An overview of recent progress in the development of flexible electrochromic devices
Bin Wng,Wu Zhng,Feifei Zho,,Willim W.Yu,Adulhkem Y.Elezzi,***,Linhu Liu,**,Hizeng Li,*
a Optics and Thermal Radiation Research Center,Institute of Frontier & Interdisciplinary Science,Shandong University,Qingdao,266237,China
b School of Chemistry & Chemical Engineering,Shandong University,Jinan,250100,China
c Ultrafast Optics and Nanophotonics Laboratory,Department of Electrical and Computer Engineering,University of Alberta,Edmonton,Alberta,T6G 2V4,Canada
Keywords: Electrochromism Flexible electrochromic devices Optoelectronics Optical materials
ABSTRACT Electrochromic materials are capable of reversibly switching their colors or optical properties through redox reactions under applied voltages,which have shown great potential applications including smart windows,nonemissive displays,optical filters,among others.Although the current rigid electrochromic devices have shown emerging interest and developed rapidly,many applications (e.g.,wearable/deformable optoelectronics) are blocked due to their inflexible features.Herein,the adaption of rigid electrochromic devices to flexible ones is of particular interest for the new era of smart optoelectronics.In this review,the current state-of-the-art achievements of flexible electrochromic devices (FECDs) are highlighted,along with their design strategies and the choice of electrochromic materials.The recent research progress of FECDs is reviewed in detail,and the challenges and corresponding solutions for real-world applications of FECDs are discussed.Furthermore,we summarize the basic fabrication strategies of FECDs and their potential applications.In addition,the development trend,the perspectives,and the outlook of FECDs are discussed at the end of this Review,which may provide recommendations and potential directions to advance the practical applications of FECDs.
1.Introduction
Electrochromism is the phenomenon whereby certain materials reversibly switch their colors or optical properties (absorbance/transmittance/reflectance)via redox reactions under a small external voltage or current [1,2].The first demonstration of the electrochromic effect could be dated back to the late 1960s,whenever Deb observed the coloration effect of amorphous WO3films via being applied an electric field[3,4].The electrochromic devices(ECDs)have shown distinguished advantages over traditional devices (e.g.,polymer-dispersed liquid crystal devices)for smart windows,displays,anti-glare rearview mirrors,and other optelectronics,due to their low energy consumption,non-emissive characters,and long-term stability[5].So,it is essential to develop electrochromic technology.Electrochromic technology has made great progress in basic principles and commercial applications during the past several decades.For example,Gentex corporation has developed auto-dimming rearview mirrors via electrochromic technologies.In academic research,the integration of electrochromism with other technologies has made breakthrough achievements [6].Our group had developed exciting zinc anode-based electrochromic devices via integrating the electrochromism and zinc ion batteries in a single platform,which realized several new applications for electrochromic devices (e.g.,electrochromic batteries,and transparent multicolor electrochromic displays) [7–16].However,most current electrochromic devices are rigid ones as conductive glasses are utilized as the substrate,which inevitably brings issues including fragility and bulky dimensions.Thus,rigid electrochromic devices limit many potential applications(e.g.,wearable optoelectronics).To further expand the practical use of ECDs,new functionalities can be unlocked by introducing flexible architectures.For example,the design of FECDs opened up new applications,including electronic skins [17],actuators [18,19],and wearable electronics [20,21].Therefore,FECDs,which enable multiple functionalities along with reduced size dimensions and enhanced mechanical robustness,have attracted surged attention from both industry and academic fields.Furthermore,the FECDs are of great importance in both academic and industry fields,due to the increasing demands of wearable,flexible,and deformable electronics.The flexible electrochromic devices(FECDs) are always lightweight,which have positioned themselves as one of the attractive candidates for human-machine interfaces,owing to their capabilities to integrate with soft biological tissue like the surface of a human body.
