枳PtMLP1启动子的克隆和表达分析
姚利晓,苏娟,郭兴茹,李凤龙,何永睿,邹修平,陈善春
枳启动子的克隆和表达分析
姚利晓,苏娟,郭兴茹,李凤龙,何永睿,邹修平,陈善春
西南大学柑桔研究所/国家柑桔工程技术中心/国家柑桔品种改良中心,重庆 400712
【目的】基因工程是柑橘品种改良的一种重要手段。本研究基于枳根消减文库中主要乳胶蛋白基因片段,克隆根特异启动子序列,为研究外源基因在柑橘根组织的特异表达奠定基础。【方法】同源克隆及启动子序列。利用ExPASy、PSIPRED、SWISS-MODEL等在线软件对编码蛋白的理化特征、二级结构和三级结构进行生物信息学分析,利用PlantCARE数据库对启动子的顺式作用元件进行预测。实时荧光定量PCR法对在不同树龄枳根和叶中的表达进行分析。构建启动子与标记基因的融合载体,利用根癌农杆菌转化法转化枳上胚轴,GUS染色观察标记基因的表达部位。【结果】枳含2个外显子和1个内含子,开放阅读框长471 bp。PtMLP1蛋白由156个氨基酸组成,分子量17.63 kDa,等电点5.49,含Bet v I功能域。其二级结构含3个-螺旋和7个-折叠,三级结构包含一个保守疏水基结合位点和一个富含甘氨酸的回环结构。5′端1 666 bp的上游调控序列不仅有TATA-box、CAAT-box等启动子结构的核心元件,还具有多个根组织特异表达元件,以及TGACG-motif、P-box和ABRE等激素应答相关的顺式作用元件。3′端非翻译区具有加尾信号AATAAA。该基因在1月龄苗、6月龄苗、20年生成年枳根中的表达量分别是叶中的46.34、74.82、110.25倍。构建启动子的融合表达载体pBI121-ProPtMLP1::,获得枳转基因植株。启动子驱动在转基因枳幼苗根中特异表达,在3个转基因枳株系的根中表达量分别为叶中表达量的124.78、11.53和7.76倍。【结论】获得柑橘主要乳胶蛋白及启动子序列,该启动子可驱动标记基因在柑橘根组织特异表达。
枳;主要乳胶蛋白;根特异性启动子;
0 引言
【研究意义】柑橘是世界第一大水果,也是我国南方地区农民脱贫致富和乡村振兴的支柱产业。我国柑橘的产量和种植面积均居世界第一位。然而,柑橘面临着严重的干旱、冻害等非生物胁迫和黄龙病、溃疡病等生物胁迫。转基因技术是改良柑橘品质和增强抗性的重要手段[1-2]。启动子是决定外源基因转录效率的关键因素。在柑橘转基因研究中,来自烟草花叶病毒的35S启动子(cauliflower mosaic virus,CaMV35S)是最常用的组成型启动子[3]。但组成型启动子驱动外源基因在植物体内持续、高效表达,不仅耗费植物大量能量和养分,也可能会改变某些性状,影响植株的正常生长发育。因此,组织特异性启动子和诱导型启动子开始受到研究者的关注,前者驱动目的基因在特定的植物组织表达,后者可在特定的条件下诱导目的基因表达[4]。在植物转基因研究中使用特异性启动子,既能够减少转基因植物能量和养分的消耗,又可以降低转基因植物环境释放的风险。【前人研究进展】柑橘中研究相对较多的组织特异性启动子是韧皮部启动子,有柑橘来源的CsPP2.B1、CsVTE2[5]和CsSUS1p启动子[6]。也有外源韧皮部特异启动子用于柑橘转基因的研究,如水稻东格鲁杆状病毒(RTBV)启动子、拟南芥蔗糖/质子同向转运体基因(AtSUC2)启动子和豇豆富甘氨酸蛋白基因(GRP)启动子[7-8]。另外,来自马铃薯的KST1启动子在柑橘保卫细胞特异表达[9],花、果实、种子、胚和木质部等组织和器官特异性启动子也有应用于柑橘转基因研究的报道[3]。诱导型启动子也用于柑橘转基因研究,如低温和光诱导的柑橘Ruby1启动子[10]和人工合成的可被柑橘溃疡病菌效应因子特异识别的启动子[11]。【本研究切入点】根是植物的支撑器官,也是植物吸收水分和营养元素及响应外界胁迫的重要器官。已经从拟南芥[12]、烟草[13-14]、大豆[15]、鹰嘴豆[16]、水稻[17]、苹果[18]等植物中分离出根组织特异性启动子。