Serotonin enrichment of rice endosperm by metabolic engineering
Qingqing Yng, Yn Tn, Ying Ye, Dongsheng Zho, Qioqun Liu,b,*
a Key Laboratory of Crop Genomics and Molecular Breeding of Jiangsu Province/Zhongshan Biological Breeding Laboratory/Key Laboratory of Plant Functional Genomics of the Ministry of Education, College of Agriculture, Yangzhou University, Yangzhou 225009, Jiangsu, China
b Jiangsu Co-Innovation Center for Modern Production Technology of Grain Crops/Jiangsu Key Laboratory of Crop Genetics and Physiology, Yangzhou University, Yangzhou 225009, Jiangsu, China
Keywords:Rice endosperm Serotonin Metabolic engineering TDCs T5H
ABSTRACT In animals, serotonin is a neurotransmitter and mood regulator.In plants, serotonin functions in energy acquisition, tissue maintenance, delay of senescence, and response to biotic and abiotic stresses.In this study, we examined the effect of serotonin enrichment of rice endosperm on plant growth, endosperm development, and grain quality.To do so, TDCs and T5H were selected as targets for serotonin fortification.Overexpression of TDC1 or TDC3 increased serotonin accumulation relative to overexpression of T5H in rice grain.Transgenic lines of target genes driven by the Gt1 promoter showed better field performance than those driven by the Ubi promoter.Overexpression of T5H showed little effect on plant growth or grain physicochemical quality.In neuronal cell culture assays, serotonin induced neuroprotective action against apoptosis.Breeding of rice cultivars with high serotonin content may be beneficial for health and nutrition.
1.Introduction
Rice(Oryza sativa L.)provides energy and nutrition for one third of the world’s population.Rice endosperm supports grain development, quality formation, and seed physiology [1].Over the past 20 years, it has been used as a bioreactor to enhance nutrition and develop functional food crops[2].One endosperm metabolite,serotonin, acts as a neurotransmitter and mood regulator in humans.Studies [3,4] have indicated its roles in plant vegetative growth, morphogenesis, germination, and abiotic and biotic stress responses, its biofortification and influence on rice endosperm have not been reported yet.
In higher plants, serotonin biosynthesis forms a branch of the tryptophan metabolism pathway.In this pathway, tryptophan is decarboxylated by tryptophan decarboxylase (TDC) into tryptamine, which is then converted into serotonin by tryptamine 5-hydroxylase (T5H) (Fig.1A).Both enzymes function in regulating serotonin levels [4].In rice, studies of serotonin tend to focus on its role in plant growth and development, as well as responses to biotic and abiotic stress.Appropriate serotonin levels are required for proper shoot growth in seedlings[3].Overexpression of the TDC gene driven by the constitutive CaMV 35S promoter in rice resulted in accumulation of serotonin, causing maldevelopment, pollen hypofertility, and a dark-brown plant phenotype [5].The rice mutant spl5 (spotted leaf 5) presents increased serotonin levels and expression of TDC and T5H, resulting in a hypersensitive leaf response to pathogens [6].Constitutive overexpression of T5H reduced melatonin, serotonin, and tryptamine levels in transgenic rice seedlings [7].
There has been no attempt to produce serotonin in the rice endosperm or identify the consequent effects on plant growth,endosperm development, and rice quality.The objective of this study was to evaluate a potential strategy for breeding high serotonin rice via regulation of the serotonin biosynthesis pathway driven by different promoters.
2.Materials and methods
2.1.Plant materials, transgene construction, and rice transformation
An elite japonica rice cultivar from China, Wuxiangjing 9(WXJ9), was used for transformation.T5H (LOC_Os12g16720),TDC1 (LOC_Os08g04540), and TDC3 (LOC_Os08g04560) genes were subcloned into the vector pSB1300 [8].Six transgene constructs were used for T5H, TDC1, and TDC3 overexpression, driven by the rice endosperm-specific glutelin (Gt1) and constitutive ubiquitin(Ubi) promoters.The GT, GD1, and GD3 constructs provided overexpression of T5H, TDC1, and TDC3 genes driven by the Gt1 promoter, and constructs UT, UD1, and UD3 provided constitutive expression driven by the Ubi promoter.
