Impact of black cherry on pedunculate oak vitality in mixed forests:Balancing benefits and concerns
Ellen Dese,Bart Muys,Jan en Ouen,Bart Nyssen,Rta Sousa-Slva,Leon van en Berg, Arnol van en Burg, Gert-Jan van Dunen, Koenraa Van Meerbeek,Maake Weters, Karen Vancampenout
a Division Forest, Nature and Landscape, KU Leuven Campus Geel, Kleinhoefstraat 4, B-2240 Geel, Belgium
b Division Forest, Nature and Landscape, KU Leuven, Celestijnenlaan 200E, Box 2411, B-3001 Leuven, Belgium
c KU Leuven Plant Institute, B-3001 Leuven, Belgium
d Forest Ecology and Management Group, Wageningen University, P.O.Box 47, Wageningen, the Netherlands
e Bosgroep Zuid-Nederland, Huisvenseweg 14, 5591 VD Heeze, the Netherlands
f Young Academy for Sustainability Research, University of Freiburg, 79104 Freiburg im Breisgau, Germany
g Aquatic Ecology & Environmental Biology, Radboud University Nijmegen, Heyendaalseweg 135, 6525 AJ Nijmegen, the Netherlands
h Biosphere / Zoological Museum Netherlands, Onderlangs 17, 6731 BK Otterlo, the Netherlands
i Stichting Bargerveen, Toernooiveld 1, 6525 ED Nijmegen, the Netherlands
j B-WARE Research Centre/Radboud University Nijmegen, Toernooiveld 1, 6525 ED Nijmegen, the Netherlands
Keywords:Rich litter species Black cherry Nutrient cycling Insect herbivory Dilution effect Humus type Pedunculate oak Plant-soil interaction
ABSTRACT The vitality of European forests continues to decline due to new pests and diseases, climate-change related disturbances and high loads of atmospheric nitrogen deposition.Deteriorating soil health is a major factor underpinning the low vitality of West-European forests.Selecting tree species with soil ameliorative traits is proposed as an avenue to counteract soil acidification and improve overall forest vitality.Here we evaluate the impact of black cherry(Prunus serotina Ehrh.),a known rich litter species,on the vitality of neighboring pedunculate oak(Quercus robur L.) in ten mixed forests on sand in Germany, Belgium and the Netherlands.We found that black cherry admixture increases foliar N and P to a surplus whereas it causes deficiencies in foliar Mg,thereby resulting in an overall negative effect on oak foliar nutrient concentrations.Contrary, defoliation of oak leaves by herbivory decreases with the proximity of black cherry.Using structural equation modelling (SEM), we tested the hypothesized ‘improved soil health’ pathway.Our analyses showed that black cherry admixture leads to lower accumulation in the humus layer, resulting in higher soil base saturation which has a positive effect on foliar Ca yet a negative effect on total chlorophyll.Moreover, the SEM illustrated that herbivory of oak leaves decreases when black cherry is admixed,both via dilution and improved soil health.Indirect effects of black cherry on oak vitality via “improved soil health” in our SEM are however small in comparison to direct relations.Hence, our study showed that the combined positive and negative impacts of black cherry on oak vitality are limited,which tempers the potential benefits of using the rich litter species to counteract oak decline via improved soil healthyet,the concern of black cherry as an invasive alien species negatively affecting the vitality of mature pedunculate oak trees may also be exaggerated.
1.Introduction
The vitality of European forests is declining(FAO and UNEP,2020).Increased defoliation, reduced growth and spiking mortality are a consequence of new pests and diseases, climate-change related disturbances and high loads of atmospheric deposition of nitrogen (Forzieri et al., 2021).The resilience of forests to withstand such external pressures is underpinned by multiple factors,of which soil health and overall biodiversity are key examples(Trumbore et al.,2015;Brockerhoff et al.,2017).
Soil health has been low in European forests since the 1970's, with acidification as one of the main drivers of degradation (Schütt, 1977),and one that requires prolonged efforts to restore (Trumbore et al.,2015).A plethora of studies have reported the negative impacts of this acidification on forest vitality (e.g.Schütt, 1977; Kauppi et al., 1986;Ulrich, 1991), mainly pointing to the loss of essential macro- and micronutrients from the soil through leaching (Likens et al., 1996;Schaberg et al., 2001).Despite efforts to cut back acidifying emissions,soil acidification is still a major problem for soil health today, further threatening the vitality of European forests (Jonard et al., 2015; Braun et al., 2020) as foliar concentrations of essential nutrients continue to decline (Jonard et al., 2015; Talkner et al., 2015).Moreover, recent research indicated how soil nutrient imbalances,linked to acidification,are making forests increasingly sensitive to other global change impacts such as drought(Braun et al.,2017,2020;Hevia et al.,2019).Addressing these deficits in the foliar nutrient concentrations, especially base cations, by alleviating the deficits directly in the soil may be one of the prime mechanisms to boost the overall resilience of forests(Battles et al.,2014;Nickmans et al.,2015).
