%–95% at low temperatures and approximately 70% at the very highest temperatures of the total MAGs [1].While for the DAGs,1,3-DAGs are more thermodynamically stable since the steric effect of the structure,and 1,2-DAGs in the edible oil will be largely converted to 1,3-DAGs to reach equilibrium at a ratio of(3–4):(7–6)between 1,2-and 1,3-DAGs[2].In addition,the energy value and digestibility of DAGs is similar to that of triacylglycerols (TAGs).The energy value is 38.9 and 39.6 kJ/g respectively for DAGs and TAGs.The digestibility of rats fed with DAGs resembles that of TAGs with digestibility value both at 96.3% (no significant difference)[3].
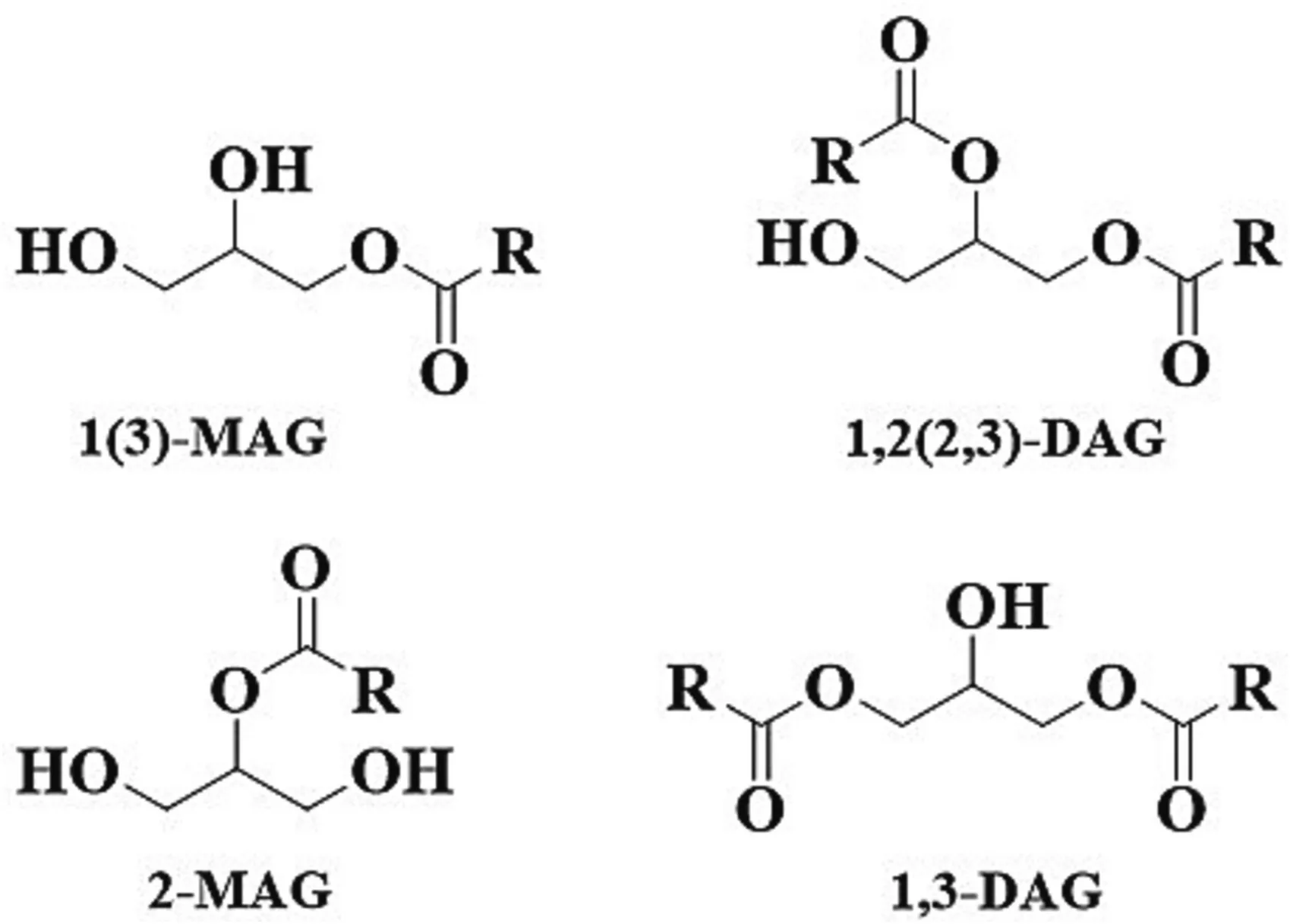
Fig.1.Structures of MAG and DAG.MAG,monoacylglycerol;DAG,diacylglycerol.
MAGs show excellent emulsifying properties,which are widely used in the food,cosmetic,pharmaceutical and chemical industries [4].MAGs or their mixtures with DAGs account for approximately 75% of the world’s annual emulsifier production [1].DAGs are recognized as functional cooking oils,they are reported to be able to suppress body fat accumulation and postprandial serum TAGs level[5].Yet the mentioned health benefits can only be observed with DAGs concentration not less than 40% [6],which is far greater than the DAGs present naturally in vegetable oil.The concentration levels of DAGs naturally in vegetable oils are less than 10%,in addition,MAGs occur naturally in various oils are less than 1%[5].Therefore,production of MAGs and DAGs has been studied extensively over the years.MAGs and DAGs can be produced chemically or enzymatically.The chemical method offers a much cheaper alternative,yet this process is conducted at high temperature up to 220–260◦C,which is not suitable for oil containing heat sensitive polyunsaturated fatty acids;moreover,the high temperature will also burden extra operating cost.On the other hand,enzymatic approach is environmentally friendly,it is at mild conditions and enzyme catalyzed reactions are selective and specific.In this review,we summarized the advances in the recent 15 years on the the enzymatic preparation of MAGs and DAGs.The critical process parameters under different reaction routes were presented and emphasized.The effects of reaction media on the production of MAGs and DAGs are included,and the reaction media shifted the reaction equilibrium was emphasized.In addition,application of MAGs and DAGs,as well as the patent evaluation were also covered.
2.Properties and applications of MAGs and DAGs
2.1.Properties and applications of MAGs
MAGs consist of a hydrophilic head and a hydrophobic tail,therefore,MAGs display excellent emulsifying properties,particularly for oil and water combination.In food industry,MAGs are safe and non-toxic emulsifiers,they have been recognized as food-grade additives by the United States of Food and Drug Administration and the European Union.They are good emulsifiers for cake,bread and margarine products.And around 75% of the total emulsifiers in food industry worldwide are MAGs [7].In addition,MAGs were also used in beverage clarifiers to help disperse the system[8].
Besides in food industry,MAGs also show biological activities.For example,monolaurin,monomyristin,monocaprin,monoolein and monolinolein were reported to display antimicrobial activities[9,10].In a study from Cho et al.(2010),MAGs containing oleic acid were reported to have antioxidant,anti-diabetic and anti-atherosclerotic effects bothinvitroand in cells [11].In addition,MAGs containing mediumchain fatty acids and the fatty acids as pig feed additives are also beneficial for gut health improvement and feed pathogen mitigation[12].Moreover,MAGs containing n-3 polyunsaturated fatty acids(PUFAs),like eicosapentaenoicacid (EPA) and docosahexaenoic acid(DHA),are beneficial for preventing different cardiovascular disorders.They are useful for human health based on their nutritive value,their regulation of inflammation,cholesterol metabolism,and brain functions,as well as their influence to erythrocyte fatty acid profiles and the expression levels of inflammatory circulating mediators [7].Fish oil is the natural source of n-3 PUFAs.Most of the natural PUFAs exist in the form of TAGs,and only a small amount of PUFAs derived from krill oil exist in the form of phospholipids(PL)[13,14].In functional oils,PUFAs mainly exist in the form of ethyl ester (EE),2-MAGs,structured TAGs and PL,and so on.Dyerberg et al.and Neubronner et al.found that n-3 PUFAs can be digested more effectively as TAGs than that as FAEE[15,16].The position of PUFAs in TAGs also plays a role on their physiological activity [17].However,high content of PUFAs in fish oil makes it easy to be oxidized [18].Furthermore,MAGs are also used in synthetic organic chemistry as synthetic intermediates or as chiral building blocks [19].