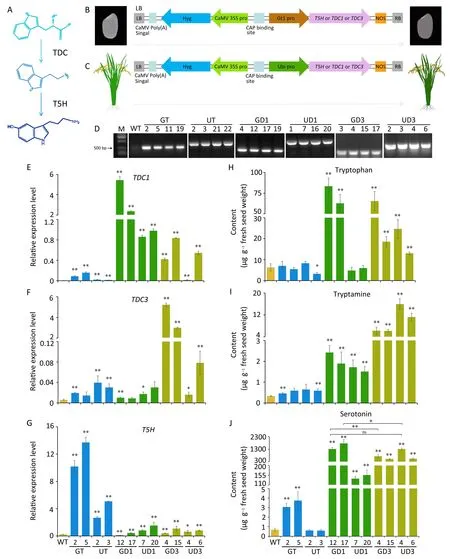
Fig.1.Serotonin accumulation via regulation of serotonin biosynthesis in the rice endosperm.(A)The serotonin biosynthesis pathway and its key enzymes TDC and T5H in rice.(B–C)Schematic representation ofthe recombinant vectors harboring T5H used for serotonin biosynthesis.(D)Target genes were amplified by PCR to screen and identify transgenic homozygous lines.(E–G) qRT-PCR analyses of TDC1, TDC3, and T5H genes in transgenic and WT seeds obtained 15 days after flowering.(H–J) Tryptophan,tryptamine and serotonin contents (μg g-1 fresh seed weight) of mature milled seeds from transgenic and WT rice.Values are mean ± SD.*, P < 0.05; **, P < 0.01; ns, not significant.(Student’s t-test).
Agrobacterium-mediated transformation was performed as described previously [9] using rice calli from mature embryos as explants.PCR screening was then performed using transgenespecific primers (Table S1) and stably transformed plants were regenerated and grown in paddy fields for subsequent analysis(Fig.1D).All selected transgenic lines were homozygous for the target genes.
2.2.Quantitative RT-PCR and enzyme activity assay
Total RNA extraction and preparation were performed as previously described [10] with three biological replicates per sample.Total RNA was purified from rice seeds sampled 15 d after flowering (DAF) and then reverse-transcribed into cDNA for quantitative reverse-transcription PCR (qRT-PCR).As an internal control, the rice housekeeping gene actin was used for measurement of relative T5H,TDC1,TDC2,and TDC3 expression levels.The gene-specific primers used for RT-PCR were designed with Primer Premier5.All primers are listed in Table S1.
TDC activity was measured as previously described[11].Developing seeds sampled 15 DAF were ground to fine powder and extracted with 50 mmol L-1K-Pi buffer (pH 6.9) containing 5 mmol L-1β-mercaptoethanol.The supernatant obtained after purification and incubation was separated by LC-MS as described below.
2.3.Metabolite analysis
Free amino acid content was determined as described previously [10].Free trptophan, tryptamine, and serotonin were extracted and quantified by LC-MS/MS following Yang et al.[12].Briefly, the extraction solution was injected into an Agilent 1200–6460 QQQ mass spectrometer equipped with a Cadenza CD-C18 column(150×4.6 mm;particle size,5 mm;Agilent;Santa Clara, CA, USA).Elution was performed with 0.1% formic acid:acetonitrile at a rate of 100:0[v/v]for 0 min,99:1 for 10 min,96:4 for 30 min, 70:30 for 60 min, 0:100 for 75 min, and 100:0 for 80 min,at a flow rate of 1 mL min-1with monitoring at a wavelength of 280 nm.