目前,尚未发现柑橘根特异性启动子的报道。虽然异源植物根特异性启动子有可能用于柑橘转基因的研究,但是,特异性启动子的异源表达存在组成型表达的风险,如草莓根特异性启动子FaRB7在烟草中异源转化显示为组成型表达特性[19],胁迫诱导型启动子AtRD29A在柑橘中丧失了诱导表达功能[20]。【拟解决的关键问题】枳属于冬季落叶性灌木或灌木状小乔木,是柑橘产区常用的砧木。枳砧柑橘一般表现树势较矮化、抗寒性强、结果时间较早、果实品质佳、抗脚腐病和衰退病等优点,但易感裂皮病,对盐碱性土壤敏感,易表现缺铁性黄化症状。本研究在前期构建枳根消减文库和全长文库[21-22]基础上,对枳主要乳胶蛋白基因和启动子进行克隆和转基因植物组织表达分析,为柑橘根部性状的改良提供有利的基因资源,也为其他植物的根组织特异表达提供候选启动子。
1 材料与方法
试验于2021—2022年进行。枳()成年树叶片和种子取自国家柑橘种质资源圃(重庆)。枳实生苗和转基因植株在国家柑桔品种改良中心网室中培养。试验所用引物见表1。

表1 试验用引物
1.1 总RNA提取方法
分别取枣阳小叶枳根和叶各100 mg,在研钵中迅速加液氮研磨成粉末。转入1.5 mL RNase-free离心管中,加入1 mL Trizol试剂,按照Trizol法提取样品总RNA。
1.2 改良CTAB法提取DNA
称取约1 g幼叶材料,置于研钵中液氮研磨至粉末状,按照改良CTAB法提取叶片总DNA。溶于200 μL TE溶液,紫外分光光度法结合琼脂糖凝胶电泳检测DNA的浓度和纯度,储存于-20 ℃备用。
1.3 PtMLP1启动子和内含子序列克隆
根据5′端上游调控序列设计引物,以枣阳小叶枳基因组DNA为模板,2PaR1和2PaS2引物对克隆启动子序列。反应体系为10×Ex Taq Buffer 5 μL,MgCl2(25 mmol∙L-1)4 μL,dNTP(10 mmol∙L-1each)1 μL,上、下游引物(20 μmol∙L-1)各1 μL,DNA模板1 μL,Ex Taq 0.5 μL,加ddH2O至50 μL。反应条件为95 ℃ 1 min;94 ℃ 30 s,55—50 ℃(0.5 ℃/循环、30 s),72 ℃ 2 min,10个循环;94 ℃ 30 s,60 ℃ 30 s,72 ℃ 1—2 min,30个循环;72 ℃ 10 min。通过琼脂糖凝胶电泳纯化回收PCR产物,与pMD19 simple载体连接,将阳性克隆送三博远志基因公司测序。
1.4 生物信息学分析
利用在线软件ExPASy(http://web.expasy.org/ compute_pi)预测蛋白分子量、理论等电点等理化特性。PSIPRED 4.0(http://bioinf.cs.ucl.ac.uk/psipred/)预测蛋白的二级结构,SWISS-MODEL(https://swissmodel.expasy.org/)预测蛋白的三级结构。TMHMM和SignalP V5.0(https://services.healthtech.dtu.dk/)预测跨膜区域和信号肽区域。利用PlantCARE数据库(http://bioinformatics.psb.ugent.be/webtools/plantcare/ html/)预测启动子序列的顺式作用元件。
1.5 实时荧光定量PCR
以500 ng总RNA为模板,采用PrimeScript®RT Reagent Kit(Perfect Real Time)在37 ℃反应15 min合成cDNA第一链,85 ℃作用5 min灭活逆转录酶。实时荧光定量PCR的反应体系为:2×SYBR®Premix Ex Taq Buffer 12.5 μL,上、下游引物(10 μmol∙L-1)各1 μL,cDNA模板1 μL,加ddH2O至25 μL。反应条件为95 ℃ 90 s;95 ℃10 s,61 ℃20 s,72 ℃20 s,40个循环;72 ℃延伸1 min。以为内参基因,采用2-ΔΔCT法计算目的基因的相对表达量。每样品设3次生物学重复。