Furthermore,it is important to harmonize management measures that optimize soil health with measures that aim at augmenting overall biodiversity.Boosting tree diversity in planted forests is generally accepted as a measure to improve forest resilience and vitality (Sousa--Silva et al., 2018; Messier et al., 2022).Although the positive impact of tree diversity on aboveground ecosystem functioning is extensively studied (Tilman et al., 2014; Civitello et al., 2015; Jing et al., 2021),many studies conclude on context-dependent effects (Jactel and Brockerhoff,2007;Eisenhauer,2012;Jing et al.,2021).It is often not diversity per se that affects ecosystem functioning but rather the functional identities within the tree composition(Scherer-Lorenzen et al.,2007).In that sense,selecting tree species with soil ameliorative traits,i.e.the so-called rich litter species that produce litter high in base cations and low C/N ratio, has been proposed many times in the context of restoring soil health (Ehrenfeld,2003; Aubert et al., 2006; Eviner and Hawkes,2008;Desie et al., 2020a) and has been widely adopted in Western Europe in the last decade by admixing so-called rich litter tree species as a measure to counteract soil acidification and boost nutrient cycling (eco2eco,2018; Desie et al., 2020a).Yet, studies that evaluate the effects of such litter trait-based approaches on forest health and overall tree vitality remain scarce(Nickmans et al.,2015).
In this study,we aim to evaluate whether the presence of black cherry(Prunus serotina Ehr.)affects the vitality of neighboring pedunculate oak trees (Quercus robur L.) on acidified sandy soils and, if so, whether the impact goes via its rich-litter effects on the soil.We focus on oak trees because of their economic and ecological relevance in Europe(L¨of et al.,2016; M¨older et al., 2019) and recent signals on negative trends in vitality (Haavik et al., 2015; Brown et al., 2018; Sousa-Silva et al., 2018;Losseau et al., 2020).As proxies for oak vitality, we will evaluate the nutrient content of the oak leaves, their chlorophyll content and leaf damage by herbivory as a measure for defoliation(Rieske and Dillaway,2008; Guyot et al., 2016).Black cherry is a common alien invasive species in Europe(Ehrenfeld,2003;Starfinger et al.,2003;Annigh¨ofer et al.,2015) with studies reporting negative effects on biodiversity (Verheyen et al., 2007; Vanhellemont et al., 2010) and neighboring tree vitality(Aerts et al.,2017).However,eradication is proven to be challenging in many cases.Therefore the species could be embraced again as an ally in restoring nutrient imbalances in soils through its rich litter(Nyssen et al.,2019; Desie et al., 2020b), which was also the reason for its initial introduction in European forests(Starfinger et al.,2003).We hypothesize that.
(H1) black cherry admixture leads to higher foliar nutrient concentrations in the oak leaves via more efficient use of the total available resources in forests with higher species richness,and via improved soil health and more efficient recycling of soil nutrients (proxied by topsoil base saturation)due to the admixture of a tree species with rich litter traits;
(H2) total chlorophyll concentrations will increase as a consequence of the admixed black cherry because of the improved soil health including increased availability of essential nutrients(such as Mg and N)needed for the synthesis of chlorophyll;
(H3) black cherry admixture will make oaks less vulnerable to herbivory, both due to dilution effects, i.e.reduced proportion of host trees (Muiruri et al., 2019), and via improved host resistance towards biotic agents due to improved soil health (Manion and Lachance,1992).
Using structural equation modelling,we aim to evaluate such causal relations and, particularly, isolate the impact of black cherry through improved soil health from other diversity effects(e.g.niche-partitioning or dilution via increased species richness).
2.Material and methods
2.1.Study region and sampling design
The study region is located in Northern Belgium, Southern Netherlands and the adjacent area in Germany(Fig.1).Pleistocene sandy aeolian deposits locally admixed with sediments from marine or riverine origin characterize the region(Kasse et al.,2007)and result in a range of textures varying from almost pure sand, over loamy or clayey sands to sandy loams(Van Ranst and Sys,2000).Our study sites were located on a gradient of soil texture(ranging from 56%to 95%sand),land use legacy(Arenosols, Podsols and Anthrosols according to WRB Soil Reference Base)and land use history(first generation forest to afforested 170 years ago)(Table S1,see Desie et al.,2020b).The climate in the study area is temperate with a mean annual precipitation of circa 800 mm and a mean annual temperature of 10.5°C (data provided by the Royal Meteorological Institute of Belgium).
Within the study region,ten locations with mixed forest stands were selected consisting of a mixture of pedunculate oak(Quercus robur L.)and black cherry (Prunus serotina Ehrh.) in the upper canopy (Desie et al.,2020b).Tree species composition varied between sites (with admixture of other tree species such as Betula pendula,Fagus sylvatica and Carpinus betulus)and within sites(varying levels of dominance of Prunus serotina in the overstory).
In each mixed forest site,four replicates of two types of co-dominant and mature oak trees were selected:an oak tree under the influence of a mature black cherry tree(at a maximum of one tree height distance from the black cherry) and a reference oak tree not directly influenced by mature black cherry trees and thus surrounded by other pedunculate oak trees(Fig.1).We ensured that all selected trees within a site grew under the same climatic and topographic environmental conditions.By sampling both types in the same forest stand,other confounding factors such as forest management history were limited to ensure that the actual differences reported can be appointed to tree species effects.In total,we selected and sampled 79 oak trees(one missing value because we could not select a fourth suitable target tree under influence in Alphen-Chaam).
2.2.Sampling and laboratory analysis

Fig.1.Left: Ten mixed forest sites located on Pleistocene sandy deposits in Belgium, the Netherlands and Germany.Right: Study design and different sampling campaigns.In each mixed forest,twelve locations(4×3 types of target trees)were sampled for soil chemical analysis in 2017.In each mixed forest eight(4×2 types)oaks were sampled to determine leaf damage by herbivory and chlorophyll content (in April 2018) and nutrient content (in July 2019) of their leaves.