Melatonin was extracted from the rice samples as previously described [7] with slight modifications.Briefly, frozen samples(200 mg) were homogenized to fine powder and extracted overnight with 1 mL methanol before centrifuging.A 10-μL aliquot was then subjected to HPLC using a fluorescence detector (Rigol,Suzhou,Jiangsu,China).The samples were separated using an Ultimate XB C18 reverse column (250 × 4.6 mm, 5 μm) and elution was performed with 0.1% formic acid:methanol (60:40 [v/v]) at a flow rate of 0.8 mL min-1.The samples were monitored at an excitation wavelength of 280 nm and emission wavelength of 348 nm.
2.4.Measurement of grain quality
Mature seeds were milled and processed into flour for grain quality and physicochemical characterization [13].Total starch,crude protein, and apparent amylose content (AAC), as well as gel consistency (GC), and Rapid Visco Analyzer (RVA) profile were determined as described previously [13,14].Purified starch samples were obtained from milled rice endosperm based on the neutral protease method [15].
2.5.Field trials and measurement of agronomic traits
Transgenic and non-transgenic wild-type (WT) plants were grown in an experimental field at Yangzhou University as part of a small-scale field trial from 2018 to 2021 under safety supervision.Plots were designed in triplicate, with tested lines planted randomly in each.Plants were grown under similar climatic conditions and managed.Main agronomic traits were recorded at maturity before harvest of mature seeds.
2.6.Cell culture
Mouse hippocampal neuron cell line (HT-22) cells were maintained in DMEM (Dulbecco’s modified Eagle’s medium) medium supplemented with 10% FBS (fetal bovine serum), 100 μg mL-1streptomycin and 100 units mL-1penicillin at 37 °C and 5% CO2.Passages 3–10 of the HT-22 cells were used.All in vitro tests also included a vehicle control group that contained only 0.1% HCl.
2.7.Assays of cell proliferation and cell viability
A Cell Counting Kit-8 (CCK-8, CP736, DOJINDO Laboratories,Japan)was used to assay cell proliferation.HT-22 cells were seeded in a 96-well plate at 10,000 cells/well and supplemented with 100 μL of 10% FBS DMEM.Based on the CCK-8 solution protocol(Dojindo, Kumamoto, Japan), 10 μL of CCK-8 solution diluted in 100 μL of complete culture medium was used to replace the original medium.After incubation in the dark at 37 °C for 2 h, cells were quantified at an absorbance of 450 nm to test cell proliferation.
HT-22 cells seeded as described above were incubated for 24 h.Thirty minutes prior to H2O2(500 μmol L-1) treatment,they were preincubated with serotonin (31.25–500 μg mL-1) or without,based on previous findings[16].Cell viability was determined after 12 h by absorbance at 450 nm using a microplate reader (BioTek ELx800, Winooski, VT, USA).The percentage of surviving cells relative to the control values was taken as representing viability.
To verify the protective effect of transgenic rice seed extract containing serotonin, HT-22 cells were incubated in extracts from each transgenic line and the WT(with 31.25 μg mL-1of serotonin as a positive control) 30 min prior to H2O2(500 μmol L-1) treatment.Final concentrations of GT-2, GT-5, GD1-12, and WT were 30, 30, 30 and 0.68 μg mL-1, respectively.After 12 h, cell viability was measured by CCK-8 assay.
2.8.Flow cytometry
Cell apoptosis was measured by the FITC-Annexin V/PI doublestaining technique as previously described[17].HT-22 cells at the logarithmic growth phase were seeded in 6-well plates.They were then washed twice in PBS, digested with 0.25% trypsin, harvested in Eppendorf tubes, and centrifuged at 1000 r min-1for 5 min at 4 °C.The supernatants were discarded and the remaining HT-22 cells were resuspended in 500 μL 1× binding buffer (BD Biosciences, Franklin Lakes, NJ, USA) at a concentration of 5×105-cells mL-1.Annexin V-FITC and propidium iodide, 5 μL each,were then added to each tube before placing in the dark at room temperature for 10 min.Flow cytometry (FCM, Beckman-Coulter CytoFLEX,Miami,FL,USA)was then performed.All data acquisition and analyses were performed with Quest software(Becton–Dickinson, San Diego, CA, USA).