As shown in Table 1,the device configurations are similar between rigid ECDs and FECDs.All these devices are comprised of conductive layers,an electrochromic layer,an electrolyte layer,and an ion storage layer (Fig.1).Although the only difference between the two types of electrochromic devices is the device architecture (i.e.,the substrate layer),the design of FECDs will bring great challenges including poor flexibility,performance decay upon deformation,and the leakage of electrolytes during mechanical strain/stress.As such,it requires developing significantly different electrode and electrolyte systems when fabricating FECDs.This brings new research aspects which differ from the rigid devices for these specific FECDs,as FECDs also belong to another research field (i.e.,flexible optoelectronics).Therefore,the development of FECDs requires interdisciplinarity knowledge,which includes mechanical engineering,optics,and materials expertise among others.

Fig.1.Schematic comparison of rigid and flexible EC devices.

Table 1Comparison of rigid and flexible ECDs.
With the rising demand for multifunctional devices [22–24],the development of FECDs is of significant importance for broadening various applications.Notwithstanding,FECDs,at their emerging stage,only very few literature reviews outlined the research progress before the year 2017 [25].In light of these investigations,we offer a critical comprehensive review focusing on the recent achievements on FECDs.In this Review,we systematically discuss recent progress in designing conductive substrates,electrochromic materials,and electrolytes for FECDs.This review first introduces the progressive fabrication of flexible conductors which have potential applications in FECDs.This is followed by an extensive literature review of recent key achievements of FECDs using inorganic and organic electrochromic materials,along with the design of deformable gel electrolytes.Finally,current challenges and future directions of FECDs are discussed,which we think may accelerate the development of FECDs.
2.The design of flexible conductors
The conductive substrate serves as an important component for electrochromic devices,the primary objective of developing FECDs is to design flexible conductors.In this section,the design strategies,fabrication approaches,and recent progress of flexible conductors are discussed,and we hope to draw a picture of the future development of flexible conductors for a broad range of applications (e.g.,FECDs,and flexible electronics).
As transparency is a vital parameter for flexible transmissive-type electrochromic devices,the fabrication of flexible transparent conductors has received more attention from both academia and industry.A transparent conductor requires a high transmittance at a certain wavelength region,a low resistivity for better charge transportation,and excellent flexibility.The current commercialized flexible transparent conductors are indium tin oxide (ITO) coated clear plastic films.However,due to the brittle nature of the ITO ceramic coatings,such ITOcoated plastic films are suffering limited flexibility [26,27].Hence,the metal materials,having excellent malleability along with admirable conductivities,have been developed for fabricating flexible transparent conductors[28].
Besides the selection of metal materials as a conductive layer for flexible transparent conductors,the choice of deformable substrate,and fabrication method are also crucial parameters that influence the performance of the FECDs.At present,the most flexible transparent conductors are comprised of precious metal nanowires(e.g.,silver nanowires,copper nanowires) and transparent polymer substrates.Such an incorporation design of metal nanowires and polymer substrate endows some advantages that are superior to the rigid conductors,which include lightweight nature,excellent flexibility,and low cost,among others[29].