MAGs are also used in textile,fiber processing and plastics production,because of their lubricating and plasticizing properties [1].In addition,MAGs can be used as a binder in drug tablets,skin moisturizing agents in pharmaceutical industry.In the cosmetics industry,MAGs are helpful to improve the quality of creams and lotions[20].
2.2.Properties and applications of DAGs
DAGs are structured lipids,they are absorbed and metabolized differently from the common oils and fats (TAGs).During digestion in the small intestine,TAGs undergo hydrolysis to form 2-MAGs and fatty acids.These products are absorbed by the epithelial cells and they can be re-esterified to generate TAGs[21].In addition,DAG has been shown to increase bone mineral density and improve bone microstructure [22].Unlike that of the TAGs,DAGs are hydrolyzed into 1(3)-MAGs and fatty acids in the presence of pancreatic lipase.However,the generated 1(3)-MAGs and fatty acids can not be re-esterified to synthesis TAGs.Therefore,the ingestion of DAGs leads to increment in theβ-oxidation of fatty acids [4].Due to the such metabolic pathway,consumption of DAGs was indicated to be capable of suppressing visceral fat accumulation [23,24],lowering postprandial serm TAGs and cholesterol [25],as well as modulating serm glucose level [26,27].
The DAGs have therefore attracted global attention,and the DAGsenriched oil was recognized as the functional cooking oil under the brand name of “Econa” and “Enova” in Japan and United States respectively.It was first developed by Kao Corporation in Japan and the DAGs content was around 80% (W/W) in the DAGs-enriched oil.However,the sales of DAGs-enriched oil were put to a halt since the presence of glycidyl fatty acid esters were confirmed,which was carcinogenic[5].Although the technology was developed to eliminate the contamination from the DAG oil products,the market,especially in Japan,did not come back.Encouragingly,DAGs oil has been launched in China recently from a few companies under different brands as small pack retail products for cooking or other culinary applications.This new market wave raised the new consideration of the products and the revisit of the nutritional values of DAG oils in the current light life.
Besides as the functional cooking oils,the DAGs have extensive applications especially in food industry.They can be used as stabilizer for oil-in-water (O/W) and water-in-oil (W/O) emulsions [28–30].In addition,they are potential anti-oxidants in oil-in-water emulsions[31].Furthermore,they also can be used as new indices to measure quality of fresh oils [32].Moreover,DAGs can be used as the frying medium in shortening[33].In addition to food industry,DAGs can also be used as building blocks for organic products synthesis in pharmaceutical industry,such as phospholipids,glycolipids,pipoprotein,pro-drugs like 1,3-DAG conjugated chlorambucil and 1,2-DAG conjugated (S)-(3,4-dihydroxy-phenyl)alanine(L-Dopa) [34,35].
3.Reaction routes for MAGs and DAGs preparations
Compared with the chemical method,enzymatic preparations of MAGs and DAGs are more attractive,due to the mild reaction conditions and reaction specificity that enzymed afforded.The main reaction routes for MAGs and DAGs preparations are esterification,glycerolysis,partial hydrolysis.In addition,ethanolysis reaction was sometimes used to produce MAGs and DAGs.
3.1.Esterification
Enzymatic esterification refers to the dehydration of glycerol and fatty acids to form acyl glycerol under the catalysis of lipase(Fig.2).The glycerol first reacts with the fatty acid to form MAG,the obtained MAG will further react with fatty acid to generate DAG and then the TAGs[36].Therefore,the substrates molar ratio is key for desired product synthesis.From the reaction equilibrium point of view,molar ratio of glycerol to fatty acid at 1:1 would probably favor MAGs formation,and at 1:2 would the DAGs formation,more fatty acids would favor the TAGs formation.Nevertheless,it should be pointed out that,since the reaction is reversible,when the molar ratio of glycerol to fatty acid is 1:1 or 1:2(or even other ratios),the reaction products are not the pure MAGs or the pure DAGs,but free fatty acid,MAGs,DAGs and TAGs are usually present in the reaction products.Table 1 and Table 2 [37–76] respectively presented the MAGs and DAGs preparation through enzymatic esterification.In the initial stage,some lipases need a small amount of water to maintain their activity.Interestingly,Candidaantarcticalipase B (CALB) can catalyze the reaction in dry state [77].In addition,Lipozyme RM IM and Lipozyme TL IM are also able to initiate the reaction without water addition.
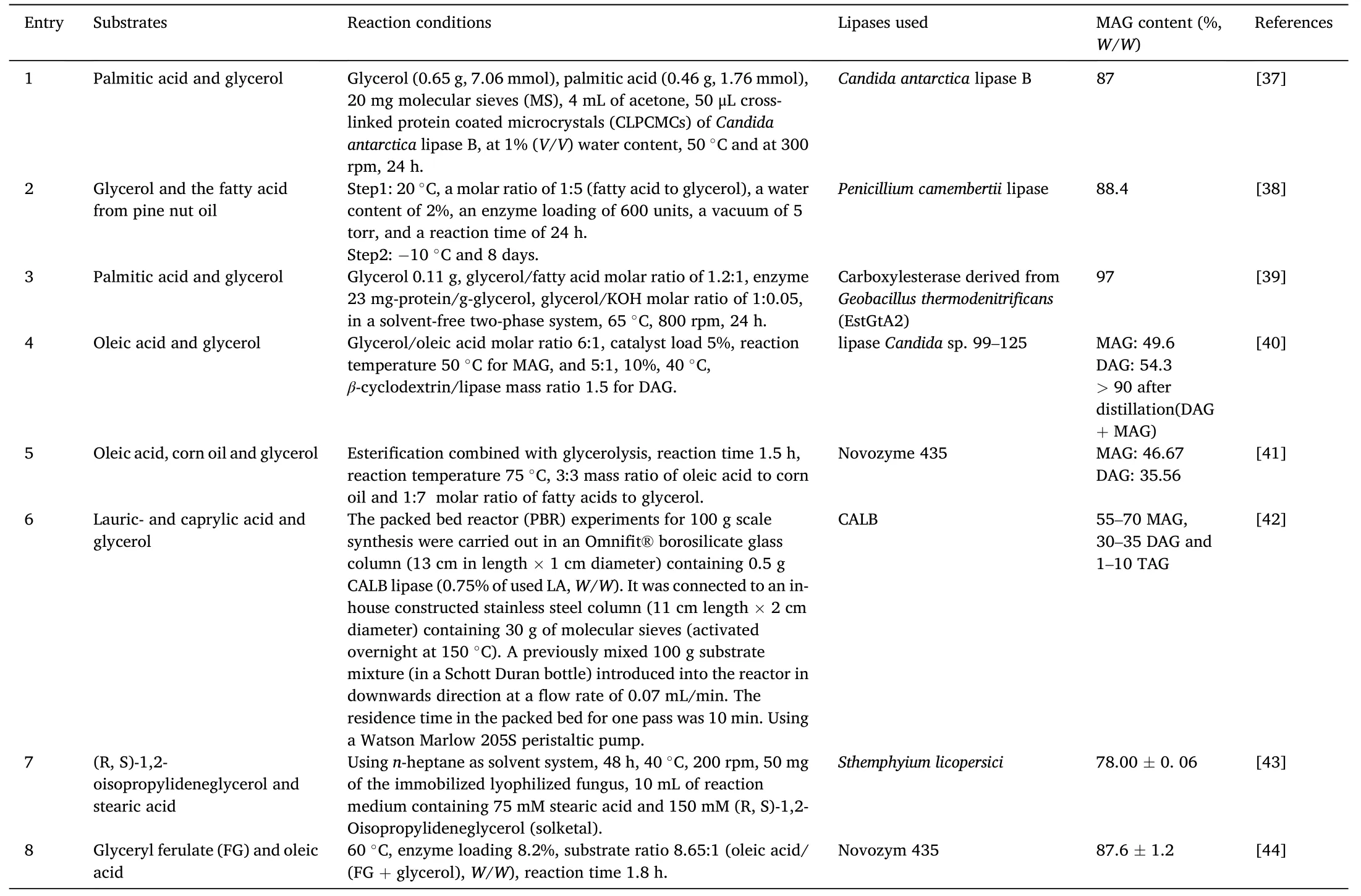
Table 1 Enzymatic preparation of MAG through esterification.
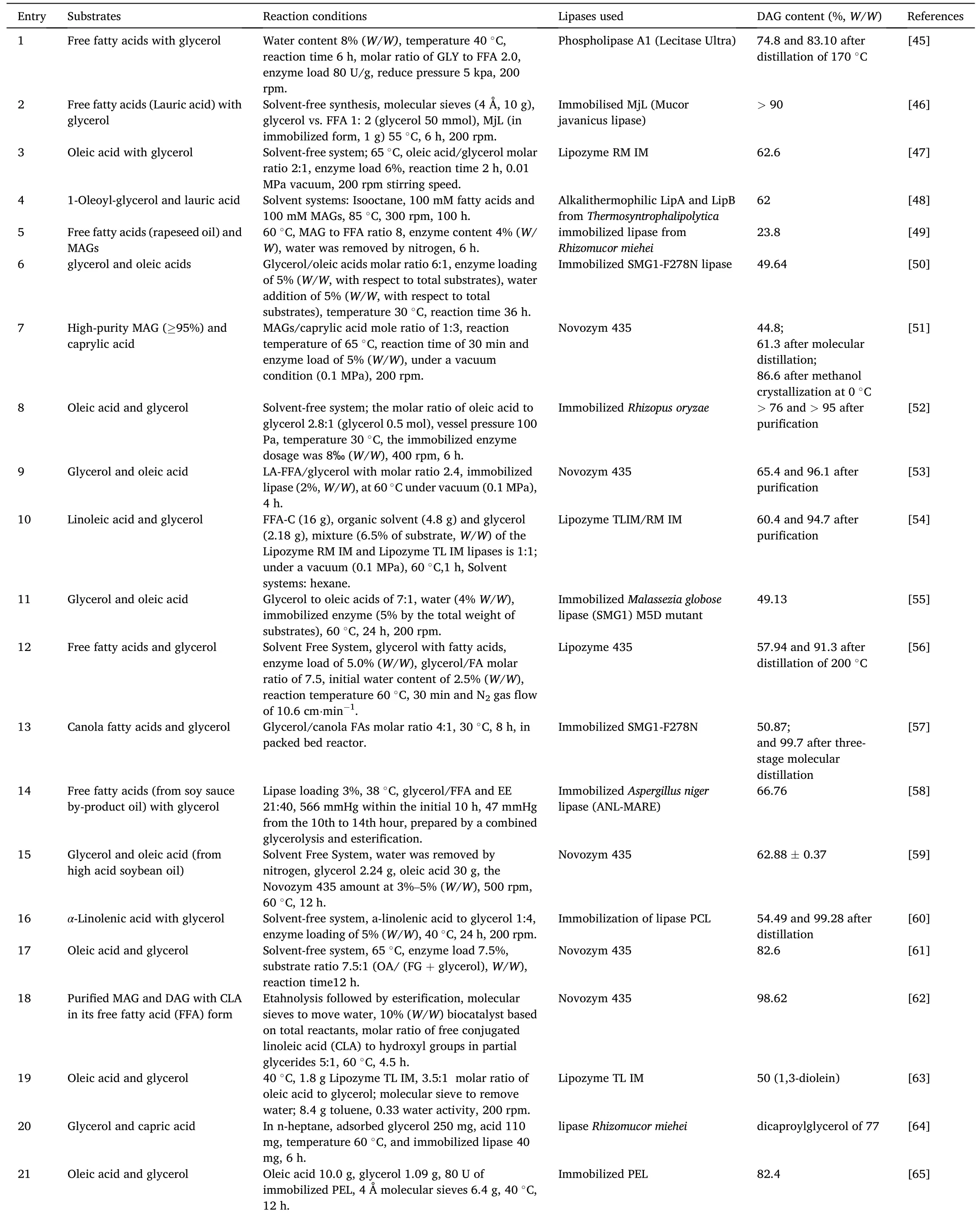
Table 2 Enzymatic preparation of DAG through esterification.