2.9.Statistical analysis
At least three replicate measurements were used for sample characterization,unless otherwise specified.Comparisons of transgenic and WT plants were performed using Student’s t-test with SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
3.Results
3.1.Overexpression and identification of transgenic plants
TDC(in particular,TDC1 and TDC3)was previously found[5]to function in the regulation of serotonin biosynthesis and the metabolic flux of tryptophan metabolism.In this study, three genes(TDC1, TDC3 and T5H) were chosen as targets for serotonin fortification in rice (in particular, rice endosperm).For this purpose, six constructs were generated using a single target gene driven by the Gt1 or Ubi promoter [7,18] (Fig.1B, C).Using these six constructs, transgenic rice lines were generated via Agrobacteriummediated genetic transformation.PCR analysis of the transgenic lines was then employed to confirm insertion of the target genes.Screening of the T1–T5generations revealed more than 20 homozygous transgenic lines per construct(Figs.1D,S1).Two T4transgenic lines per construct were selected for qRT-PCR,confirming the high expression of each target gene in the endosperm at 15 DAF.Expression levels were higher with the Gt1 promoter than with the Ubi promoter (Fig.1E–G), as was further confirmed by measurement of enzyme activity (Fig.S2).Overall, expression of TDC2 was least affected in all transgenic lines (Fig.S2).
3.2.Metabolite analysis in transgenic lines expressing different constructs
Determination of tryptophan, tryptamine, and serotonin(Fig.S3), revealed a significantly higher tryptophan content in the T4mature rice endosperm than in the WT(Fig.1H),and significant increases in tryptamine levels in all transgenic lines (Fig.1I).The transgenic line GD1 showed the highest level of serotonin(1765 ± 326 μg g-1fresh milled seeds) followed by the WT (0.68± 0.17 μg g-1fresh milled seeds) and then by UD3 and GD3, suggesting that the regulatory effect of TDC on serotonin accumulation was greater than that of T5H in mature rice seeds(Fig.1J).Overall,the serotonin content of mature milled GT rice seeds was 5.48-fold higher than that of the WT (Fig.1J).T3generations of the transgenic lines showed similar results (Fig.S4).The serotonin content of brown rice followed the same trend(Fig.S5).The serotonin contents of brown and milled rice from the Ubi-driven transgenic lines differed more than those of the Gt1-driven brown and milled rice(Fig.S5).
Melatonin,a downstream metabolite of serotonin,is a bioactive molecule.To determine the effects of serotonin enrichment on its derivatives, we measured melatonin levels in mature transgenic rice seeds.Melatonin content increased in the transgenic compared to WT seeds, with a slightly greater increase in the T5Hoverexpressing lines (Fig.S6).
3.3.Effects of serotonin accumulation on field performance and grain physicochemical qualities
Main agronomic traits were strongly affected by overexpression of TDC(Fig.2A–D;Table S2).In contrast,the main agronomic traits of GT transgenic rice differed negligibly from those of the WT(Fig.2A–D; Table S2).Contents of tryptamine and serotonin in the UT transgenic rice were similar to or lower than those of the GT transgenic lines, and changes in plant and panicle morphology were found only in the UT rice(Fig.1I–J;Table S2).These findings suggest that selection of the Gt1 promoter exerted a greater effect on the level of serotonin in the resulting transgenic plants than that of Ubi promoter.These results suggest that regulation of T5H expression represents a potential strategy for breeding functional rice with a high serotonin level.