2.1.Polyimide (PI) as substrate for flexible conductors
Recently,polyimide (PI) is considered one of the most promising flexible substrates due to its excellent heat resistance,superior optical transparency,good mechanical properties,and low dielectric constant.Park et al.introduced a flexible transparent PI substrate that was synthesized by 2,2′-bis-(3,4dicarboxyphenyl) hexafluoropropanedianhydride(6FDA) and 2,2′-bis-(trifluoromethyl)-4,4'diaminobiphenyl (TFDB) [30].The indium tin oxide(ITO)thin layers were coated onto the PI substrates via a magnetron sputtering method at different temperatures,thus obtaining the ITO-coated PI transparent conductors.However,such ITO-coated PI transparent conductors exhibited inferior mechanical performance due to the fragile nature of the ITO coatings.Contrastly,the utilization of Ag NWs is promising for PI-based flexible transparent conductors,due to the ease of synthesis of Ag NWs and excellent electrical conductivity of Ag NWs.In 2015,Huang et al.fabricated flexible transparent conductors via coating composite aluminum-doped zinc oxide(AZO)-AgNWs conductive layer onto PI substrate [31].Such a composite conductive layer on PI substrate enables an excellent thermostability(<10% increase of resistivity (Rc) when exposed to 250°C for 1h),distinguished electrical conductivity(Rsh=8.6 Ohm/sq),and good flexibility of the transparent conductors.In addition,J.A.Spechler et al.synthesized a colorless PI substrate from a fluorinated dianiline moiety and a typical dianhydride moiety[32].Such a colorless PI substrate can be used to accommodate Ag NWs and thus forming a transparent conductor.To further increase the thermal stability of the silver nanowire networks,a titania coating was cast onto the top of the Ag NWs to serve as a protective layer (Fig.2).Thus,the conductive polyimide exhibited a high conductivity (3.4 Ω/sq with a 69% visible transparency) and excellent thermal stability(thermally stable within 360°C).
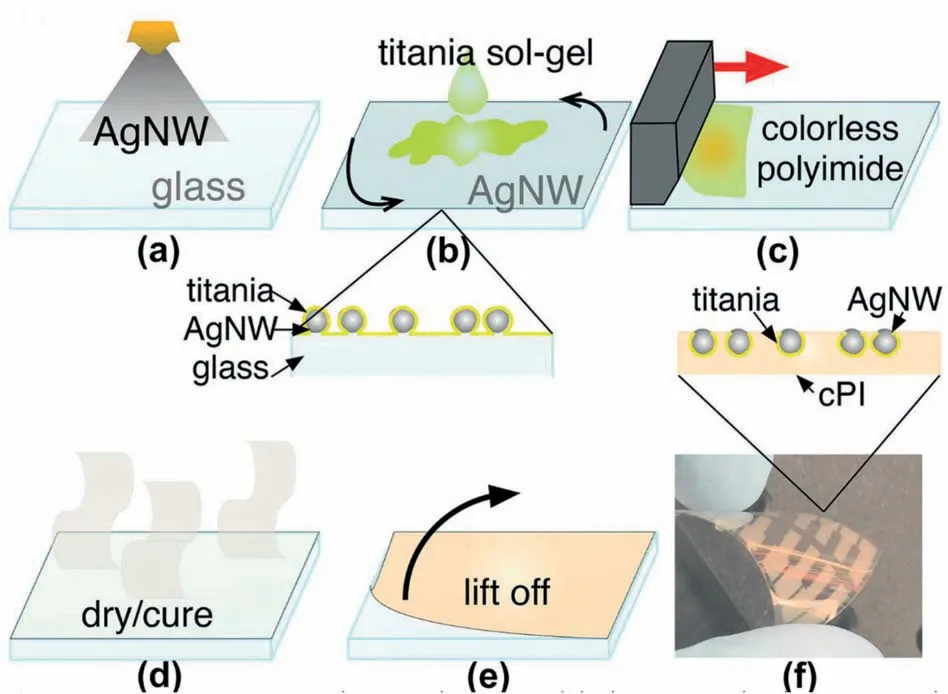
Fig.2.Schematic illustration of the fabrication process of silver nanowires (Ag NWs)-coated polyimide(PI) flexible transparent conductors.a) Spray-coating Ag NWs onto the glass substrate.b) Casting Titania sol.c) Coating colorless polyimide (PI) liquid precursor.d) Drying and curing process.e) Lifting the polyimide film from the glass,and thus forming a transparent conductor.f) A photograph of the Ag NWs-coated PI flexible transparent conductor.Reproduced with permission [32].Copyright 2015,Wiley-VCH.