1.6 pBI121-ProPtMLP1::GUS表达载体构建
PCR克隆启动子序列,构建中间载体pMD19-ProPtMLP1。然后用限制性内切酶d Ⅲ和Ⅰ对pMD19-ProPtMLP1和pBI121分别进行酶切,琼脂糖凝胶纯化回收目的片段。将回收的pBI121载体片段和目的片段用T4连接酶连接,转入大肠杆菌DH5α中,提取单克隆抽提质粒进行酶切检测试验,结果显示pBI121-ProPtMLP1::载体构建成功。电击法转化到感受态根癌农杆菌EHA105细胞。
1.7 枳的遗传转化
用2%次氯酸钠对枳成熟种子消毒,将其播种于灭菌的MS固体培养基中。待上胚轴生长至8—10 cm,光照培养5 d。将含有pBI121-ProPtMLP1::载体的农杆菌EHA105侵染切割成1 cm小段的枳上胚轴,同时以含pBI121空载体的农杆菌侵染枳作为对照组。当枳不定芽长到1—2 cm的时候,将其转移到MS生根培养基(0.25 mg·L-1-萘乙酸、0.25 mg·L-1吲哚-3-丁酸、500 mg·L-1羧苄青霉素)生根培养4—6周,取叶片提取DNA,以GUSF/GUSR引物进行候选转基因植物的PCR检测。
1.8 GUS组织化学染色
柑橘转基因株系的根、叶按照Peng等[23]的方法进行GUS组织化学染色。GUS染液成分如下:100 mmol∙L-1NaH2PO4,100 mmol∙L-1Na2HPO4,0.5 mmol∙L-1K4[Fe(CN)6],0.5 mmol∙L-1K3[Fe(CN)6],10 mmol∙L-1EDTA-Na2,1 mmol∙L-1X-Gluc,0.1% Sodium azide,0.1% TritonX-100。37 ℃下孵育过夜,用70%酒精进行脱色,最后进行显微观察拍照。
2 结果
2.1 PtMLP1序列及生物信息学分析
通过核苷酸序列比对,发现枳根消减cDNA文库中contig 22和singleton 297[22]与枳根全长文库中的JK316196[21]为同一基因。三者经序列拼接后得到含完整开放阅读框的基因,编码主要乳胶蛋白(major latex protein),将其命名为。利用2PaS2和2PaR1引物克隆启动子序列,2IS和2IR引物克隆内含子序列。分析结构,发现含2个外显子(186 bp和285 bp)、1个内含子(104 bp)及5′端上游调控序列(1 666 bp)和具有poly(A)信号AATAAA的3′端非翻译区序列(207 bp)。
该基因开放阅读框长471 bp,以ATG为起始密码子,以TAG为终止密码子,共编码156个氨基酸(图1)。分析发现其编码蛋白具有保守Bet v 1功能域(2—152氨基酸残基),分子量为17.63 kDa,等电点为5.49,不含信号肽和跨膜区。预测PtMLP1蛋白二级结构中有3个-螺旋和7个-折叠,三级结构中包含一个保守疏水基结合位点和一个富含甘氨酸的回环结构。
启动子序列中具有TATA-box、CAAT- box等启动子结构的核心元件和多个根特异表达的顺式作用元件,还具有MYBHv1结合位点CCAAT、分生组织表达元件CAT-box、MeJA反应元件TGACG-motif,赤霉素反应元件P-box,脱落酸反应元件ABRE等应答元件(图1)。
2.2 PtMLP1组织表达分析
实时荧光定量PCR分析结果表明,1月龄幼苗根中的表达量是叶的46.34倍,6月龄苗根的表达量是叶的78.42倍,20年生成年树根的表达量是叶的110.25倍(图2-A)。
为进一步确认在根中的特异表达,下载其同源基因Cs2g_pb010910在甜橙不同组织中的转录组数据(http://citrus.hzau.edu.cn/)并进行分析。发现该基因在根转录组FPKM平均值为9 930.67,远高于早期胚珠、晚期胚珠、种子、幼果果肉、成熟果果肉和叶等组织(图2-B)。