In July 2017,composite bulk soil samples were taken from the 0-10 cm mineral soil layer and humus descriptions were made under each selected tree(see also Desie et al.,2019).We measured the thickness of the organic fragmentation (OF) and organic humic (OH) layers as a measure for litter accumulation which are less seasonally dependent than the organic litter (OL) layer (Zanella et al., 2014).Composite samples consisted of five auger points and humus descriptions were repeated three times per target tree.Soil pH,nitrate(NO3-),ammonium(NH4+)and available ion concentration were determined in 0.2 mol·L-1NaCl extracts (after mixing fresh soil (17.5 g dry soil equivalent) with 50 mL solution).The pH was measured using a combined pH electrode (radiometer and a TIM840 pH meter(Hach,Loveland,USA)).NO3-,NH4+and phosphate(PO43-)concentrations were determined colorimetrically with a Seal auto-analyser III (Seal, Norderstedt Germany), using salicylate,hydrazin sulphate and ammoniummolybdate/ascorbic acid reagent,respectively.Cation exchange capacity (CEC) and base saturation (BS)were determined by extraction of 5 g dried soil in 200 mL 0.2 mol·L-1SrCl (Liu et al., 2001).Base saturation was calculated, after measuring the extracts on ICP-OES, radial view Seaspray or Crossflow nebulizer at 1300 W (ARCOS MV II, Spectro, Kleve, Germany), as the sum of exchangeable Ca2+, Mg2+and K+(in terms of charge equivalents)divided by the CEC and expressed as%.Soil and leaf litter total nitrogen(N)and carbon(C)concentrations were measured by dry combustion at 1020°C with a CNS analyzer(Model NA 1500; Carlo Erba Instruments,Milan, Italy).Soil texture was analyzed by laser diffractometry (LS 13 320, Beckman, Brea, USA) as percentage of clay (<8 μm; Konert and Vandenberghe,1997),silt(8-50 μm)and sand(50 μm-2 mm)according to the Belgian and USDA systems (USDA, 1975; Beuselinck et al.,1998;Buurman et al.,2001;Taubner et al.,2009).
In the winter of 2018-2019,we mapped the forest structure of all sites using the FieldMap system (FieldMap, IFER, Czech Republic).All trees with a diameter at breast height(DBH)higher than 15 cm and within a radius of 15 m around the target tree were spatially mapped and species,DBH and height of the tree were included in the map.Based on this stand structure data, the influence of black cherry (further called Prunus influence)was calculated for each target tree based on the basal area(BA)and distance of black cherry trees in 15 m radius relative to the total amount of neighbors of any species(equation 1,in which dist i=,dist j=)(Desie et al.,2020b).Larger trees and trees that are standing closer to the target tree are given more influence in this equation.
In spring 2018, we sampled 50 individual leaves from the lower branches of the canopy for each target oak tree (newly flushed leaves were excluded because not all species and individuals were flushing).Branches were selected randomly from different cardinal directions.Total chlorophyll was measured in vivo using the Apogee MC100 Chlorophyll meter (Apogee, Santa Monica, USA).Leaf herbivory was estimated per leaf using an ordinal scale of defoliation classes: 0%, 0-1%,1%-4%,5%-25%,25%-50%,50%-75%and 75%-100%(Martini et al.,2022).We calculated total leaf damage by herbivory per tree by using the median of each class of defoliation(based on the 50 sampled leaves)and we considered damage as leaf area reduction in tree crown,also referred to as defoliation.Herbivory was scored by a single observer (AVB).In summer 2020, all oak trees were re-sampled for nutrient analysis.Per target tree,10 leaves from different branches in the crown were collected with a pole saw.Leaf samples were oven dried at 60°C and ground before chemical analysis.Total element contents(P,Ca,Mg,K,Na,Cu,Fe,Mn)of the leaves were determined using ICP-OES (radial view Seaspray nebulizer at 1300 W, ARCOS MV II, Spectro, Kleve, Germany) after digesting 200 mg of dried (48 h, 70°C) and homogenized (by mortar)sample in 5 mL concentrated HNO3and 2 mL 30% H2O2(Ethos One or Ethos Easy Milestone,Sorisole,Italy)(Kingston and Haswell,1997).
2.3.Statistical analysis
The effect of Prunus influence on different soil and oak leaf properties was tested by means of mixed models with site as a random effect using the package nlme in R.The normality of the residuals and their relation to the fitted values were evaluated graphically.All predictors were standardized(to a mean of 0 and sd of 1)so that the coefficients of the mixed models could be compared.Subsequently, we used structural equation modelling(SEM)to evaluate the impact of Prunus influence on nutrient content(Fig.5a),chlorophyll content(Fig.5b)and defoliation(Fig.5c)directly and indirectly via improved soil health (here proxied by increased decomposition of litter leading to higher topsoil base saturation-which we consider a good proxy for soil health(Desie et al.,2020a,2021)).We created separate SEMs as nutrient content data were not collected in the same season as the data on defoliation and chlorophyll content and could therefore not be directly linked.As a first step, we created conceptual models encompassing our hypotheses: (H1) the nutrient content of oak leaves will increase,(H2)that chlorophyll content will increase and (H3) leaf damage by herbivory will decrease, both directly and indirectly through the improved soil conditions as a consequence of Prunus influence.Based on the a priori specified causal relationships, we built three SEMs by populating each component of the conceptual model with our measured variables.Based on the results of individual mixed models (Fig.4), we grouped leaf N + P as “macro-nutrients” and only included Ca and Mg as important base cations to limit the number of relationships in our model.Correlations between different foliar nutrient concentrations were included in the SEM.The linearity of the proposed relationships was investigated.The SEMs were fit using the PiecewiseSEM package (Lefcheck, 2015).Significance and goodness-of fit of the final model were assessed using Fisher's C statistic (see Tables S3-S5 for full output).All analyses were performed in R version 4.0.5(R Core Team,2019).