Protein content and starch properties are the main components of rice endosperm affecting nutritional and cooking qualities [13].In this study, contents of protein, starch and apparent amylose were measured along with viscosity.Differences of grain endosperm of the transgenic GT and UT lines from the WT were not significant,except for an increase in protein(Fig.2E–H).Our previous study [12] revealed an increase in serotonin levels in the endosperm of high-lysine rice.In agreement with this finding,most free amino acid contents increased,with a significant increase in lysine(Fig.S7), further suggesting a correlation between lysine metabolism and serotonin metabolism.Mature seeds of TDCoverexpressing lines with a dramatic increase in serotonin also showed greater changes in amino acid levels than the T5Hoverexpressing lines and the WT (Fig.S7).A significant increase in threonine(Fig.S7),and significant decreases in alanine and serine were observed (Fig.S7).Together, these results suggest that moderate serotonin fortification did not impair the nutritional or physical–chemical qualities.
3.4.Protective effect of transgenic rice seeds extracts on oxidative stress-induced apoptosis in HT-22 cells
Natural remedies act both prophylactically and by alleviating symptoms of diseases such as depression and gastrointestinal disease.The number of people suffering from such diseases in the future is expected to increase [19],increasing the need for natural remedies.Given that serotonin is known to have neuroprotective properties, we performed a series of in vitro assays to determine whether serotonin fortification of the transgenic seeds could also affect neurons.High doses of serotonin inhibited cell proliferation following preincubation of HT-22 cells with several concentrations of serotonin (Fig.2I).As a control, 31.25 μg mL-1serotonin was used to determine the effect of rice seed extracts containing serotonin on hydrogen peroxide-induced cell death of HT-22 cells.Rice seed extracts from transgenic lines GT-5, GT-2, and GD1-12 blocked the influence of the peroxide on cell activity and proliferation, protecting the neuronal cells from hydrogen peroxideinduced cytotoxicity (Fig.2J).The number of apoptotic bodies was reduced in comparison with the WT, and the effect was only slightly lower than that of the serotonin control(Fig.2K–M).These results suggest that the rice seed extracts exerted neuroprotective action against oxidative stress-mediated apoptosis in the neuronal cells.This finding accords with a previous study[20]of the effect of 10 μmol L-1serotonin on the virulence of enteric pathogens.We infer that a 1-g GD1-12 seed extract is sufficient to meet serotonin requirements.Overall,high-serotonin rice not only provides nutrition but may alleviate various disease conditions.
4.Discussion and conclusions
Since the first identification of serotonin in the legume Mucuna pruriens, its widespread distribution in plants has been confirmed[3].It has been suggested that serotonin functions in plant developmental processes as well as biotic and abiotic stress responses[21].TDC and T5H genes,which regulate serotonin levels,have also been identified in several species [3].But no previous attempt has been made to increase serotonin levels in the rice endosperm.In this study, we identified an increase in serotonin content in rice endosperm following endosperm-specific Gt1 promoter-driven overexpression of T5H (Fig.1J).Optimal agronomic performance was also observed in the transgenic plants (Fig.2A–D; Table S2).The increase in serotonin was also found to have neuroprotective action,supporting the value of serotonin biofortification.The serotonin content of the GD3 transgenic rice was similar to or lower than that of the UD3 transgenic lines, although greater changes in panicle and grain morphology were found in the GD3 rice(Fig.2A–D; Table S2).We attribute panicle and grain morphology changes in GD3 rice to the differing effects of promoters and to the high accumulation of tryptamine.