2.3 ProPtMLP1::GUS在枳根中特异表达
同源克隆启动子序列,用其取代pBI121载体上d III和I酶切位点之间的CaMV35S启动子片段,成功构建pBI121-ProPtMLP1::载体(图3)。
对候选转基因枳抽提DNA进行标记基因的PCR检测,结果显示获得10株有ProPtMLP1启动子插入基因组的幼苗。组织化学染色结果表明转基因枳的叶中无色,根显现蓝色,且根组织纵切观察显示维管束组织染色深于表皮。而对照组(CaMV35S启动子)枳幼苗的根和叶中均显示蓝色。对部分枳转基因苗进行实时荧光定量PCR分析,结果表明转基因苗根中的表达量分别是叶中的124.78倍、11.53倍、7.77倍(图4)。
3 讨论
3.1 PtMLP1是柑橘中新发现的一种主要乳胶蛋白基因
主要乳胶蛋白(major latex protein,MLP)是植物特有的一种蛋白家族,其三级结构可形成疏水空腔结构和富含甘氨酸的回环结构,与疏水化合物相结合[24]。MLP首次从罂粟的乳胶中鉴定出来[25],广泛存在于其他植物中,目前已经从葡萄、苹果、黄瓜、西葫芦等园艺植物基因组中鉴定出多个家族成员[26-29],在柑橘中尚未见关于主要乳胶蛋白基因的报道。本研究从枳中克隆出,其编码蛋白不仅具有MLP蛋白二级结构中存在的-螺旋和-折叠,而且三级结构含有疏水基结合位点和富含甘氨酸的回环结构,是一种主要乳胶蛋白的编码基因。转录组数据和实时荧光定量PCR结果显示在柑橘根中特异表达。这与拟南芥和、棉花、西葫芦在根中优势表达[26,30-32]结果相一致。MLP基因家族成员在植物的其他组织中也存在优势或特异表达,如苹果主要在花中表达[33]。关于MLP功能的解析尚处于初级阶段,研究显示MLP正向调控种子休眠、营养生长,抑制生殖生长[31-32],在植物中过表达可增强对冻害、干旱等非生物胁迫的抗性[33-34],但具体调控机制未知,其在柑橘中的生物学功能值得关注。

黑色小写字体表示启动子序列,棕色横线为顺式作用元件:①根特异基序,②TGACT基序,③ P盒子,④CCAAT盒子,⑤CAT盒子,⑥ABRE,⑦CAAT盒子,⑧TATA盒子。红色大写字体表示外显子区域和编码氨基酸;绿色小写字体表示内含子;蓝色小写字体表示3′非翻译区,具有加尾信号AATAAA
The black lowercases mean promoter sequence, with the cis-acting elements marked with brown horizontal lines: ① root specific motif, ② TGACT-motif, ③ P-box, ④ CCAAT-box, ⑤ CAT-box, ⑥ ABRE, ⑦ CAAT-box, ⑧TATA-box. The red uppercases mean exons and their encoded amino acids. The green lowercases mean intron sequence. The blue lowercases mean a 3′-terminal untranslated region with the poly (A) signal (AATAAA)
图1及启动子序列
Fig. 1 The DNA sequence of

A:PtMLP1在不同生长期枳根和叶中的表达结果;B:PtMLP1同源基因Cs2g_pb010910在甜橙不同组织转录组中的FPKM值(数据来源于http://citrus.hzau.edu.cn/)A: The expression of PtMLP1 in roots and leaves of 1-month, 6-month, and 20-year-old Poncirus trifoliate; B: FPKM value of orthologous gene with PtMLP1 from RNA-seq data of sweet orange (Data from http://citrus.hzau.edu.cn/)

