Effect of Human Umbilical Cord Mesenchymal Stem Cells on GRP78/ATF4 Pathway in Alzheimer’s Disease Model Mice
Fuhong LI, Tianyu WANG, Junjie CAI, Zhuorui HE, Yufan ZANG, Liqun REN
Hebei Key Laboratory of Nerve Injury and Repair, Chengde Medical University, Chengde 067000, China
Abstract [Objectives] To study the effect of human umbilical cord mesenchymal stem cells (hUC-MSCs) on GRP78/ATF4 pathway in APP/PS1 mice. [Methods] Twelve 6-month-old female APP/PS1 mice were randomly divided into model group (MOD, n=6) and human umbilical cord mesenchymal stem cell treatment group (MSC, n=6); six 6-month-old C57BL/6N mice were used as control group (CON, n=6). The mice in each group were treated with the fourth generation of human umbilical cord mesenchymal stem cells through tail vein. Four weeks later, the mice in each group were killed. The expression of GFP78 and ATF4 in the cortex of mice in each group was detected by Western blotting and real-time fluorescence quantitative PCR. [Results] The results of immunoblotting and real-time fluorescence quantitative PCR showed that the expression of GRP78 in MOD group was lower than that in CON group and the expression of ATF4 increased. The expression of GRP78 protein in MSC group was higher than that in MOD group, but the expression of ATF4 protein was lower. The results of real-time fluorescence quantitative PCR showed that the mRNA level of GRP78 decreased and the mRNA level of ATF4 increased in MOD group compared with CON group. The mRNA level of GRP78 in MSC group was higher than that in MOD group, while the mRNA level of ATF4 in MSC group was lower than that in MOD group. [Conclusions] Human umbilical cord mesenchymal stem cells can regulate the expression of GRP78/ATF4 pathway in APP/PSI mice, which may be related to the stress level of endoplasmic reticulum in the brain of APP/PS1 mice mediated by human umbilical cord mesenchymal stem cells.
Key words Alzheimer’s disease, Human umbilical cord mesenchymal stem cells, APP/PS1 mice, Endoplasmic reticulum stress
1 Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease with occult onset. Its clinical manifestations are progressive cognitive decline, memory dysfunction and other symptoms, which seriously affect the quality of daily life of patients. Its pathological features include the deposition of β-amyloid (Aβ) outside neurons and the hyperphosphorylation of Tau protein in neurons to form Neurofibrillary Tangles (NFTs)[1]. Endoplasmic reticulum is the key site of protein synthesis, modification, folding and transport in cells, and it is an important organelle to promote signal transduction within or between cells. Some studies believe that Aβ deposition is related to protein misfolding and abnormal protein clearance pathway damage, and endoplasmic reticulum homeostasis is out of balance, thus activating endoplasmic reticulum stress (ERS), regulating damaged organelles by mediating the levels of glucose regulated protein 78 (GRP78) and activating transcription factor 4 (ATF4), and releasing protease or ubiquitin through lysosomal fusion to degrade contents, so as to maintain the stability of intracellular environment[2-3]. Human umbilical cord mesenchymal stem cells (hUC-MSCs), as multifunctional stem cells derived from neonatal cord blood, can cross the blood-brain barrier, reduce Aβ deposition and NFTs, promote autophagy, restore mitochondrial transport, regulate endoplasmic reticulum homeostasis,etc.[4-7], and are considered to have great potential in the treatment of AD. Studies have shown that hUC-MSCs injection can significantly improve the spatial learning function of AD model mice, delay memory loss and reduce the deposition of Aβ in the brain of AD patients[8-9].
Based on the correlation between AD and ERS, this study aims to explore the effect of hUC-MSCs on GRP78/ATF4 pathway in APP/PS1 mice, thus enriching the experimental basis of drugs targeting ERS to treat AD.
2 Materials and methods
2.1LaboratoryanimalsandgroupingTwelve 6-month-old SPF female APP/PS1 mice with a body weight of (20-25) g were purchased from Beijing Huafukang Biotechnology Co., Ltd. with the certificate of "SCXK (Beijing) 2019-0008"; six 6-month-old SPF female C57BL/6N mice, weighing (22-25) g, were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. with the certificate of "SCXK (Beijing) 2021-0006" and raised in the Experimental Animal Center of Chengde Medical University, with the certificate of "SCXK (Hebei) 2022-002". All experiments were approved by Animal Ethics Committee of Chengde Medical University (CDMULAC-20220401-001). The mice were raised in a barrier environment at temperature of (25±2) ℃ and humidity of (55±5) %, with a light and dark cycle of 12 h/12 h, and the mice ate and drank freely. After 7 d of adaptive feeding, the experiment was carried out. C57BL/6N mice were used as control group (CON group,n=6); twelve APP/PS1 mice were randomly divided into model group (MOD group,n=6) and hUC-MSCs treatment group (MSC group,n=6).