3.Results
3.1.Prunus influence on the vitality of neighboring oak
3.1.1.Foliar nutrient concentrations
In terms of nutrient concentrations of the oak leaves,Prunus influence has a significant (P <0.05) positive effect on total N and Fe concentrations in the oak leaves and a significant negative impact on Na and Mg concentrations (Fig.2).The relation between prunus influence and P concentrations in the oak leaves is marginally significant (P = 0.06).There was no statistically significant(P >0.1)direct impact on C,Ca,Mn,Si and Al concentrations in the oak leaves(Fig.S1).
3.1.2.Foliar total chlorophyll
Average chlorophyll concentration was 261 μmol·m-2and ranged from 143 to 380 μmol·m-2.We found no impact of Prunus influence on the chlorophyll concentration of the oak leaves(P= 0.36)(Fig.3).
3.1.3.Leaf damage by herbivory
All sampled oak trees were damaged by herbivory, with the proportion of completely intact leaves (i.e.0% defoliation class) ranging from 0 to 52%per target oak tree and estimated total herbivory ranging from 5%to 54%per target oak tree.Prunus influence had a direct impact on leaf damage by herbivory(P= 0.008).

Fig.3.Prunus influence on the chlorophyl content of oak leaves(a)and oak leaf damage by herbivory (b).Leaf damage is expressed as a percentage and chlorophyll is expressed μmol·m-2.Raw data are indicated by circles.Statistically significant relations are indicated by solid lines whereas relations that are not significant are indicated by dashed lines.The shaded interval indicates the 95%confidence interval.Significance levels of mixed models accounting for site are indicated in the top right corner of each panel.
3.2.Prunus influence via the improved soil health pathway
3.2.1.Impact on belowground ecosystem
Our results indicate a subtle ‘improved soil health’ effect with increasing Prunus influence:the thickness of the organic(OF+OH)layer decreases significantly(P=0.03)and topsoil pH increases marginally(P= 0.09, Fig.4).We found no direct impact on topsoil base saturation,topsoil nitrate, ammonium and phosphorus concentrations (Fig.4 and Fig.S5).

Fig.2.Prunus influence on the foliar concentrations of oak leaves:a)total nitrogen,b)phosphorus,c)potassium,d)calcium,e)magnesium and f)sodium.Raw data are indicated by the circles.Statistically significant relations (P <0.05) and marginally significant relations (P <0.1) are indicated by black solid lines whereas relations that are statistically not significant are indicated by black dashed lines.The shaded interval indicates the 95%confidence interval.Available reference critical foliar concentration ranges (max and min) for oak are indicated by red dotted lines (according to Mellert and G¨ottlein, 2012).Significance levels of mixed models accounting for site are indicated in the top right corner of each panel.(For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)

Fig.4.Prunus influence on the accumulation in the OF+OH layer(a),topsoil pH(b),topsoil base saturation(c)and topsoil nitrate concentration(d).Raw data are indicated by the circles.Statistically significant relations(P <0.05)are indicated by solid lines whereas relations that are not statistically significant are indicated by dashed lines.The shaded interval indicates the 95%confidence interval.Significance levels of mixed models accounting for site are indicated in the top right corner of each panel.
3.2.2.Structural equation models
Our structural equation illustrates an ambivalent impact of Prunus influence on nutrient content: there is an indirect effect on foliar Ca concentration of the oaks via the OF + OH layer and topsoil base saturation(i.e.“the soil health pathway”)(Fig.5a,Table S3).Contrary,foliar Mg concentrations decrease with increasing Prunus influence.The indirect impact of Prunus influence on foliar Ca is however small in comparison to the direct negative impact on Mg (Table 1).We only find a significant indirect effect of Prunus influence on oak foliar chlorophyll content via increased topsoil NO3-concentrations (Fig.5b, Table S4).Furthermore, we find that Prunus influence leads to decreased defoliation(expressed as leaf damage by herbivory)both directly and indirectly via the improved soil health pathway(Fig.5c, Table S5).
4.Discussion
In this study,we investigated whether the rich litter impact of black cherry affects the vitality of neighboring pedunculate oak trees,proxied by foliar nutrient and total chlorophyll content and leaf damage by herbivory.We hypothesized that black cherry would have a positive effect on oak vitality via improved soil health,for which we consider base saturation a good proxy as it gives an indication of the soil acidity status and nutrient availability, and it can be linked to belowground functioning(Desie et al.,2019;2021).Our results,however,emphasize the complexity of black cherry effects on oak vitality.