图3 PtMLP1启动子克隆(A)和植物表达载体构建示意图(B)
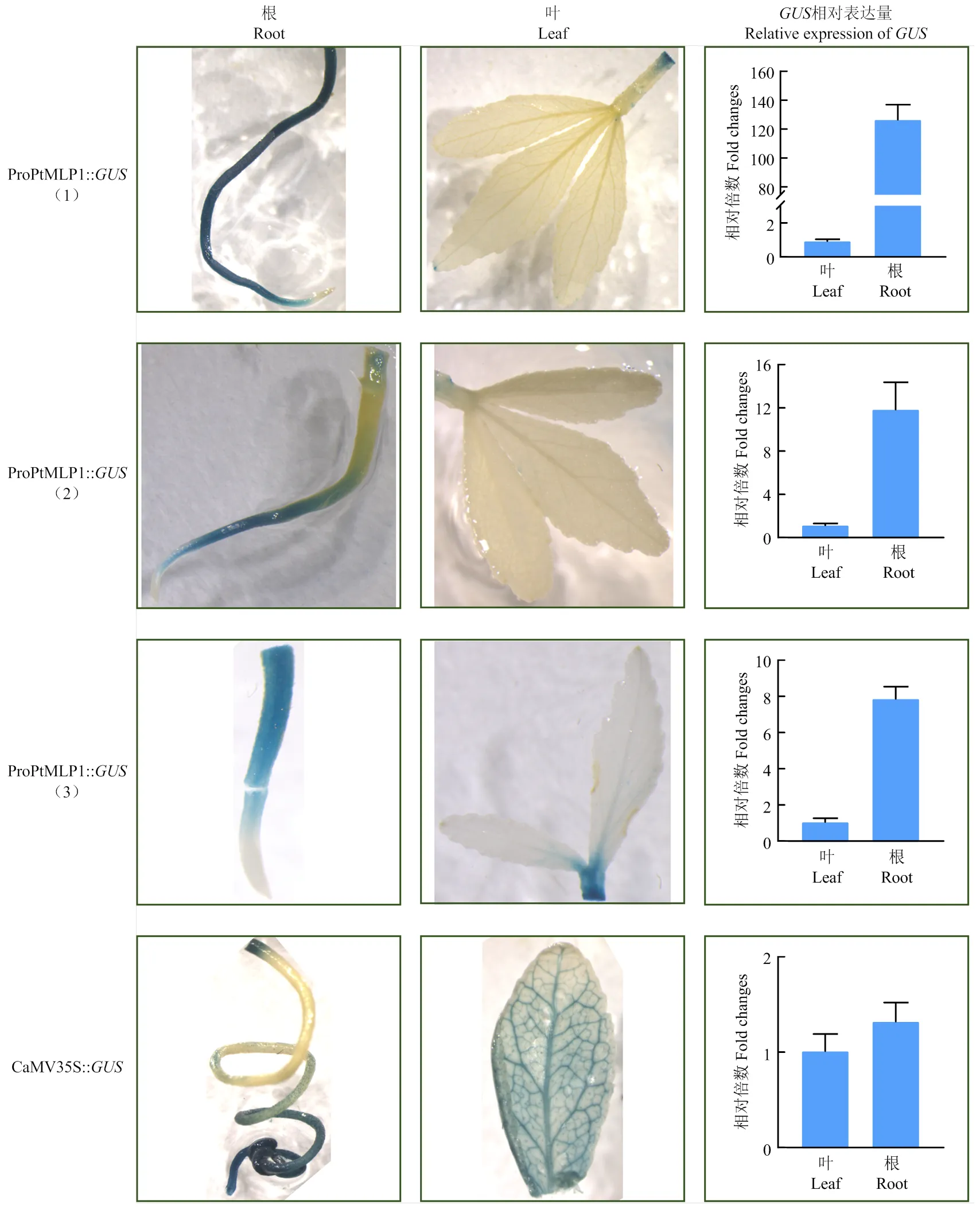
图4 部分ProPtMLP1::GUS转基因枳中GUS的表达分析
3.2 PtMLP1启动子是一种根特异表达启动子
在柑橘转基因研究中,常用烟草花叶病毒的35S启动子作为外源基因的组成型启动子,也利用诱导型启动子和韧皮部、木质部、花、果实、种子和胚等组织和器官特异性启动子[3]。根是植物生长、发育和抵抗不良环境的重要组织,关于柑橘根特异性表达基因已有研究成果,尚未发现关于柑橘根特异性启动子的报道或其他植物根特异性启动子应用于柑橘转基因研究的报道。
柑橘根中高表达或优势表达的基因常常来自功能基因家族的研究。海藻糖-6-磷酸合成酶(trehalose-6- phosphate synthase,TPS)在柑橘基因组中有8个成员,其中6个在根组织高表达,另外2个在茎中高表达[35]。6个多胺氧化酶基因在柑橘根中都存在优势表达[36]。和是8个H+-ATPase家族基因中在根中优势表达的成员[37]。笔者课题组在资阳香橙中发现与铁吸收和转运相关的铁螯合还原酶和5在根中优势表达,并受到缺铁胁迫的诱导[38]。另外,笔者课题组前期构建了枳根的消减cDNA文库,丰富了根组织中优势表达基因的资源[22]。MLP家族基因的启动子如拟南芥启动子可驱动标记基因在拟南芥根中特异表达[31]。本研究结果增加了MLP根特异性启动子的种类,也为柑橘转基因功能研究和种质创制提供了本源的根组织特异启动子。
4 结论
通过序列比对,发现枳根消减文库中contig22和singleton 297为的片段。的启动子可在转基因枳中驱动标记基因在根中特异表达。这种从根的特异表达基因发掘根特异启动子的研究思路可筛选和鉴定更多的柑橘组织特异性启动子,为柑橘砧木的改良提供更多的候选启动子种类,也为该启动子在柑橘和其他植物基因改良中的应用奠定了理论基础。
[1] 姚利晓, 何永睿, 邹修平, 雷天刚, 许兰珍, 彭爱红, 陈善春. 柑橘基因工程育种研究策略及其进展. 果树学报, 2013, 30(6): 1056-1064.
YAO L X, HE Y R, ZOU X P, LEI T G, XU L Z, PENG A H, CHEN S C. Advances and strategies in citrus genetic engineering and breeding. Journal of Fruit Science, 2013, 30(6): 1056-1064. (in Chinese)
[2] SOARES J M, TANWIR S E, GROSSER J W, DUTT M. Development of genetically modified citrus plants for the control of citrus canker and huanglongbing. Tropical Plant Pathology, 2020, 45(3): 237-250.
[3] CONTI G, XOCONOSTLE-CÁZARES B, MARCELINO-PÉREZ G, HOPP H E, REYES C A.genetic transformation: An overview of the current strategies and insights on the new emerging technologies. Frontiers in Plant Science, 2021, 12: 768197.