2.2MainmaterialsThe hUC-MSCs were purchased from Beijing Hongxin Stem Cell Biotechnology Co., Ltd.
2.3hUC-MSCscellresuscitationandcellcountThe hUC-MSCs cell cryopreservation tube was taken out from the ultra-low temperature freezer at -80 ℃, and quickly put into the constant temperature water tank at 37 ℃ after the cell generations and cryopreservation time were checked. It was gently shaken, and after it melted, its surface was disinfected with alcohol, it was transferred to the biosafety cabinet and the cryopreservation tube was opened. The cell suspension was pipetted into a centrifuge tube filled with 1 mL of normal saline. The centrifugation condition was 1 000 rpm, 5 min, the supernatant was discarded, and the washing was repeated for three times. Finally, the cells were re-suspended with 1 mL of normal saline and beaten evenly to prepare hUC-MSCs normal saline suspension. 10 μL of sample was taken from the above cell suspension and diluted to 1 mL with normal saline. The diluted cell suspension was stained with equal volume of trypan blue reagent for 1 min, and the number of living cells (N) was calculated by cell counting plate. Calculation formula: Total number of cells/mL=N/4×104.
According to the number of living cells, the cell suspension was made into hUC-MSCs normal saline suspension with a concentration of 2.5×106/mL, which was used for subsequent injection.
2.4IntravenousinjectionintorattailThe mice in each group were placed in a mouse fixator, and the tail of mice was wiped with alcohol cotton ball to make the tail vein clearly dilate. The thinner skin of 1/3 rat tail under the middle vein was selected and the suspension was injected into the rat tail vein.
Mice in MSC group were given 0.4 mL of hUC-MSCs saline suspension (106) by 1 mL disposable syringe, and the cell suspension was slowly injected without resistance. After injection, a small amount of blood returned can be seen in the syringe tube, indicating that the rat tail vein injection was successful. CON group and MOD group were given the same amount of sterile saline for tail vein injection. After injection, cotton balls were pressed to stop bleeding, and all mice were gently put back into cages. The above treatment was carried out once a week, with a total of 4 interventions in 4 weeks.
2.5BraintissuesamplingAfter anesthesia, the fur of mice in each group was cut along the midline at the foramen magnum to expose the whole skull. The skull was carefully separated, the rat cerebral cortex was stripped, placed in a sterile EP tube, packaged and stored in a refrigerator at -80 ℃.
2.6WesternblottingtestThe cortical tissues of mice (20 mg) were ground, the protein concentration was measured, the protein was separated by electrophoresis, then transferred to PVDF membrane, and sealed for 2 h by skim milk. GRP78 and ATF4 primary antibodies (1:3 000) were added overnight at 4 ℃, respectively, and the secondary antibodies (1:3 000) were added to incubate at 4 ℃ for 50 min. ECL was added dropwise to develop color, the chemiluminescence imaging system was used to take photos, and Image J software was used to analyze the relative expression of proteins.
2.7Real-timefluorescencequantitativePCRexperiment
20 mg of mouse cortical tissue was ground, and RNA was extracted by TaKaRa MiniBEST Universal RNA Extraction Kit. The concentration andOD260/280optical density of the extracted RNA were measured. The qualified RNA was reverse transcribed into cDNA by TaKaRa PrimeScriptTMRT Reagent Kit with gDNA Eraser (Perfect Real Time) Kit, and the cDNA was used as template for real-time fluorescence quantitative PCR experiment. The primer sequences are detailed in Table 1.

Table 1 Primer sequence
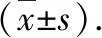
3 Results and analysis
3.1ResultsofWesternblottinginmiceAccording to the expression results of GRP78 and ATF4 protein in each group, the histogram of relative expression of GRP78 and ATF4 protein was drawn. Fig.1 shows that the expression of GRP78 decreased and the expression of ATF4 increased in MOD group compared with CON group. The expression of GRP78 in MSC group was higher than that in MOD group, but the expression of ATF4 decreased. The difference was statistically significant (P<0.05).