Fig.2.Enzymatic esterification (of glycerol with fatty acid) reaction routes for glycerides preparation.MAG,monoacylglycerol;DAG,diacylglycerol;TAG,triacylglycerol.
Water will be generated as the esterification proceeds,therefore,removal of the generated water will shift the equilibrium towards the glycerides formation side [49].The excess water could be removed by ventilation[45,49,56,59],vacuum pump[38,47,51–54,58],silica gel or molecular sieve desiccants [37,62,63,65–67,72].As shown in Table 1 and Table 2,the maximum water content in enzymatic esterification is no more than 10%.Study indicated that vacuum can interestingly increase the amount of MAGs and inhibit the DAGs formation.However,too high vacuum would result in the lack of water to maintain the lipase activity[9].Wang et al.explored the effect of water content(2%–14%,W/W) on the esterification catalyzed by Lecitase Ultra.It showed that the esterification efficiency(EE)of free fatty acids increased slowly with the increase of water content.However,EE decreased when the water content exceeded 8%[45].
Sometimes solvents were used as reaction media to increase the compatibility of glycerol and fatty acids and in turn accelerating the reaction.Salameh et al.investigated the effects of several organic solvents including isooctane,octane,toluene,acetonitrile on the enzymatic esterification.The results showed that isooctane was the most efficient solvent [48].Actually as the esterification proceeds,the generated MAGs and DAGs would emulsify the reaction system and increase the contact of substrates.The compatibility of glycerol with fatty acids changes in real time with the advance of the reaction [61].To our experience,it is unnecessary to introduce medium into the reaction system in most cases.In some cases,the organic solvent system may lead to acyl transfer and other side reactions [46].Moreover,some lipases activity would be impaired in organic solvents.For example,tert-butanol is a amphiphilic solvent,it can combine oils and glycerol efficiently.However,most common commercial lipases (except for Novozym 435)exhibit lower esterification activity intert-butanol system [46,66].Therefore,solvent-free was more attractive for enzymatic esterification.While in the case of adding solvents can produce significantly higher industrial benefits,this reaction system with little solvent separation price will be acceptable and attractive.
3.2.Glycerolysis
Glycerolysis is the most widely used reaction route for MAGs and DAGs production,because of the easy access to the glycerol.In recent years,production of glycerol is increasing,especially from the biodiesel industry.It is therefore necessary to transform glycerol into valuable products,and glycerides are the such bioproducts.Table 3 and Table 4[78–130] listed the enzymatic production of MAGs and DAGs through glycerolysis in recent 15 years.