[4] ZHONG V, ARCHIBALD B N, BROPHY J A N. Transcriptional and post-transcriptional controls for tuning gene expression in plants. Current Opinion in Plant Biology, 2023, 71: 102315.
[5] DOS ANJOS BEZERRA Y C, MARQUES J P R, STIPP L C L, ATTÍLIO L B, FREITAS-ASTÚA J, DE ASSIS ALVES MOURÃO FILHO F. How to drive phloem gene expression? A case study with preferentially expressed citrus gene promoters. Revista Brasileira De Fruticultura, 2021, 43(4): e-005.
[6] SINGER S D, HILY J M, COX K D. The sucrose synthase-1 promoter fromdirects expression of the-glucuronidase reporter gene in phloem tissue and in response to wounding in transgenic plants. Planta, 2011, 234(3): 623-637.
[7] DUTT M, ANANTHAKRISHNAN G, JAROMIN M K, BRLANSKY R H, GROSSER J W. Evaluation of four phloem-specific promoters in vegetative tissues of transgenic citrus plants. Tree Physiology, 2012, 32(1): 83-93.
[8] 许兰珍, 彭爱红, 何永睿, 姚利晓, 雷天刚, 刘小丰, 姜国金, 邹修平, 陈善春. 异源韧皮部特异启动子在转基因枳中的表达. 园艺学报, 2014, 41(1): 1-8.
XU L Z, PENG A H, HE Y R, YAO L X, LEI T G, LIU X F, JIANG G J, ZOU X P, CHEN S C. Expression analysis of three phloem-specific promoters in transgenic. Acta Horticulturae Sinica, 2014, 41(1): 1-8. (in Chinese)
[9] KELLY G, LUGASSI N, BELAUSOV E, WOLF D, KHAMAISI B, BRANDSMA D, KOTTAPALLI J, FIDEL L, BEN-ZVI B, EGBARIA A, ACHEAMPONG A K, ZHENG C L, OR E, DISTELFELD A, DAVID-SCHWARTZ R, CARMI N, GRANOT D. TheKST1 partial promoter as a tool for guard cell expression in multiple plant species. Journal of Experimental Botany, 2017, 68(11): 2885-2897.
[10] HUANG D, YUAN Y, TANG Z Z, HUANG Y, KANG C Y, DENG X X, XU Q. Retrotransposon promoter of Ruby1 controls both light- and cold-induced accumulation of anthocyanins in blood orange. Plant, Cell & Environment, 2019, 42(11): 3092-3104.
[11] SHANTHARAJ D, RÖMER P, FIGUEIREDO J F L, MINSAVAGE G V, KRÖNAUER C, STALL R E, MOORE G A, FISHER L C, HU Y, HORVATH D M, LAHAYE T, JONES J B. An engineered promoter driving expression of a microbial avirulence gene confers recognition of TAL effectors and reduces growth of diversestrains in citrus. Molecular Plant Pathology, 2017, 18(7): 976-989.
[12] GONG J M, LEE D A, SCHROEDER J I. Long-distance root-to-shoot transport of phytochelatins and cadmium in. Proceedings of the National Academy of Sciences of the United States of America, 2003, 100(17): 10118-10123.
[13] CAI T C, CHEN H, YAN L M, ZHANG C, DENG Y, WU S X, YANG Q, PAN R L, RAZA A, CHEN S H, ZHUANG W J. The root-specific NtR12 promoter-based expression of RIP increased the resistance against bacterial wilt disease in tobacco.Molecular Biology Reports, 2022, 49(12): 11503-11514.
[14] ZHANG C, PAN S F, CHEN H, CAI T C, ZHUANG C H, DENG Y, ZHUANG Y H, ZENG Y H, CHEN S H, ZHUANG W J. Characterization of NtREL1, a novel root-specific gene from tobacco, and upstream promoter activity analysis in homologous and heterologous hosts. Plant Cell Reports, 2016, 35(4): 757-769.
[15] CHEN L, JIANG B J, WU C X, SUN S, HOU W S, HAN T F. The characterization of, a root-specific gene from soybean, and the expression analysis of its promoter.Plant Cell, Tissue and Organ Culture, 2015, 121(2): 259-274.
[16] KHANDAL H, GUPTA S K, DWIVEDI V, MANDAL D, SHARMA N K, VISHWAKARMA N K, PAL L, CHOUDHARY M, FRANCIS A, MALAKAR P, SINGH N P, SHARMA K, SINHAROY S, SINGH N P, SHARMA R, CHATTOPADHYAY D. Root-specific expression of chickpea cytokinin oxidase/dehydrogenase 6 leads to enhanced root growth, drought tolerance and yield without compromising nodulation. Plant Biotechnology Journal, 2020, 18(11): 2225-2240.