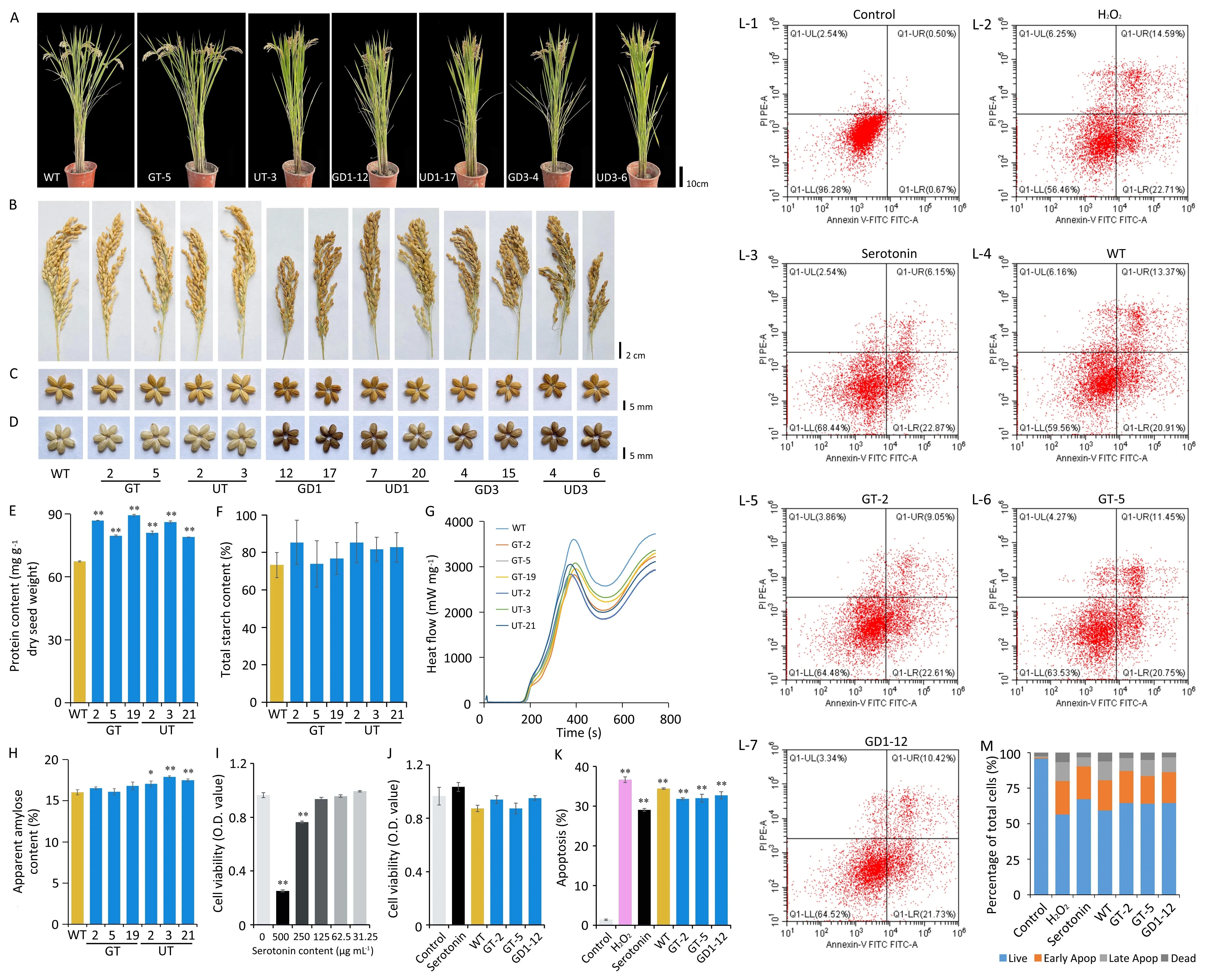
Fig.2.Effects of serotonin accumulation on field performance,grain physicochemical qualities,and cell activity.(A)Morphological observations of transgenic lines and WT.(B–D) The appearance of spikes and seeds from transgenic and WT rice.(E) Total protein and (F) starch contents (mg g-1 dry seed weight) of mature milled seeds.(G) RVA spectra of the rice flours.(H)The percentage of apparent amylose content(AAC)in mature milled rice flour.(I)Effect of concentrations(32.25–500.00 μg mL-1)of serotonin on cell proliferation by CCK-8 in HT-22 cells.(J) Inhibitory effect of transgenic rice seed extract on oxidative stress-induced cytotoxicity in HT-22 cells.(K–L) The effects of transgenic rice seed extract on cell apoptosis in HT-22 cells by flow cytometry.(M) Inhibitory effects on oxidative stress-induced apoptosis with HT-22 cells.Values are mean ± SD.*, P < 0.05; **, P < 0.01; ns, not significant (Student’s t-test).