Table 3 Enzymatic preparation of MAG through glycerolysis.

Table 4 Enzymatic preparation of DAG through glycerolysis.

Table 5 Enzymatic preparation of MAG through partial hydrolysis.
Glycerolysis is a multi-step and reversible reaction,as shown in Fig.3.DAGs are the intermediate products,as DAGs can also react with glycerol to form MAGs.More glycerol would help shift the reaction equilibrium towards MAGs generation.Therefore,molar ratio of glycerol to TAGs over 3,at 4 or even at 5,was usually used to obtain high content of MAGs.And MAGs content up to 70%[99–101]or even at 80%[83] and 90% [94]was observed from enzymatic glycerolysis.
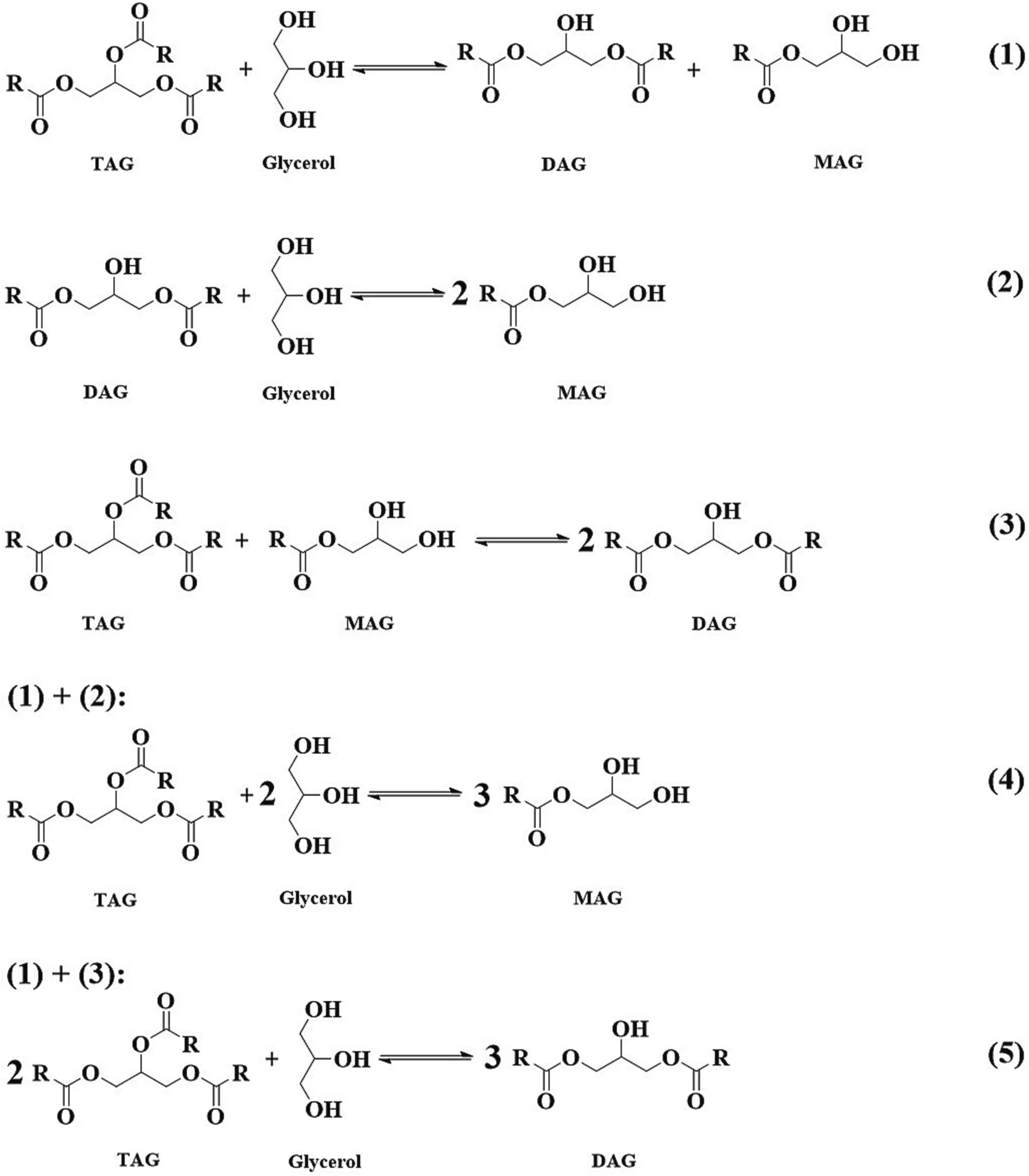
Fig.3.Enzymatic glycerolysis reaction routes for glycerides preparation.MAG,monoacylglycerol;DAG,diacylglycerol;TAG,triacylglycerol.
However,the excess use of glycerol would cause immiscibility with oils in the reaction system.Moreover,lipases tend to be trapped by the glycerol,which prevents the contact of lipases with oils,and in turn the glycerolysis reaction difficult to proceed.To improve the miscibility and enhance the reaction,organic solvents,ionic liquids,surfactants were introduced into the reaction systems.Solvents such astert-butanol,tertpentanol,isopropanol,n-butane,hexane,et al.were introduced into the reaction system.Due to their amphiphilicity,tert-butanol,tert-pentanol,isopropanol were sufficient to mix oils and glycerol,and MAGs content up to 70% and even 90% could be obtained,as above-mentioned[83,94,99–101].It is true that,the use of solvents would increase the production cost as well as safety issues in some cases,due to the added separation process and release of solvent into air.Yet such a high content of MAGs is still attractive and potential.Thus,a special section 4 is included to discuss the details of medium applications.
In addition to the organic solvents,surfactants,such as TritonX-100,Tween65,Tween40,Tween80,soy lecithin and sodium (bis-2-ethylhexyl)sulfosuccinate(Aerosol-OT or AOT),are also used to improve the compatibility of the system.They can form micellar systems and both hydrophilic and hydrophobic substances can be dissolved at high concentrations.The increase of interface area afforded by the surfactants significantly promotes the preparation of glycerides catalyzed by interfacial lipases [79,81,84,85,93,104].For example,study from Valerio et al.indicated that,the addition of TtitonX-100 increased the conversion rate,reduced the amount of the enzyme,and shorten the reaction time,during the enzymatic glycerolysis of olive oil [79].
If the desired products are DAGs,then the molar ratio of substrates is another scenario.When combine the reaction routes 1 and 3 in the Fig.3,we can simply obtain route 5,that is,1 mol glycerol with 2 mol of TAGs could be theoretically obtained 3 mol DAGs.Therefore,molar ratio of glycerol to TAGs usually at about 0.5 (1:2) was used for DAGs preparation.Unlike in the case of MAGs preparation through glycerolysis,in which molar ratio of glycerol to TAGs is usually over 3,the use of glycerol is quite less(molar ratio of glycerol to TAGs at about 0.5).In this case,the glycerolysis reaction can proceed without solvents or surfactants introduction at the enzymatic temperatures (usually 50 to 70◦C) under the vigorous stirring.Of course,the introduction of reaction media would enhance the reaction rate,however,it would add the production cost at the same time.To our experience,considerable content of DAGs from 50%to 60%could be obtained from solvent-free enzymatic glycerolysis.Actually it is unnecessary to introduce reaction media into the glycerolysis reaction system for DAGs preparation.Interestingly,Xu et al.found that ionic liquids can not only efficiently improve the miscibility of substrates but also increase the DAGs selectivity.DAGs content over 70%was firstly encouragingly observed from binary ionic liquid system of [TOMA]⋅[Tf2N]/Ammoeng102 [112].
Another strategy to increase the contact of substrates in solvent-free enzymatic glycerolysis is with the aid of ultrasonic radiation,especially for DAGs production.Since in DAGs preparation,the required glycerol amount is less and the glycerolysis could proceed under extensive stirring;while for MAGs preparation,more glycerol is required and the lipase tends to be trapped by the glycerol in this case,resulting in the glycerolysis reaction being difficult to proceed even under vigorous stirring.The cavitation collapse afforded by the ultrasonic radiation can increase the surface area and reduce the mass transfer resistance,and therefore speed up the reaction [110].Results from Fiametti et al.indicated that,even under the weak radiation,the ultrasonic increased the reaction rate and reduced the enzyme usage,moreover,no obvious loss of enzyme activity was observed under the ultrasonic radiation[85].
3.3.Partial hydrolysis
Hydrolysis of oils would liberate fatty acids from TAGs.As showed in Fig.4,MAGs and DAGs could be obtained under partial hydrolysis;while if totally hydrolysis,only free acids and glycerol were obtained.Therefore,it is key to control the hydrolysis degree for MAGs or DAGs preparation.Tables 5 and 6 [131–145] listed the recent advances for MAGs and DAGs preparation from partial hydrolysis.Water content is crucial in the hydrolysis reaction.It would affect the reaction equilibrium since it participates the hydrolysis reaction[132,137,139,140].In addition,it also influences the enzyme structure and in turn the activity.In the initial stage,the oil-water interface is the main rate-limiting factor.Vigorous stirring,ultrasonic radiation or the introduction of surfactants,could help to increase the interface.Extensive stirring can keep the substrates in emulsion state,which is beneficial to speed up thereaction,however,high-speed shearing may destroy the enzyme structure and reduce the enzymatic activity[140,142].The use of surfactants or ultrasonic radiation can reduce the substrates viscosity and increase the mass transfer,which improve the hydrolysis [137,142].It is worth noting that,under the ultrasonic radiation,reaction temperature should be controlled,usually with the aid of circulating cooling water.Otherwise,high temperature caused by the ultrasonic would incur the enzyme inactivation [137].