[17] LI Y Y, LI C X, CHENG L Z, YU S S, SHEN C J, PAN Y. Over- expression ofunder a rice root specific promoter. Plant Physiology and Biochemistry, 2019, 136: 52-57.
[18] LV D M, ZHANG Y H. Isolation and functional analysis of appleandgene promoters in transgenic. Plant Cell, Tissue and Organ Culture, 2017, 129(1): 133-143.
[19] VAUGHAN S P, JAMES D J, LINDSEY K, MASSIAH A J. Characterization of FaRB7, a near root-specific gene from strawberry (× ananassa Duch.) and promoter activity analysis in homologous and heterologous hosts. Journal of Experimental Botany, 2006, 57(14): 3901-3910.
[20] ORBOVIĆ V, ALI RAVANFAR S, ACANDA Y, NARVAEZ J, MERRITT B A, LEVY A, LOVATT C J. Stress-induciblepromoter constitutively drivesandexpression and precocious flowering in transgenicspp. Transgenic Research, 2021, 30(5): 687-699.
[21] 姚利晓, 王金萍, 何永睿, 雷天刚, 许兰珍, 彭爱红, 邹修平, 陈善春. 枳根cDNA全长文库的构建与分析. 园艺学报, 2015, 42(1): 149-156.
YAO L X, WANG J P, HE Y R, LEI T G, XU L Z, PENG A H, ZOU X P, CHEN S C. Construction and analysis of a root full-length cDNA library of. Acta Horticulturae Sinica, 2015, 42(1): 149-156. (in Chinese)
[22] 姚利晓, 何永睿, 许兰珍, 雷天刚, 彭爱红, 邹修平, 陈善春. 应用抑制性消减杂交技术从枳中筛选根特异表达基因. 园艺学报, 2014, 41(12): 2481-2488.
YAO L X, HE Y R, XU L Z, LEI T G, PENG A H, ZOU X P, CHEN S C. Identification of root-specific genes with subtractive suppression hybridization from. Acta Horticulturae Sinica, 2014, 41(12): 2481-2488. (in Chinese)
[23] PENG A H, ZOU X P, XU L Z, HE Y R, LEI T G, YAO L X, LI Q, CHEN S C. Improved protocol for the transformation of adultOsbeck ‘Tarocco’ blood orange tissues.Cellular & Developmental Biology-Plant, 2019, 55(6): 659-667.
[24] FUJITA K, INUI H. Review: Biological functions of major latex-like proteins in plants. Plant Science, 2021, 306: 110856.
[25] NESSLER C L, KURZ W G W, PELCHER L E. Isolation and analysis of the major latex protein genes of opium poppy.Plant Molecular Biology, 1990, 15(6): 951-953.
[26] FUJITA K, CHITOSE N, CHUJO M, KOMURA S, SONODA C, YOSHIDA M, INUI H. Genome-wide identification and characterization of major latex-like protein genes responsible for crop contamination in. Molecular Biology Reports, 2022, 49(8): 7773-7782.
[27] ZHANG N B, LI R M, SHEN W, JIAO S Z, ZHANG J X, XU W R. Genome-wide evolutionary characterization and expression analyses of major latex protein (MLP) family genes in. Molecular Genetics and Genomics, 2018, 293(5): 1061-1075.
[28] YUAN G P, HE S S, BIAN S X, HAN X L, LIU K, CONG P H, ZHANG C X. Genome-wide identification and expression analysis of major latex protein () family genes in the apple (Borkh.) genome. Gene, 2020, 733: 144275.
[29] KANG Y Y, TONG J L, LIU W, JIANG Z L, PAN G Z, NING X P, YANG X, ZHONG M. Comprehensive analysis of major latex-like protein family genes in cucumber (L.) and their potential roles inblight resistance. International Journal of Molecular Sciences, 2023, 24(1): 784.
[30] YANG C L, LIANG S, WANG H Y, HAN L B, WANG F X, CHENG H Q, WU X M, QU Z L, WU J H, XIA G X. Cotton major latex protein 28 functions as a positive regulator of the ethylene responsive factor 6 in defense against. Molecular Plant, 2015, 8(3): 399-411.
[31] CHONG S N, RAVINDRAN P, KUMAR P P. Regulation of primary seed dormancy by major latex protein-like protein329 inis dependent on dna-binding one zinc finger6. Journal of Experimental Botany, 2022, 73(19): 6838-6852.