4.1.Impact of black cherry trees on foliar nutrition of oak
As a first proxy for oak vitality,we evaluated foliar nutrient content.We consider this a good proxy as balanced foliar nutrition and uptake of essential elements from the soil is vital for tree growth and health(Mellert and G¨ottlein, 2012; Jonard et al., 2015).Regardless of Prunus influence, most oaks in our study showed deficiencies in their foliar Ca and Mg concentrations, whereas N, P and K were not limiting or even above the normal range described by Mellert and G¨ottlein(2012)(Fig.2 and Table S2).This trend in foliar nutrient content mirrors the relative nutrient availability in the studied soils where there is an overload of nitrogen and shortage of base cations(Desie et al.,2020a,2020b).Hence,our results correspond with previous findings illustrating low base cation content and relatively high N and Al content in foliar tissues for oaks on poor soils(Jonard et al.,2012;2015)(Table S2).This also corroborates earlier findings on declining and/or poor forest vitality in Western Europe (Sioen et al., 2021), especially in areas with poor soil health(Schaberg et al., 2001; Lucassen et al., 2014) and high atmospheric nitrogen depositions (H¨ogberg et al., 2006; Jonard et al., 2012).The hypothesized positive impact of black cherry admixture on the nutrient content of oak leaves (H1) was only partially confirmed in our study:black cherry admixture led to increased total N and total P contents of the oak leaves but lower Na and Mg contents(Fig.2).This is contradictory to the results of Aerts et al.(2017),who found that Prunus serotina invasion results in lower foliar N concentrations in neighboring trees.The study of Aerts et al.(2017)was executed in a region where nitrogen deposition is considerably lower compared to our study region.The increased availability of foliar N and P concentrations linked to black cherry in our study did not alleviate any nutrient constraints as foliar N and P concentrations were already within the normal range for oak trees in Europe (Mellert and G¨ottlein, 2012).Foliar Ca and Mg concentrations in the oak trees were low(Mellert and G¨ottlein,2012)and became deficient for Mg with increasing Prunus influence (Fig.2).This suggests that, despite its rich litter profile, black cherry is a competitor for the available Mg.This corresponds with previous findings, which showed a particular strategy of Prunus serotina and Prunus padus combining high C/N ratios with high Mg concentrations in their litter(Desie et al.,2020a).Almost all oak trees in our study had extremely low foliar Ca concentrations whereas foliar K concentrations were within the critical range (and were not affected by black cherry) (Fig.4).In that regard, our study illustrates that the high litter quality of a certain species and its evidenced soil ameliorative properties do not necessarily translate to higher nutrient availability for neighboring trees of another species.However,we did not evaluate total litter quantity and therefore foliar nutrient concentrations cannot be compared over crown volumes, meaning that we could not evaluate potential dilution effects of nutrient contents related to increased productivity.
4.2.Impact of black cherry trees on foliar chlorophyl and herbivory of oak
We evaluated foliar total chlorophyll values as a second proxy for oak vitality.Total chlorophyll concentrations give a good indication of photosynthetic activity and reductions in total chlorophyll (i.e.crown discoloration)has often been used as an indicator for forest condition in Europe(Adams and Demmig-Adams,2004;Rossini et al.,2006).Overall total chlorophyll contents in our study were comparable to other studies in West-European oak forests(De Vries et al.,2019), indicating that the crown condition in our sampled forests was overall good.We found no direct statistically significant effect of Prunus influence on chlorophyll content (Figs.3 and 5b).This is in line with previous literature that illustrates low variability in total chlorophyll for oak stands that do not show clear signs of acute oak decline or high rates of mortality(De Vries et al., 2019).We also found no impact of foliar nutrient concentrations(Mg, Fe, N as most important elements in chlorophyll synthesis (Beale,1999; Verbruggen and Hermans, 2013)) on chlorophyll concentrations which suggests that deficiencies in foliar Mg do not (yet) result in reduced synthesis of chlorophyll or vice versa that surplus in foliar N leads to more chlorophyll.Note that these analyses were executed on the same trees but on leaves sampled in different years and seasons, which does not allow a good comparison between chlorophyll and nutrient contents.Future research could look into the impact of Mg deficiencies on leaf pigment content (e.g.chlorophyll a, chlorophyll b, total chlorophyll and carotenoids) and functioning of the photosynthetic apparatus by means of chlorophyll fluorescence parameters (e.g.Fv/Fm, PIabs,different energy fluxes per active reaction centre, sensu (Daems et al.,2022)) in the same leaves to determine whether the current nutritional disbalances will in time translate into crown discoloration and thereby growth loss.