A slight difference in serotonin levels was also observed between brown and milled rice; that is, a greater difference was observed in the serotonin content between Ubi-driven than between Gt1-driven brown and milled rice (Fig.S5).One possible explanation is that the Gt1(GluA2)promoter is expressed mainly in endosperm [22], whereas Ubi-driven transgenic lines are greatly influenced by the seed coat,aleurone layer,and embryo.The highest content of serotonin in mature seeds of T4transgenic rice was observed in GD1, while the highest content in T3transgenic rice was observed in UD3(Figs.1J,S4),owing possibly to environmental factors and the effect of the light/dark cycle on serotonin biosynthesis [23].In our previous study [12], expression of TDC3 was more affected by temperature than that of TDC1.
The melatonin level increased in mature seeds of the transgenic lines relative to the WT(Fig.S6).Irrespective of the differing effects of the promoters, this finding suggests that the balance between melatonin and serotonin as well as the dose-dependent effect of serotonin are important for the melatonin accumulation, with serotonin and melatonin metabolism represented by a complex metabolic network [24,25].
Previous studies[7,18]used three independently overexpressed TDC1, TDC2, TDC3) and one T5H gene to assess the effects on serotonin and melatonin, but effects on mature seeds were not reported.Mutant rice overexpressing TDC1 and TDC3 showed severely stunted growth and low fertility [5].But tryptamine and serotonin contents in rice calli expressing OASA1D and TDC were much higher than those in rice seedlings expressing only TDC[26,27].Further study of the effect of TDC and T5H co-expression on grain serotonin content is required.
To summarize, regulation of the serotonin metabolic pathway increased serotonin content,and T5H gene expression in rice endosperm showed no effects on transgenic plant growth or grain quality relative to the effects of TDC expression.Serotonin showed neuroprotective action against apoptosis of neuronal cells.Increasing serotonin accumulation via T5H regulation using an appropriate endosperm-specific promoter could result in serotonin biofortification of milled rice, benefiting both human health and nutrition.
CRediT authorship contribution statement
Qingqing Yang:Formal analysis,Investigation,Writing– original draft, Project administration, Funding acquisition.Yan Tan:Investigation, Writing – original draft.Ying Ye:Investigation.Dongsheng Zhao:Validation, Supervision, Writing – review &editing.Qiaoquan Liu:Conceptualization, Resources, Supervision,Project administration, Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (32270586, 31825019, and 31801322) and the Department of Science and Technology of Jiangsu Province (BM2022008-02 and BE2022336).
Appendix A.Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2023.07.003.
- The Crop Journal的其它文章
- OsSPL10 controls trichome development by interacting with OsWOX3B at both transcription and protein levels in rice (Oryza sativa L.)
- Ectopic expression of OsNF-YA8, an endosperm-specific nuclear factor Y transcription-factor gene, causes vegetative and reproductive development defects in rice
- Mechanisms of autophagy function and regulation in plant growth,development, and response to abiotic stress
- ZmDRR206 functions in maintaining cell wall integrity during maize seedling growth and defense response to external stresses
- The plasmodesmata-associated β-1,3-glucanase gene GhPdBG regulates fiber development in cotton
- The MabHLH11 transcription factor interacting with MaMYB4 acts additively in increasing plant scopolin biosynthesis