[32] GUO D, WONG W S, XU W Z, SUN F F, QING D J, LI N.-cinnamic acid-enhanced 1 gene plays a role in regulation ofbolting. Plant Molecular Biology, 2011, 75(4/5): 481-495.
[33] LIU H, DU B Y, MA X C, WANG Y, CHENG N N, ZHANG Y H. Overexpression of major latex protein 423 () enhances the chilling stress tolerance in. Plant Science, 2023, 329: 111604.
[34] WANG Y P, YANG L, CHEN X, YE T T, ZHONG B, LIU R J, WU Y, CHAN Z L. Major latex protein-like protein 43 (MLP43) functions as a positive regulator during abscisic acid responses and confers drought tolerance in. Journal of Experimental Botany, 2016, 67(1): 421-434.
[35] LIU K H, ZHOU Y. Genome-wide identification of the trehalose-6- phosphate synthase gene family in sweet orange () and expression analysis in response to phytohormones and abiotic stresses. PeerJ, 2022, 10: e13934.
[36] WANG W, LIU J H. Genome-wide identification and expression analysis of the polyamine oxidase gene family in sweet orange (). Gene, 2015, 555(2): 421-429.
[37] SHI C Y, SONG R Q, HU X M, LIU X, JIN L F, LIU Y Z.PH5-like H(+)-ATPase genes: Identification and transcript analysis to investigate their possible relationship with citrate accumulation in fruits. Frontiers in Plant Science, 2015, 6: 135.
[38] YAO L X, HE Y R, FAN H F, XU L Z, LEI T G, ZOU X P, PENG A H, LI Q, CHEN S C. Identification and expression analysis of multiple ferric chelate reductases in. Journal of the American Society for Horticultural Science, 2017, 142(6): 419-424.
Cloning and Expression Analysis ofPromoter in
YAO LiXiao, SU Juan, GUO XingRu, LI FengLong, HE YongRui, ZOU XiuPing, CHEN ShanChun
Citrus Research Institute, Southwest University/National Citrus Engineering Technology Research Center/National Center for Citrus Varieties Improvement, Chongqing 400712
【Objective】Genetic transformation plays a significant role in exploring gene function and improving traits in citrus. Tissue-specific promoters is a key to regulate the expression of transgenes in particular tissues. Here, expression characteristics of thepromoter, isolated from the root subtractive library of, was thoroughly examined, which could lay a foundation for the specific expression of exogenous genes in citrus root tissue. 【Method】The complete sequence ofgene was cloned by PCR using DNA as a template. The physiochemical attributes, secondary and tertiary structures of PtMLP1 protein were predicted by ExPASy, PSIPRED, and SWISS-MODEL tools. Cis-acting elements inpromoter were predicted by PlantCARE. The expression pattern oftrees of diverse ages was examined by employing real-time qPCR. Furthermore, to investigate the tissue-specific expression of thepromoter in citrus, a pBI121-ProPtMLP1::plasmid, in whichexpression was controlled by thepromoter, was constructed and then introduced intothrough-mediated hypocotyl transformation. 【Result】consisted of two exons and one intron, which possessed a 471 bp open reading frame encoding a protein with 156 amino acid residues. This protein had a molecular weight of 17.63 kilodaltons with an isoelectric point of 5.49 and contained a Bet v I functional domain in its primary structure. Moreover, the secondary structure of PtMLP1 contained three α-helices and seven β-folds, while its tertiary structure had a conserved hydrophobic binding site and a cyclic domain, which was rich in glycine. Thepromoter was 1 666 bp long. Multiple root-specific expression elements, phytohormone response elements (such as the TGACG motif, P-box, and ABRE), and the TATA box and CAAT box core elements were predicted in the promoter. Additionally, the 3-terminal untranslated region ofwas predicted to contain a poly (A) signal AATAAA. Notably, the expression ofwas significantly higher in the roots of 1-month, 6-month, and 20-year-old, with fold changes of 46.34, 74.82, and 110.25, respectively, compared with those in leaves. GUS expression analysis of pBI121-ProPtMLP1::transgenic plants showed thatpromoter exhibited specific and high expression in roots, and its expression levels were 7.76 to 124.78 times of that in the leaves. 【Conclusion】The sequences of thegene and its promoter were successfully obtained, and the promoter demonstrated the ability to drive specific expression ofgene in citrus roots.
; major latex protein; root-specific promoter;

10.3864/j.issn.0578-1752.2023.24.009
2023-05-31;
2023-08-04
国家重点研发计划(2021YFD140080,2021YFD160080)、国家现代农业(柑橘)产业技术体系(CARS-26)
姚利晓,E-mail:yaolixiao@cric.cn。通信作者陈善春,E-mail:chenshanchun@cric.cn
(责任编辑 赵伶俐)