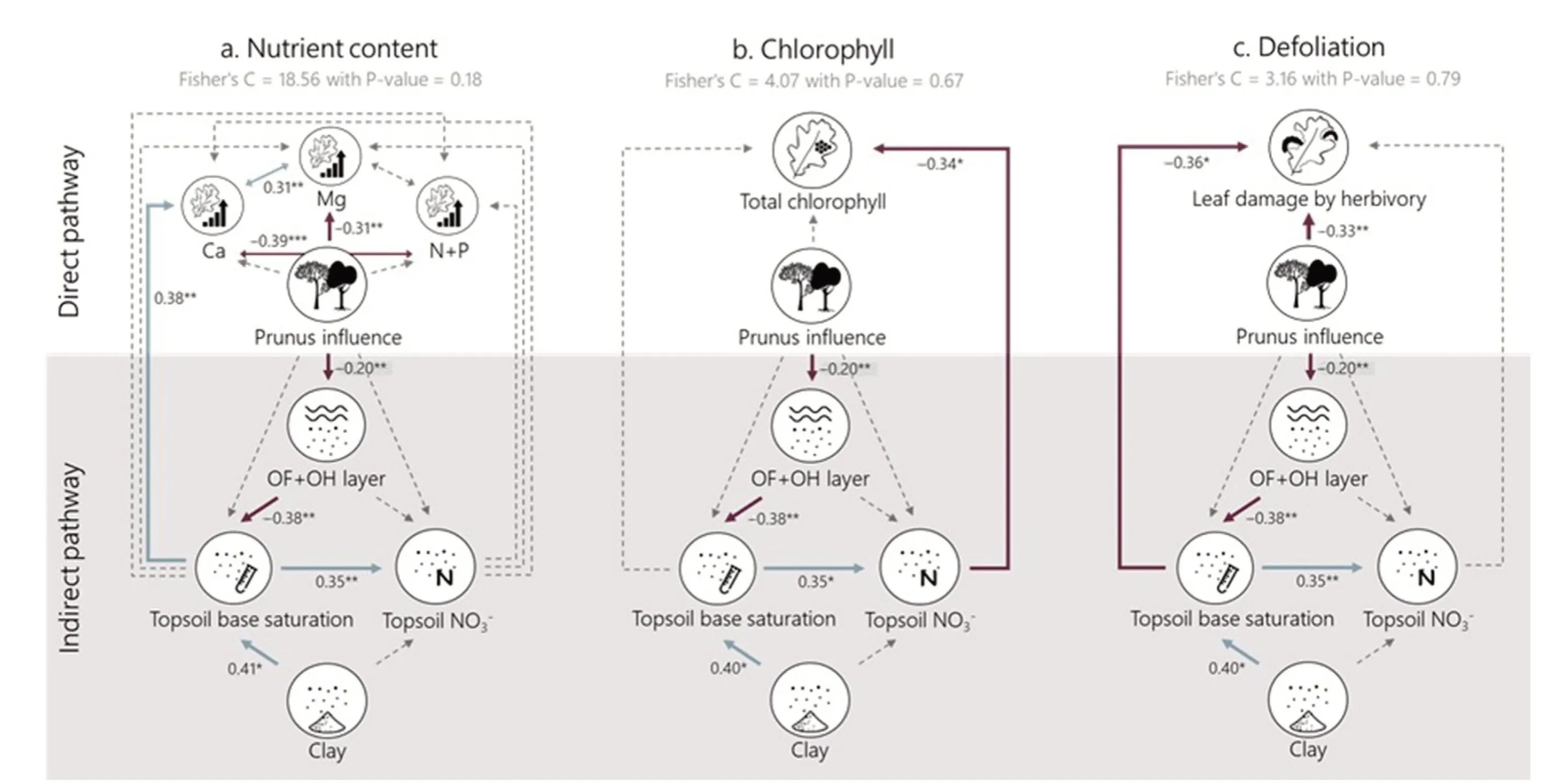
Fig.5.Tested PiecewiseSEM structural equation models for(a)foliar nutrient content,(b)foliar total chlorophyll content and(c)defoliation by herbivory of the oak leaves.Foliar nutrient content is split into base cation contents(Ca andMg)and macro nutrient content(sum of Nand P).The‘improvedsoilhealthpathway(shadedgrey area)is represented bythe OF+OH layerand topsoilbase saturation.Numbers nextto arrows give standardized path coeffciients withtheirstatistical signifcianceindicated as***P <0.001,**P <0.01,’*P <0.05.Solid arrows represent signifciant effects,dashedarrows represent non-signifciant(α <0.05)relations.Positive relations are indicated in light blue and negative relations in dark red.(For interpretation of the references to color in this fgiure legend, the reader is referred to the Web version of this article.)
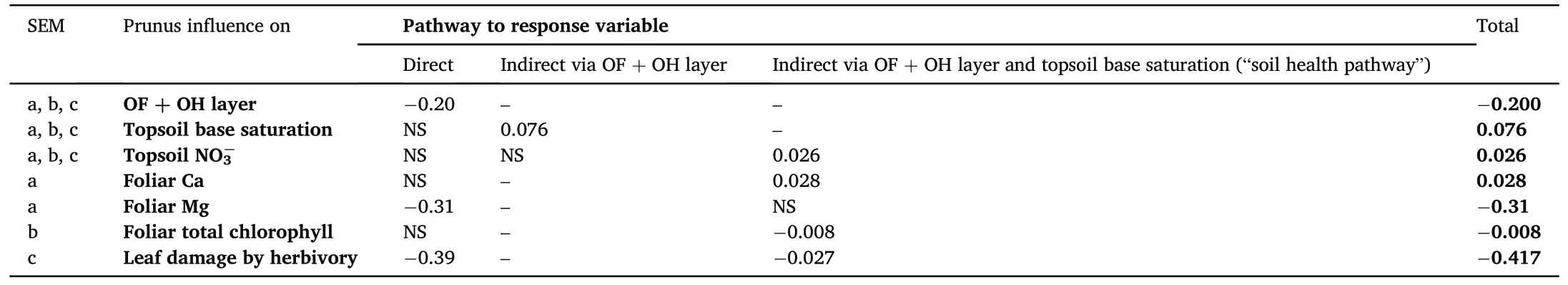
Table 1The direct, indirect and total standardized effects of Prunus influence on OF + OH layer, topsoil base saturation, topsoil NO3-, foliar nutrient content, foliar total chlorophyll content and leaf damage by herbivory for significant relations(P <0.05)from the respective structural equation models(SEM:a.nutrient content,b.total chlorophyll content and c.defoliation).