Note: CON: control group; MOD: model group; MSC: human umbilical cord mesenchymal stem cells treatment group, *P<0.05.
3.2Real-timefluorescencequantitativePCRtestresultsofmiceineachgroupAccording to the relative quantitative expression results of GRP78 and ATF4 mRNA in each group, the histogram of relative expression of GRP78 and ATF4 mRNA was drawn.
Fig.2 shows that the expression of GRP78 decreased and the expression of ATF4 increased in MOD group compared with CON group. The expression of GRP78 in MSC group was higher than that in MOD group, but the expression of ATF4 decreased, and the difference was statistically significant (P<0.05).

Note: CON. control group; MOD. model group; MSC. human umbilical cord mesenchymal stem cells treatment group; *P<0.05; ns: P>0.05.
4 Discussion
The pathogenesis of AD is complex, and it is found to be related to abnormal neuroinflammatory corpuscles, abnormal autophagy and endoplasmic reticulum stress[10]. In the course of AD, the imbalance of endoplasmic reticulum homeostasis is a long-term pathological state, which gradually changes ERS from temporary and pro-tective stress response to long-term and destructive stress process, resulting in the activation of a series of downstream factors and pathological damage[3, 11-12]. When ERS occurs, both GRP78 and PERK dissociate from immunoglobulin binding protein (Bip) and self-phosphorylate, which leads to synaptic damage and memory impairment[13]. ERS can also induce autophagy formation, digestion and aggregation of abnormally folded proteins and damaged organelles through ATF4 and Ca2+release. When endoplasmic reticulum stress continues, ATF4 transcriptional activity is activated in the late stage. This factor initiates apoptosis by driving the expression of C/EBP homologous protein. If the cell injury is aggravated, especially in the pathogenesis of AD, the unfolded protein response (UPR) enters the decompensation stage, ERS continues to be stressed, and finally induces apoptosis of damaged cells[14-17]. Our results showed that ATF4 levels in APP/PS1 mice were significantly higher than those in CON mice, suggesting that it might be related to the activation of ERS, and hUC-MSCs injection could effectively reduce ATF4 levels in the brain of APP/PS1 mice. In addition to AD, ERS-mediated ATF4 level reduction is also found in other central system diseases. For example, Zhang Aihuaetal.[19]found that ERS-mediated ATF4 pathway was closely related to Parkinson’s disease, which could cause expression of a large number of oxygen free radicals and cause damage to the synthesis of macromolecular substances such as DNA, protein and fat, participating in the regulation of transcription of various target genes, and regulating various physiological and pathological transcription regulation processes. In addition, the expression level of ATF4 protein in intestinal mucosa of many intestinal diseases such as ulcerative colitis is also significantly increased[20]. Under normal circumstances, GRP78 is located in the endoplasmic reticulum lumen, while PERK, ATF-6 and IRE1 remain inactivated due to GRP78 binding. Our results showed that the expression of GRP78 in MOD group was lower than that in CON group, and the expression of GRP78 in MSC group was higher than that in MOD group, which was consistent with the clinical observation of a significant decrease in GRP78 in the brain of AD patients[21]. In addition, some studies have found that the expression of GRP78 in AD model mice is up-regulated[22].
To sum up, the level of UPR in human AD samples proved by endoplasmic reticulum stress markers was also inconsistent, and the analysis of ERS markers should be carefully considered in accordance with AD model. This study found that hUC-MSCs could regulate the expression level of GRP78/ATF4 pathway in the brain cortex of APP/PS1 mice, which might be related to the endoplasmic reticulum stress level mediated by hUC-MSCs, enriching the theoretical research on stem cell therapy for AD with endoplasmic reticulum stress as the research direction, and providing experimental basis for drugs targeting ERS to treat AD.
- Medicinal Plant的其它文章
- Progress in the Application of Network Pharmacology in Mongolian Medicine Research
- Anti-tumor Effect of Paclitaxel Enhanced by Psoralen at the Cellular Level
- Preparation Process of Plumbagin Nanomicelle In-situ Gel
- Therapeutic Effect of Daphnetin on Mastitis Induced by Staphylococcus aureus in Mice
- Current Status and Prospects of Drugs for Ischemic Stroke Treatment
- Activity Screening Study on the Anti-tumor Effects of Extracts from Mahoniae caulis