Fig.4.Enzymatic hydrolysis of TAGs reaction routes for glycerides preparation.MAG,monoacylglycerol;DAG,diacylglycerol;TAG,triacylglycerol.
3.4.Partial ethanolysis
Ethanolysis can also be used to prepare MAGs and DAGs.As shown in Fig.5,partial ethanolysis would produce glycerides and fatty acid esters,while totally ethanolysis generates only fatty acid esters and glycerol.Like in the partial hydrolysis,ethanol content here is a key parameter.By controlling the reaction process and the ethanol amount,approximately 30%–50%of MAGs was observed (Table 7[146–153]).

Table 7 Enzymatic preparation of MAG through ethanolysis.
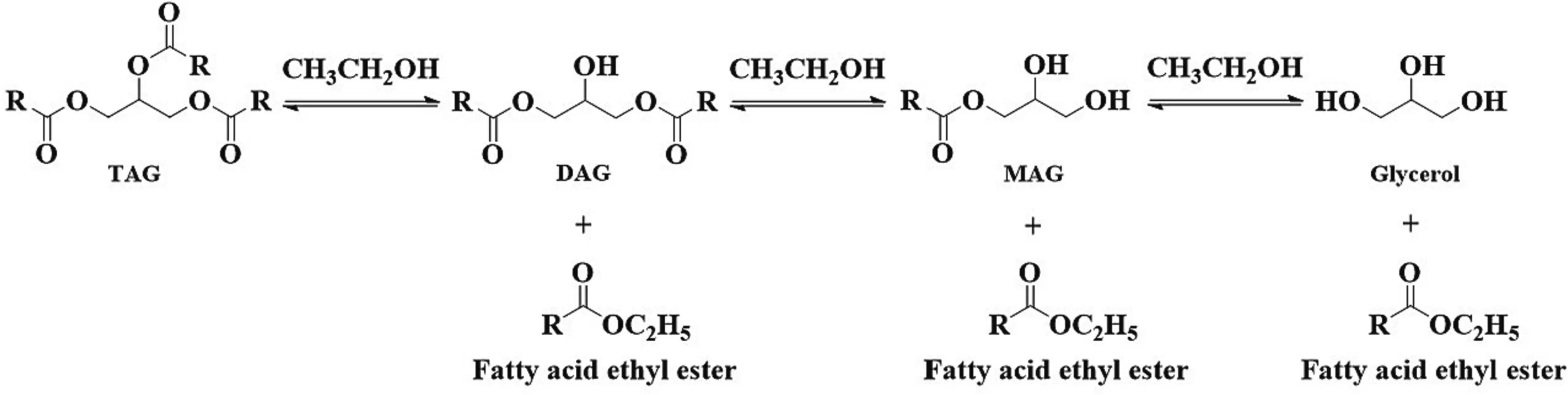
Fig.5.Enzymatic ethanolysis reaction routes for glycerides preparation.MAG,monoacylglycerol;DAG,diacylglycerol;TAG,triacylglycerol.
Overall,enzymatic glycerolysis was the most used reaction route for MAGs and DAGs production,due to its high time-space cost efficiency as well as the easy access of glycerol and TAGs.In addition,no or little glycidyl fatty acid ester would be generated at low temperatures.Since the immiscibility existence between TAGs and glycerol,the glycerolysis was difficult to proceed when more glycerol was added into the reaction system.And in this case,solvents were usually introduced to improve the miscibility and enhance the reaction.
Followed was the esterification,and it was the ideal route for pure MAG or pure DAG synthesis,which used pure fatty acid as the substrate to esterify with glycerol.Besides,MAGs or DAGs containing mixed fatty acids were also sometimes obtained from esterification,especially for DAGs preparation (Table 2).In the esterification process,water would be generated,and it was necessary to remove the water to shift the reaction equilibrium towards the glycerides formation side.
As for the partial hydrolysis and the partial ethanolysis routes,they can be used for MAGs and DAGs preparation,but not widely.Partly because free fatty acids or fatty acid esters would be formed from these reaction routes and they should be removed from the products.
4.Reaction media are important in defining the processing technology
The corresponding reaction mechanisms of esterification,glycerolysis,hydrolysis and ethanolysis have been mentioned earlier.As shown in Fig.6,the reaction equilibrium stage is reached in each reaction route,only for different reactions with different proportions of the various components(MAGs and DAGs specially as the targets).This gives us an opportunity to produce the desired substance in a rational way.If we need more DAGs or MAGs,it is time to add reaction media or adjust the ratio to interfere (glycerol/oil) to achieve our final desired purpose.

Fig.6.The substances contained in the reaction equilibrium system.TAG,triacylglycerol;DAG,diacylglycerol;MAG,monoacylglycerol;FFA,free fatty acid.
4.1.Solvent-free systems
The production of MAGs and DAGs is feasible in solvent-free systems[154–157].As shown in Fig.7,the whole system consists mainly of glycerol,oil (or fatty acids) and lipase,which are reacted by uninterrupted stirring in an intermittent reactor and finally reach equilibrium in principle.To further purify the products,distillation technologies can be used for further processing.Solvent-free systems are a very good choice for some systems because they do not require post-processing of the reaction medium compared totert-butanol or ionic liquids [158].However,the solvent-free system also has its disadvantages,with a relatively long reaction equilibrium time (about 10–24 h) [159],a relatively low yield of the resulting product,and a relatively poor miscibility due to the low homogeneity of this system [160].