The third proxy for oak vitality that we evaluated was leaf damage by herbivory (and thus crown defoliation)which can severely limit carbon assimilation thereby resulting in growth loss (Eichhorn et al., 2016;Losseau et al.,2020).Herbivory on oak leaves by caterpillars depends on a range of factors, including population size, phylogenetic diversity,nutrient content, and location of the tree within the forest (Rieske and Dillaway,2008).Defoliation of oak leaves by herbivory is very common in European forests(Moore et al.,1991),despite oak leaves being rich in defensive compounds such as tannins(Rieske and Dillaway,2008).It was not in the scope of our research to identify trophic cascades within the studied forest ecosystems nor to compare herbivore pressure to other European studies - yet to use the degree of defoliation by herbivory within our study as a proxy for oak vitality in order to evaluate the impact of black cherry admixture.We found that black cherry admixture reduces defoliation by herbivory of neighboring oak leaves,which suggests there is a dilution effect of insect manifestations in mixed stands (Jactel and Brockerhoff,2007;Civitello et al.,2015;Guyot et al.,2015;Muiruri et al.,2019).Dilution or decreased host availability (here oak leaves) in monocultures leads to smaller populations sizes of oak-specialist herbivores (also called the spatial concentration effect) (Yamamura, 2002).Contrary,more diverse forests can induce physical or chemical barriers to foraging herbivores (for example, black cherry volatiles could work repellent for oak herbivores or its dispersal may be limited due to limited landing options while foraging)and generally provide environments that are more suitable to harbor multiple natural enemies of herbivores compared to host monocultures(Jactel and Brockerhoff,2007).Since we have no data on the defoliation of black cherry itself,we cannot conclude whether the same population size of insect herbivores was sustained by feeding relatively more on black cherry leaves or the dilution resulted in overall lower infestation for the mixed stands.Although alien invasive plants typically escape natural enemies, recent studies have indicated that black cherry is rapidly adopted by local communities and it experiences at least as much intensive herbivory as do native species closely related to it (Agrawal and Kotanen, 2003; Halarewicz and Jackowski,2011).However, since herbivores are most often species-specific, it is unlikely that the same species of caterpillars that exploit oak,feed more on black cherry.
4.3.Indirect effects of black cherry via the belowground ecosystem compartment
In this study,we aimed to evaluate one mechanistic diversity effect in more detail, i.e.the improved soil health due to the admixture of trees with soil ameliorative traits(e.g.rich litter tree species).First,we found that black cherry admixture reduces the accumulation of organic material in the humus layer and consequently impacts soil pH positively(Fig.5) (Desie et al., 2020b), thereby positively affecting soil health.These soil ameliorative properties of black cherry were illustrated in multiple studies and are mainly linked to the higher litter quality of black cherry(Lorenz et al.,2004;Dassonville et al.,2008;Desie et al.,2020a).In our study,the gradient in black cherry litter admixture ranges from 0%under the reference oaks to 60% under the oaks influenced by black cherry explaining the marginal effects on soil base saturation and pH(Fig.4).This emphasizes once more that considerable admixture of rich litter tree species (>80%) is needed to significantly counteract soil acidification(Van Nevel et al.,2014;Desie et al.,2020b).
Our structural equation model illustrated that soil base saturation might increase indirectly due to Prunus influence and that,subsequently,the improved soil health leads to increased Ca concentrations in the oak leaves(Fig.5a),reduced chlorophyl concentrations(Fig.5b)and reduced oak defoliation by herbivory (Fig.5c).The higher foliar Ca concentrations due to improved soil health could be linked to the counteracted soil acidification,augmenting the concentrations of base cations available to the tree.However, all indirect effects on oak vitality are small in comparison to the direct impact of black cherry admixture(Table 1).In that regard, the negative direct effect on foliar Mg content (Fig.2) is more dominant than the positive indirect effect through improved soil health(Fig.5).The indirect effect on defoliation might be linked to improved host resistance towards biotic agents due to more suitable site conditions(Manion and Lachance, 1992).Since we evaluated herbivory and nutrient foliar concentrations in different years and seasons, it is not strictly possible to directly link them to one another.Coupled data on foliar concentrations and herbivory would have allowed us to evaluate the feeding preferences of herbivores and potential feedbacks (Muiruri et al.,2019).For example,previous studies have illustrated the impact of nitrogen deposition on decreasing foliar C/N ratios, favoring herbivory(Ferretti et al., 2015).Moreover, herbivory is ideally evaluated over multiple years to take into account the impact of population dynamics of herbivores (Muiruri et al., 2019), this was however not in the scope of our study.