Fig.7.Multi-phases exist in the solvent-free system.
From Table 8 [159,160],we observed that the yield of DAGs was around 45%,while the yield of MAGs was around 20%,and the yield of DAGs was more than two times higher than the amount of MAGs,which indicates that the preparation of DAGs is favored in the solvent-free system,while it is very inefficient for the production of MAGs since more glycerol in the system makes the system less homogenuous.If one wants to prepare MAGs,other methods should be chosen for the production of MAGs [161].However,it was found from the table that the reaction equilibrium time required for the preparation was 24 h.The reaction time was long compared to the system with reaction medium,which may be due to the poor miscibility of hydrophilic glycerol and oil[160].The conversion in solvent-free systems is also limited,but an increase in stirring speed will improve the conversion,while too high stirring speed can cause damage to the catalyst and also increase energy consumption [162],so proper reaction conditions will improve the conversion.Excessive glycerol can cause the formation of a glycerol layer around the enzyme pellet,which limits the contact with other substrates [163].Therefore,some appropriate conditions such as glycerol ratio and temperature will increase the yield of DAGs in the solventfree system and also facilitate the subsequent processing.

Table 8 Reaction systems in solvent-free systems.
4.2.Tertiary alcohols
Although solvent-free systems are also a good system,to enhance the cleavage of glycerol,amphiphilic compounds are usually introduced into the system,which will increase the miscibility between glycerol and TAGs [164] and also enrich a product by breaking the reaction equilibrium,so as to speed up the reaction rate.Since glycerol has enzymatic hydrophilicity,it can bind to enzyme particles,while oil molecules become difficult to bind to the enzymes and mass transfer of glycerol is thus limited [160].Moreover,high enzyme content may form aggregates,increasing mass transfer limitations and avoiding contact between the substrate and the active site [165].Therefore,the addition of the reaction medium to the whole system may induce the formation of a more homogeneous and less viscous reaction mixture and also avoid acyl migration [166].It can also increase the yield of the product and shorten the reaction time.While there are various types of reaction media involved in the reaction,the most commonly used reaction media are organic solvents.
The common organic solvent reaction medium in enzymatic reactions istert-butanol (TB),a small amount of TB as a polar co-solvent can improve the miscibility and reduce the viscosity of the medium,increase the homogeneity of the solvent system and facilitate mass transfer [167],while mass transfer is one of the important factors affecting the rapidity of enzymatic reactions,an appropriate increase in temperature will also have a positive effect on the mass transfer process[168],a higher temperature can activate the substrate molecules,reduce the viscosity and accelerate the reaction rate [169].No side reactions occur in biocatalytic reactions using TB as a solvent [170],as the reaction rate between TB and fatty acids is zero [171],proving that no reaction occurs between the two.After comparing the logpvalues of different reaction media [172],it was also found that TB is the most suitable solvent for efficient glycerol cleavage with high levels of MAGs yield(Table 9[160,170,173–175]).

Table 9 Reaction systems in tert alcohol.
In the experiment of lipase cleavage of sunflower oil glycerol,as in Fig.8,sunflower oil (20 g),glycerol,and TB were added to a capped beaker for mixing,and lipase was added to initiate this reaction.The reaction temperature was maintained at 40◦C with a glycerol/oil molar ratio of 4.5:1 and TB/oil of 2.2:1 and a lipase (Novozym435 derived fromPseudomonasaeruginosa,produced by deep fermentation of a transgenicAspergillusOryza[173,176],loading of 15%(W/W,oil base)proven in several experiments to be the most efficient enzyme for MAGs production [170,177,178]).Equilibrium is reached in about 2 h,and yields of up to 70%MAGs can be obtained,along with a DAG content of about 26% [160].However,it was found that the sum of the obtained MAGs and DAGs yields did not change much by increasing the temperature up to 70◦C and changing the glycerol/sunflower oil ratio for glycerol cleavage,and the yields were both around 65%,so the too high temperature and glycerol/oil ratio did not significantly improve the yields[160].The temperature of the reaction system should not be too high either,because the boiling point of TB is 83◦C [174],too high temperature can also have side effects on the reaction,so an appropriate temperature is necessary for the reaction.The yield of DAGs and MAGs production in solvent-free system was 50%–55%,but the reaction equilibrium time was as long as 24 h,which slowed down the reaction rate,but the solvent-free system is favorable for the formation of DAGs.The long reaction time and low conversion of DAGs and MAGs were mainly due to the poor miscibility of hydrophilic glycerol and lipophilic oil at low temperature,so the addition of reaction medium was very necessary[160].

Fig.8.Glycerolysis time courses of sunflower oil in tert-butyl alcohol.Adapted from[174].MAG,monoacylglycerol;DAG,diacylglycerol;TAG,triacylglycerol;FFA,free fatty acid.
In the enzymatic cleavage with camellia oil as raw material,98.02%conversion was achieved and up to 82%MAGs content was obtained by adding 25 mL of TB and 0.5 g of lipase in the system under the conditions in Table 8 [170].This is not significantly different from the yield obtained with MAGs produced from sunflower oil,indicating that the effect of different oils or fatty acids on the yield of MAGs and DAGs is not a major factor [160].As the reaction medium is the key factor on the yield,the amount of reaction medium must have an effect on the yield obviously.Zeng et al.[170]used the same reaction substrates(camellia oil and glycerol) in different volumes of TB to reach reaction equilibrium.It was concluded that a substrate concentration of 40%in TB was optimal to achieve 98.7%conversion with 82%MAGs content after 10 h.In the algal oil and olive oil cases with TB as a reaction medium[179,180],it was found that lower glycerol/oil ratios favored the formation of DAGs while the higher glycerol/oil ratios favored the formation of MAGs [165].
Among the tertiary alcohols,TB is often used as a reaction medium in addition totert-pentanol(TP).TP is an intermediate polar solvent(logp=1.5) that maintains high lipase activity due to its large spatial site barrier effect [181].Solaesa et al.(2016) [175] were able to produce MAGs rich inω-3 polyunsaturated fatty acids (PUFA) by enzymatic glycerolysis of sardine oil using TP(99%TP purity)as a solvent followed by molecular distillation,achieving a yield of 67% and 91% purity.Tertiary alcohols were chosen as the reaction medium because they have low logp(0.35 and 0.89),exhibit better hydrophilicity and hydrophobicity,ensure the mixability of the reactants,and provide a mild reaction site for the reaction.It was found that TB has a higher selectivity and TP has a higher conversion rate,so some studies also choose a mixture of TB and TP as the reaction medium[173].Furthermore,it was also found that the price of TB was cheaper than TP,so a single TB or TB/TP (4:1)could be selected for an effective reaction system [182].
4.3.Ionic liquids
As a new type of reaction media,ionic liquids (ILs) possess many unique properties [183],such as near-zero vapor pressure,high chemical and thermal stability,and tunable physicochemical properties,made these compounds popular as a medium for chemical andbiochemical reactions [184].Some of the other tunable properties include polarity,hydrophobicity and sol-sol miscibility behavior by modifying the included cationic and anionic groups [185].ILs are reported to have protective effects on enzymes for stability enhancement and to be recoverable and recyclable [186,187].Perhaps the most attractive feature of ILs is the tunability of their properties for specific applications through the selection of suitable cations and anions[188,189].The long-chain alkyl or acyl groups of the cationic portion of the ILs can significantly increase the solubility of the oils and fats and improve the initial reaction rate (manifested as a shortened induction period) [190].Therefore,this is the reason why some of the enzymatic reaction experiments use ILs as a reaction medium to produce MAGs and DAGs.
There are also various types of ILs,such as the ionic liquid shown in Fig.9 is a tetraammonium-based IL(CPMA.MS).Some of the ILs are able to form hydrogen bonds with hydroxyl groups on glycerol or ester groups,and the strong hydrogen bonding interactions of glycerol greatly reduce the thermodynamic activity of glycerol,corresponding to an improved TAGs conversion and products(more than 90%of MAGs)with strong tolerance[191].Pure ILs are not as perfect as Newtonian liquids,and the viscosity of the mixture of ILs and substrate decreases significantly with increasing rate and shear time,where the behavior is somewhat similar to thixotropic liquids[192].
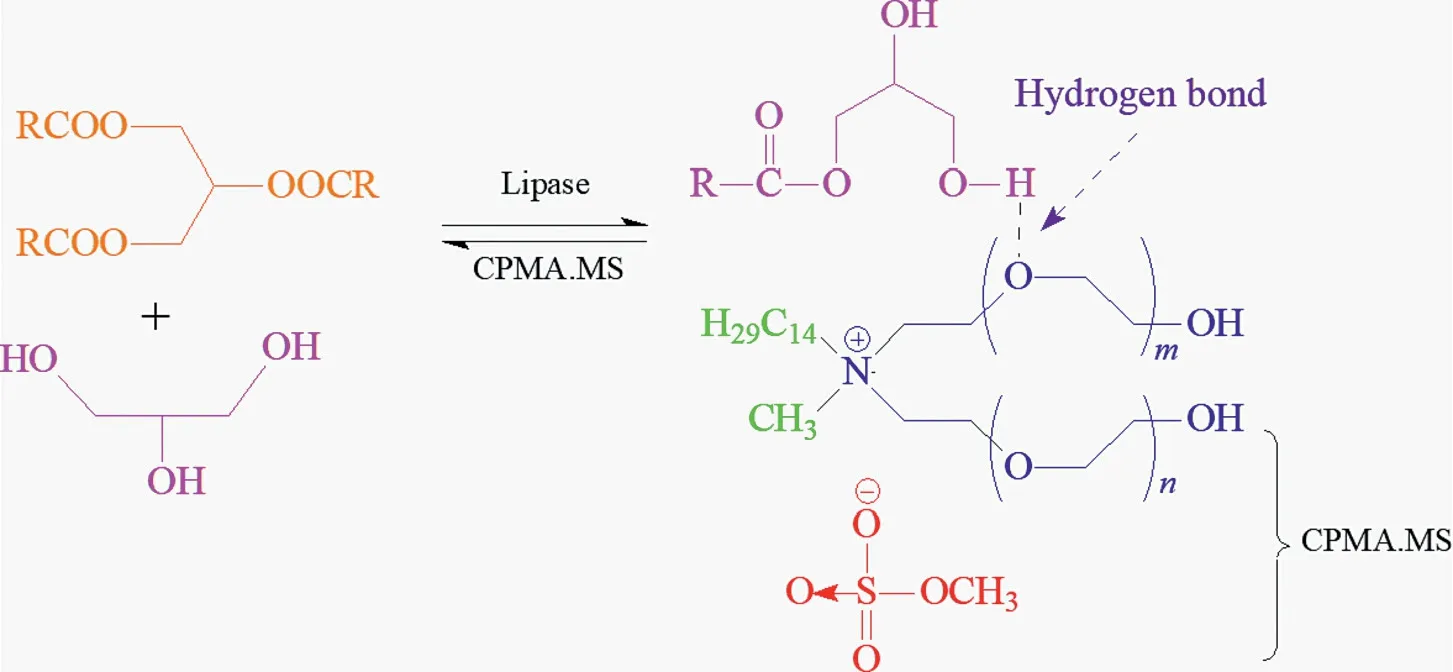
Fig.9.Proposed mechanism for the ionic liquid molecule inducing reaction equilibrium shifting.Adapted from [186,187].
By testing many kinds of ILs,a comparison selected several of the most remarkable ones,such as[TOMA]⋅[Tf2N],Ammoeng 120,and the reaction systems of Ammoeng 100 and 102 deserve further study[193].[TOMA]⋅[Tf2N]and Ammoeng 120 preferably promote the formation of DAGs because the three octyl groups in the cation increase the hydrophobicity of [TOMA]⋅[Tf2N],which then increases the oil solubility of the reaction,as indicated in Table 10.The TAGs conversions under Ammoeng 100 and Ammoeng 102 were very high,and the glycerol in Ammoeng 102 was much faster and more reactive,reaching almost equilibrium at 4 h and up to 90%TAGs conversion after 8 h.Since it was found that a single IL system was not sufficient to achieve much higher yields of the desired products than conventional solvents,a binary IL reaction system was introduced,consisting of an IL with better selectivity for DAGs production and another IL capable of achieving high oil conversion rates[156,195].The[TOMA]⋅[Tf2N]/Ammoeng 100 system resulted in high DAGs yields and TAGs conversions with relatively low MAGs concentrations compared to the pure Ammoeng 100 system[196].[BMIM]⋅[BF4] and [BMIM]⋅[PF6] are deeply studied ILs [194].Lozano et al.(2016) [167] studied eight different ionic reaction media with lauric acid as substrate,using immobilizedPseudomonasaeruginosalipase B at a temperature of 60◦C with a glycerol/oil ratio of 4:1.It was found that an equilibrium reaction in an IL reaction medium of[C12mim]⋅[BF4](1-dodecyl-3-methylimidazolium tetrafluoroborate)for 8 h resulted in the formation of a mixture of 85% MAG and 15% DAG,while 2 mmol of TB was used during this reaction to reduce the viscosity of the solution and speed up the reaction rate.COSMO-RS is a simulation program to integrate the main interactions between ionic liquid systems(electrostatic (polarity),hydrogen bonding and van der Waals (dispersion)),fully summarizing the multiple solventization interactions of ILs[197].It could be used for the pre-screening of ionic liquids for a specific reaction system when all the reaction systems were clarified or defined[198].

Table 10 Reaction systems in Ionic liquids.
Glycerol cleavage in CPMA.MS exhibited different characteristics,i.e.significantly high yield of MAGs and high oil conversion,which were not observed in conventional solvent and solvent-free systems[156].As shown in Fig.10,a comparison of the three systems,ILs,solvent-free and TB,showed the ability of ILs to promote MAGs ester formation and thus reach higher MAGs yields.This may stem from the fact that ILs not only have negligible volatile pressure,but also act very similarly to common solvents,dissolving both polar and non-polar compounds,even performing much better than common solvents in many cases [199,200].However,so far,the use of ILs as reaction media is still rarely reported,which may result from the longer equilibration time of ILs due to the lower initial reaction rate.The regulations on ionic liquids for food ingredients processing are also highly needed.
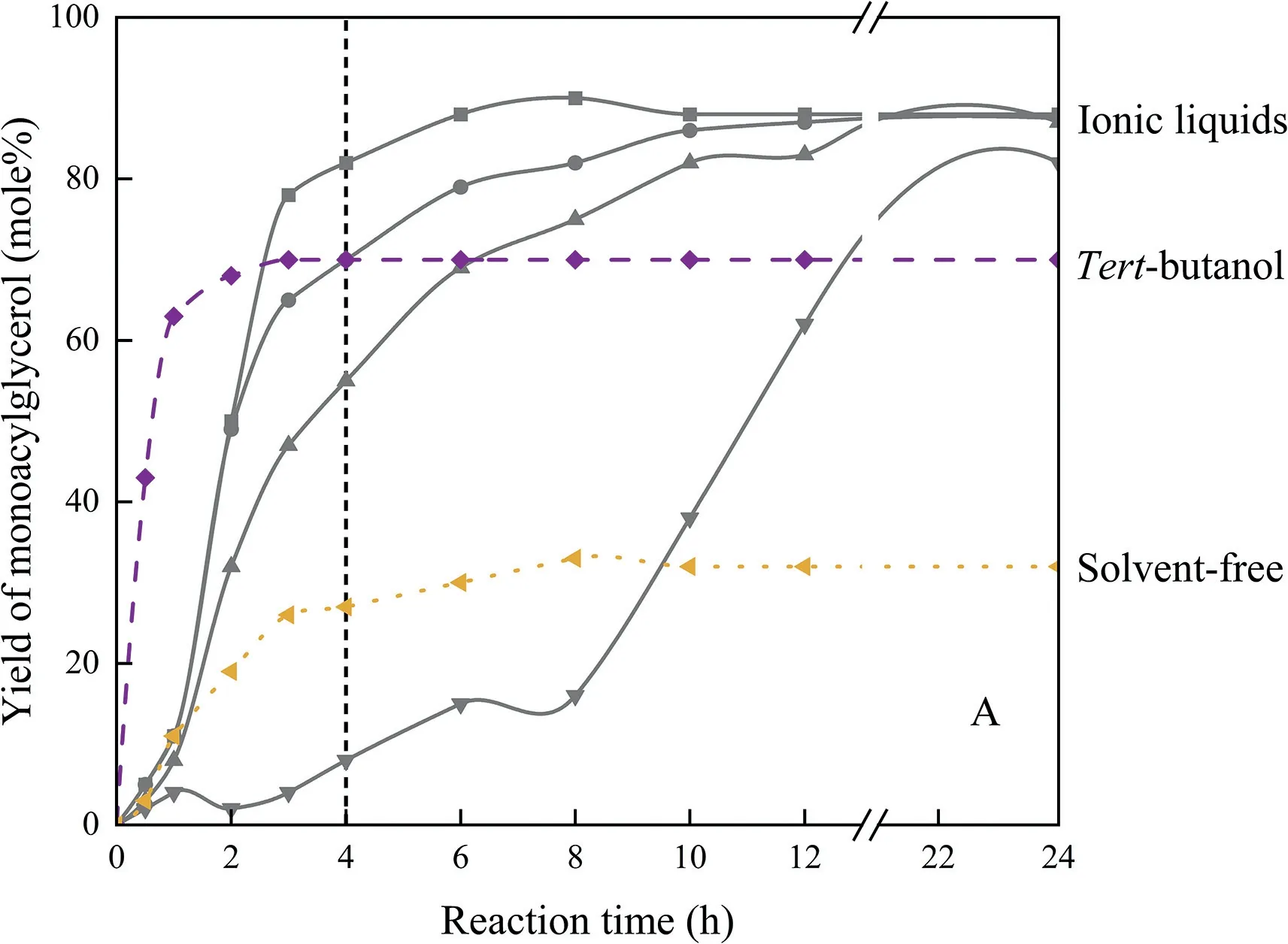
Fig.10.Ionic liquids shift the reaction to monoglycerides beyond equilibrium.Adapted from [186].
The use of ILs as a reaction medium for the production of MAGs and DAGs has a high citation prospect,but the research on ILs is so limited in companies or industries that there are few ionic liquid reactions that can be referenced.It is highly needed to continue to explore the technology in terms of feasibility and practical applications as well as the ionic liquids development for food uses.Surely some of the research has put up the foundation for the screening and further optimization of ILs[186].
4.4.Other reaction media
In addition to tertiary alcohols and ionic liquids,there are many substances used as reaction media,such as alkanes,acetones[200],deep eutectic solvents (DES),etc.Alkanes such asn-hexane are also used as reaction media in some experiments because of their easy recovery properties [201].However,the amount of glycerol dissolved in hexane alone is very small,so a polar organic solvent mixed with hexane is often used for glycerol cleavage to produce MAGs [202],where can also achieve high yields [203].Some other studies have shown that conventional organic solvents are not favorable for the enzymatic synthesis of DAGs,while a deep eutectic solvent [204] was found having good aspects as a reaction medium [205].Glycerol cleavage was then performed with soybean oil and the yield of DAGs was found to be in the range of 40%–60%,while the maximum yield of DAGs in the solventfree system was around 68%,which is higher than the discussed system.However,the DES system is more selective than the solvent-freesystem,and the DES system decreases the activity of glycerol,leading to a decrease in the yield of MAGs,which in turn favors the selective formation of DAGs [205].Therefore,the choice of media or not,or what type of reaction media to choose is based on the needs of the producers.
Thus,whether choose or not to choose a solvent system or which solvent system is chosen as well as what optimal solvent system or solvent-free system in rational designs are very much dependent on your target of the products,post-reaction purification or processing,product quality/cost ratio,facility investment,as well as sustainability performance.The possibilities are there for your planning in terms of the optimal performance as targeted.
5.Patent evaluation
This evaluation was conducted using patent data for DAGs and MAGs extracted from the Orbit(www.orbit.com)database,which is one of the most trustworthy and comprehensive patent information providers worldwide.Firstly,MAGs related patents application was summarized in Table 11 [206–213].Indeed,a relatively low number of inventions have been reported in this area (eight patents),mainly focusing on functional MAGs preparation,MAGs quality or yield improvement,and cost reduction.
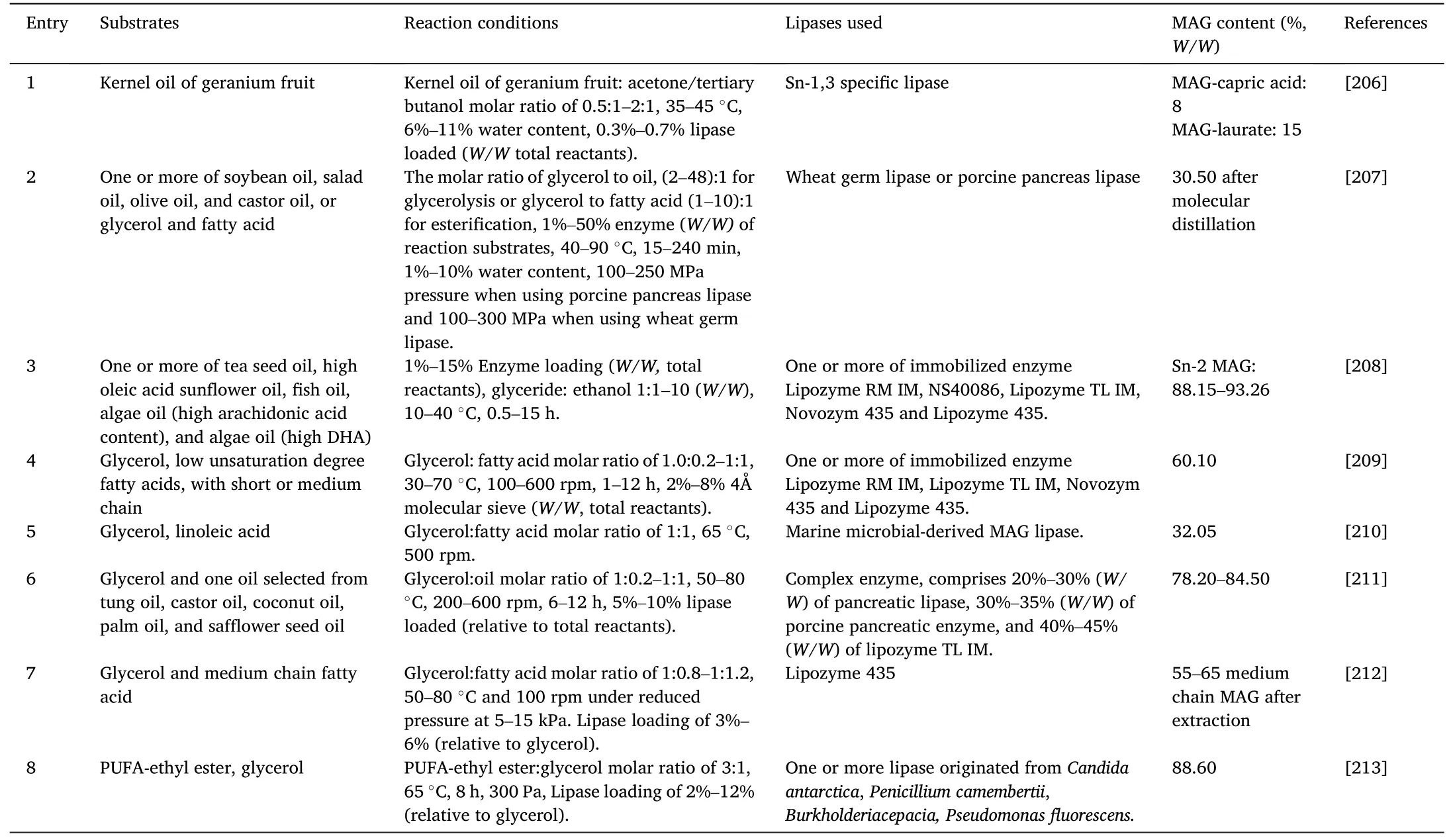
Table 11 Enzymatic preparation of MAG in patents.
On the other hand,a total of 55 patent families of DAGs published between 1986 and 2022 were retrieved.In the following,an overview of these patents in terms of invention year and regional distribution is given.Finally,a summary of the research interests,product forms,and process routes used is presented in the hope of providing a further insight into the innovation profile of DAGs.
Fig.11 illustrates the annual application numbers of DAGs patents since 1986.Overall,the number of DAGs related patent applications is gradually growing over time.Particularly,the number of patents in 2019–2022 has increased by 100% compared to the total application number in 2015–2017.And there is a clear trend that the earliest technological inventions were mainly originated or protected in other regions,while,from 2003 on,Chinese companies have also started filing DAGs patent applications as well.Note that the inventions from other regions have gradually declined in recent years,and the current research has mainly originated or been filed in China.The geographic coverage of places where DAGs patent applications have been filed is shown in Fig.12.As shown in Fig.12,more than half of the patents were filed in China with 29 patents,followed by Japan with 16 patents,Korea with five patents and the United States and other regions with three patents and two patents,respectively.This gives a picture of the targeted geographic markets where the technology is currently most prominent and commercially available,helping researchers to scan their filing strategy to ensure that their portfolios cover the top jurisdictions,and then make them an attractive acquisition target.Moreover,this may also help to identify the untapped markets for the technology.In summary,it can be inferred that the DAGs research area is currently a growing sector.And these data also point to an active market and a promising future for the DAGs industry in China.In addition,the extension of this technology into other regions may also be a potential opportunity.