4.4.Management implications
Black cherry is widely considered to be an alien invasive species in Western Europe(Ehrenfeld,2003;Annigh¨ofer et al., 2015) with studies reporting negative effects on biodiversity (Verheyen et al., 2007; Vanhellemont et al.,2010)and neighboring tree vitality(Aerts et al.,2017).Long-term efforts(and resources)were directed to eradicate the species;yet, many of these large-scale campaigns have proven unsuccessful(Starfinger et al., 2003; Nyssen et al., 2019).With the discouragement(and in some cases even a ban) of the use of glyphosate, it has become increasingly difficult for managers to combat the species successfully.Thus,some have suggested to instead embrace the species for the purpose it was initially introduced for:its soil ameliorative properties(Starfinger et al., 2003; Nyssen et al., 2013; Nyssen et al., 2019).These soil ameliorative properties can be linked to the high litter quality of black cherry (i.e.high base cation content and low lignin content) promoting overall decomposition of fresh organic matter(Lorenz et al., 2004; Dassonville et al., 2008; Desie et al., 2020a).Our study however illustrates that the effects of black cherry on pedunculate oak are ambivalent:N and P foliar concentrations of oak increase, yet Mg contents, the main limiting factor in our study sites,decrease in the foliage of oaks.On the other hand, there is a positive effect on soil nutrient availability and a decrease in defoliation of neighboring oaks.A study in the same sites and including a subset of the trees sampled here,showed no effects of Prunus influence on the diameter growth of oak(Haas et al.,2020).This tempers both the fear for black cherry as an alien invasive species negatively affecting the vitality of mature oak trees as well as the potential to use this species for improved vitality of oak.Both eradication as restoration strategies using black cherry are potentially of low impact.However,this impact depends considerably on site characteristics.For example, we previously found larger positive effects on black cherry admixture on sites with finer soil texture(Desie et al.,2020b).In that sense,we need to use our fundamental understanding of plant-soil interactions when optimizing restoration (Eviner and Hawkes, 2008), by adapting management strategies to context.Furthermore, the evidenced positive indirect effect of improved soil health could be translated to other rich-litter species that might have overall more positive impact on oak vitality.Managers should therefore also take into account other traits,besides litter quality, that impact nutrient cycling within the ecosystem(e.g.rooting architecture, growing regime, mycorrhiza, soil faunal composition) and the context of the site (Desie et al., 2020b, 2021).Further research could, for example, evaluate the impact of admixing other rich litter species, corresponding with the management goals, on oak vitality.In that regard, finding suitable study sites of mature rich litter trees admixed in oak forests,however,remains a challenge.
5.Conclusions
Black cherry admixture has multiple and contrasting effects on proxies for oak vitality.We found that foliar N and P concentrations increase due to black cherry admixture while Mg and Na concentration decrease.In general,the oaks in our studies are showing deficiencies in foliar base cation content which are not alleviated by black cherry admixture (and its positive impact on soil health).Defoliation by herbivory decreases when black cherry is admixed,both directly due to host dilution and indirectly due to increased soil health and improved resistance towards pests.In conclusion, our study shows that the combined positive and negative impacts of black cherry on oak vitality are limited,which tempers the potential benefits of using this rich litter species to counteract acute oak decline via improved soil health - yet, also the concern of black cherry,an invasive alien species negatively affecting the vitality of mature pedunculate oak seems exaggerated.
CRediT authorship contribution statement
Ellen Desie:Conceptualization, Data curation, Formal analysis,Funding acquisition, Investigation, Methodology, Visualization, Writing- original draft, Writing - review& editing.Bart Muys:Conceptualization, Investigation, Supervision, Writing - review & editing.Jan den
Ouden:Conceptualization, Investigation, Methodology, Supervision,Writing - review & editing.Bart Nyssen:Conceptualization, Data curation, Funding acquisition, Methodology, Project administration,Resources, Writing - review & editing.Rita Sousa-Silva:Visualization,Writing-original draft,Writing-review&editing.Leon van den Berg:Conceptualization, Data curation, Formal analysis, Funding acquisition,Methodology, Project administration, Resources, Writing - review &editing.Arnold van den Burg:Data curation,Formal analysis,Writingreview & editing.Gert-Jan van Duinen:Writing - review & editing,Conceptualization.Koenraad Van Meerbeek:Formal analysis, Investigation, Methodology, Supervision, Writing - review & editing.Maaike Weijters:Conceptualization, Data curation, Methodology, Writing - review & editing.Karen Vancampenhout:Conceptualization, Supervision,Writing- review&editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Bosgroep Zuid Nederland for the coordination of the applied umbrella project and the Dutch province Noord-Brabant for funding.We would like to acknowledge the support of B-Ware research center regarding the laboratory analysis and Eric Van Beek, Remi Chevalier, Fien Decoster, Simon Baert, Bjorn Rombouts, Elisa Van Cleemput,Stef Boogers and Jan Vanstockem for their support during the data collection.Finally, we thank Ilié Storms and Sanne Verdonck for their constructive feedback.E.D.held a SB-doctoral fellowship of the Research Foundation Flanders (FWO, 1S43617N) at the time of data collection.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.i.org/10.1016/j.fecs.2023.100148.
- Forest Ecosystems的其它文章
- Divergent responses of Picea crassifolia Kom.in different forest patches to climate change in the northeastern Tibetan Plateau
- Tree-based ecosystem services supply and multifunctionality of church forests and their agricultural matrix near Lake Tana, Ethiopia
- Influence of climate fluctuations on Pinus palustris growth and drought resilience
- Nutrient retranslocation strategies associated with dieback of Pinus species in a semiarid sandy region of Northeast China
- Book review “Continuous Cover Forestry - Theories, Concepts, and Implementation” by Arne Pommerening
- Sensitivity of forest phenology in China varies with proximity to forest edges