Fig.11.The numbers of application patents between 1986 and 2022.(Update to Apr.2,2023).

Fig.12.The number and distribution of patent application across the world.(Update to Apr.2,2023).
In terms of the final form of products,apart from the conventional DAGs products(37 patents),the attentions in this filed were also paid to DAGs with different configurations as well as special functional characteristics,including 1,3-DAG,1,2-DAG,DAG-poly unsaturated fatty acid(PUFA),and DAG-monounsaturated fatty acid(MUFA)(Fig.13).It is well known that DAGs show a different metabolic pathway from normal TAGs in human body,thus suppress body fat accumulation and postprandial serum TAGs level[5].This healthy effect could be further enhanced when their configuration or composition is changed,making the physiological functional advantages of the product more pronounced.From a data point of view,most of the research on functional DAGs has focused on 1,3-DAG with eight patents and DAG-PUFA with seven patents.On the other hand,only a small number of technologies targeted at 1,2-DAG (one patent) and DAG-MUFA (two patents) have been focused on or filed.This happens because some enzymatic methods(e.g.,enzymatic hydrolysis) mainly produce 1,2-DAG rather than 1,3-DAG,whereas,in fact,1,3-DAG exhibited higher physiological activities than 1,2-DAG [214,215].Therefore,there are more research interested in preparing 1,3-DAG.On the other hand,DAG-PUFA have many attractive effects,including improving memory,reducing cholesterol,preventing cardiovascular system diseases and the like,while the high polyunsaturated fatty acid content also leads to poor stability and many side reactions,such as oxidation,polymerization,etc.[216].That would certainly add to production requirements and cost concerns.Thus,a high-efficiency DAGs production process,especially for DAGs with particular functions or conformations,is needed to be found.
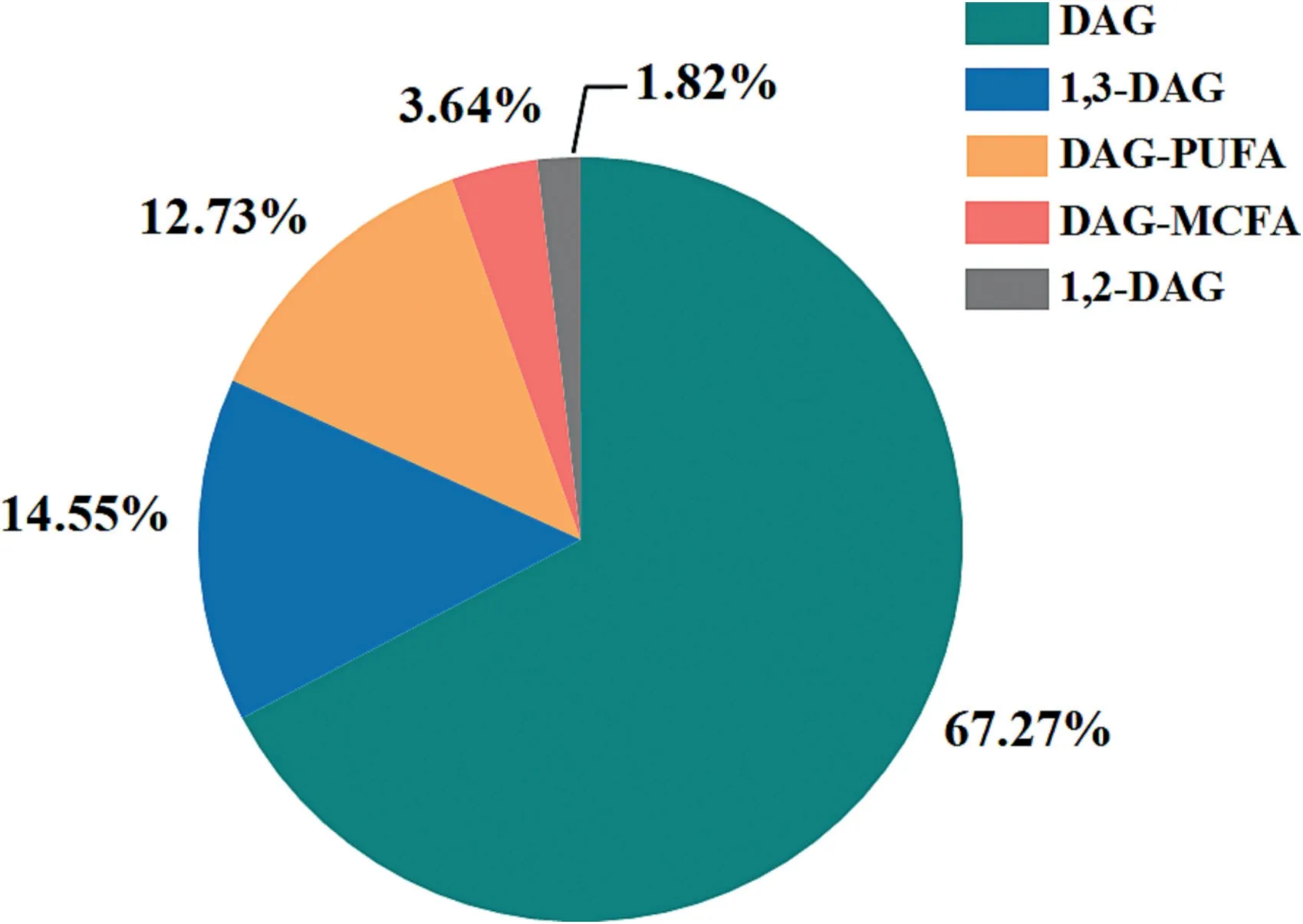
Fig.13.The distribution of DAGs research interests in product form.(Update to Apr.2,2023).DAG,diacylglycerol;PUFA,polyunsaturated fatty acids;MCFA,medium chain fatty acids.
In fact,the performance of products and the impact of progress in production are the driving forces that promote the technological innovations.In the domain of DAGs invention,as shown in Fig.14a,thearea of most interests is the purity of the final product,followed by the efficiency and yield of the methods,the cost of production,and the functions of product.Other topics include co-production,flavor,color.In more detail,at an early stage,researchers mainly focused on finding ways to increase the overall yield and product purity,while avoiding discoloration of the DAGs at high temperatures.Subsequently,as basic production methods have matured,research interests have shifted towards the practical use of DAGs products,such as the flavor,functional properties,and capacity for co-production of the obtained products.

Fig.14.The trend of (a) research interests (b) technical effects (c) key issues for process optimization of DAGs invention.(Update to Apr.2,2023).DAG,diacylglycerol.
This fact also shapes the trajectory of the development of the process routes.Considering simplicity and cost,the earliest commercialized methods utilized in this field are hydrolysis,glycerolysis and esterification (Fig.14b).Since 2000,the use of esterification and glycerolysis has opened up new developments in the preparation of DAGs.Currently,esterification,hydrolysis and multi-step preparation of DAGs are now the three most widely used methods in this field.Wherein,the multi-step process method is mainly achieved by combining hydrolysis and esterification,acetylation and transesterification,or glycerolysis and transesterification.Besides,hydrolysis method provides a new possibility for natural oils to be converted into chemical raw materials through a simple process,which greatly shortens the preparation process[4].Among these methods,esterification is the most concerned one for its high efficiency and yield,mild reaction conditions,environmental-friendly,etc.[217].
The importance of optimizing the production process has been emphasized repeatedly above.Finally,combining the current points of interest in this area,and the key words that are involved the most times in updating the techniques,it can be inferred that the improvement of purity is mainly achieved by optimizing reaction processes,solvents and enzymes (Fig.14c).On the other hand,reaction processes also play a crucial role in increasing the overall yield.In addition,the substrate acts as a critical factor that largely determines the final functionality of the product and,moreover,is an important factor for increasing the efficiency of the reaction.At the same time,the efficiency also can be improved by a reasonable selection of enzymes.
6.Conclusion and outlook
MAGs and DAGs are partial glycerides which are derived from oils and fats in general.MAGs are typical food emulsifiers and DAGs are lately developed into a nutritional oil and fat product for edible uses.Various reactions can be applied for the synthesis or production of them including glycerolysis,esterification,hydrolysis,and ethanolysis.The reaction mechanisms are different and the choice of the reaction systems relates to many issues including the choice of fatty materials,product quality and specifications,product yields,medium selection,reactor designs,processing steps,purification technology,etc.Both esterification and glycerolysis have been used in industry.
Enzymatic production of acylglycerols to replace chemical processes has been in industry also particularly for DAGs production.MAGs are traditionally produced using chemical processes as food emulsifiers.In the case of polyunsaturated fatty acids such as DHA and EPA,the MAG products are not suitable with chemical processes for the production as the high temperature as well as the use of chemical catalysts is not friendly to the stability of the fatty acids.Furthermore,enzyme technology has many more advantages for common products such as the selectivity property leading to less by-products,mild temperature leading to higher quality and less energy consuming,less CO2production leading to better sustainability,etc.
Partial glycerides are part of reaction equilibrium as demonstrated in Fig.6 in all the reaction routes.To increase either MAGs or DAGs portion in the end of the products,the first strategy is to create a system that can reach equilibrium.An efficient reaction system is required with a common incentive to make it homogeneous for the reactants and catalysts to meet freely for reactions to happen.In this case,media are important when a hydrophobic oil or fatty acids phase and a hydrophilic water,glycerol,or ethanol phase are in the reaction system.When an efficient system is possible,the yield of either MAGs or DAGs is quite dependent on the ratios of substrates.The ratios of the hydrophobic phase and hydrophilic phase is also influencing the homogeneity of the system.For the DAGs focusing case,the portion of hydrophilic phase is minor which can be dispersive in the oil phase so that a homogeneity can be reached.In an equilibrium containing MAGs and DAGs,higher yields could be also reached if the targeted products could be locked or separated from the system so that the reaction equilibrium is broken,and further reactions are continued beyond equilibrium.This is confirmed by the case using ionic liquids as a green solvent.
There is huge potentiality nowadays for the production of DAG oils with 1,3-DAGs as the main components as a nutritional oil product.For the current production of a few commercial products in the market,the cost seems high as the prices of the products are 3–10 times higher than normal oils and fats products.There should be space for development of a further efficient system with more effective cost terms of the technology.Besides the consideration of reaction routes,the media creation as well as the generation of industrial feasibility of breaking the reaction equilibria to lead a higher product turnover could be potential breakthrough of the technology,with the consideration of separation challenge of using a solvent medium in the reaction system.A smart medium with green solvents and no volatility could be an interesting target of development.
CRediT authorship contribution statement
Jiawei Zheng:Writing– original draft,Validation,Investigation,Conceptualization.Yudong Liang:Writing–original draft,Validation,Investigation.Jiaxi Li:Writing– original draft,Validation,Investigation.Shuping Lin:Validation,Investigation,Formal analysis.Qiangyue Zhang:Validation,Investigation,Formal analysis.Kanghua Zuo:Investigation,Formal analysis.Nanjing Zhong:Writing– review &editing,Writing– original draft,Supervision,Conceptualization.Xuebing Xu:Writing– review &editing,Supervision,Conceptualization.
Declaration of Competing Interest
The author Xuebing Xu is an Associate Editor for Grain&Oil Science and Technology and was not involved in the editorial review or the decision to publish this article.The authors declare that there are no conflicts of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31772000).Wilmar (Shanghai) Biotechnology Research and Development Ltd.is acknowledged for permission for the publication of the paper.
 Grain & Oil Science and Technology2023年4期
Grain & Oil Science and Technology2023年4期
- Grain & Oil Science and Technology的其它文章
- Natural and modified food hydrocolloids as gluten replacement in baked foods: Functional benefits
- Functional butter for reduction of consumption risk and improvement of nutrition
- Phosphatidylserine: An overview on functionality,processing techniques,patents,and prospects
- Optimizing techno-functionality of germinated whole wheat flour steamed bread via glucose oxidase (Gox) and pentosanase (Pn) enzyme innovation