2.2.Polyethylene terephthalate (PET) as substrate for flexible conductors
Besides the thermally stable PI that can be used for the substrate of conductors,PET is another promising substrate material for conductors due to its colorless characters,chemically inert nature,and good mechanical properties.Most importantly,PET possesses good thermal stability as it can stand a high temperature of 200°C,which enables the fabrication of PET-based transparent conductors under mild temperature conditions.Recently L.Zhao et al.presented a flexible transparent conductive electrode with an admirable electrical performance by using a PET substrate[33].Such a flexible conductor,having Cu/Ni/PEDOT:PSS(CNP) as the conductive layer on PET substrate,was fabricated by combining lithography and electroplating techniques.In this regard,such a flexible conductor exhibited an excellent electrical conductivity(~0.13 Ω/sq) along with well visible transparency (~86%).Furthermore,the conductor presented good deformability,which enables only a small increment of the resistance after 10000 bending cycles.As lithography technique requires strict nanofabrication equipment,the development of solution-processed techniques is promising for cutting the cost.
Therefore,G.Cai et al.[34]presented a transparent PET-based conductor composed of self-assembled silver grids and PEDOT:PSS(Fig.3).The utilization of PEDOT:PSS could significantly improve the anti-oxidation property of the silver grid,which leads to a negligible increment in resistance of the silver grid/PEDOT:PSS/PET conductor after being exposed to air at ambient temperature for two months.In addition,the silver grid/PEDOT:PSS/PET conductor exhibited good electrochemical cycling stability and remarkable mechanical flexibility as used for electrochromic applications,which showed great promise in emerging various applications including flexible electronics and optoelectronic devices.
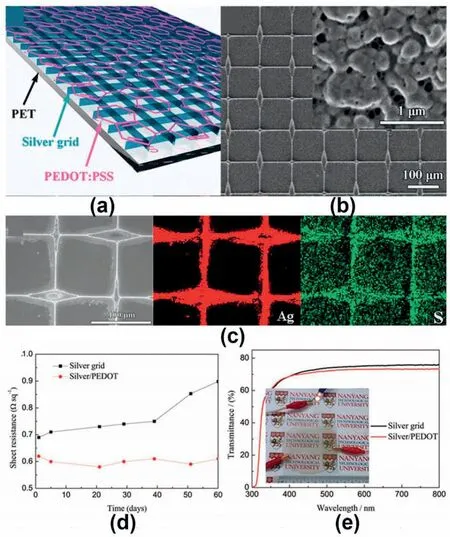
Fig.3.a) The structure of the silver grid/PEDOT: PSS hybrid film.b) SEM images of the silver grid/PEDOT: PSS conductive layer.c) EDS mapping of the film,revealing a uniform distribution of PEDOT:PSS.d)The demonstration of anti-oxidation properties of the conductors.e)Transmittance spectra of the two types of PETbased conductors.Reproduced with permission [34].Copyright 2015,Wiley-VCH.
As the above-mentioned self-assembled silver grid/PEDOT:PSS/PET conductor suffers poor compatibility with the large-scale fabrication of flexible conductors,the development of a candidate strategy to fabricate large-areal conductors has attracted extensive attention from both academia and industry.Spray coating is a versatile and facile method to fabricate large-scale films at a fast production speed,which has shown promising potential for commercialization [9,36,37].Therefore,the spray coating method is also of great interest in fabricating flexible conductors.A.R.Madaria et al.demonstrated a simple spray coating method to assemble Ag NWs on PET substrate,which showed a sheet resistance of 33 Ω/sq along with high transparency (85%) [38].Unfortunately,the spray-coated Ag NWs suffer several shortcomings that hinder their practical applications,which include poor chemical/electrochemical stability,low mechanical strength,and the ease of shedding.To address these obstacles,proper interface engineering modifications on PET substrate are required.K.Li et al.developed a surface modification method of PET substrate for fabricating Ag NW/PET conductors,which significantly improved the mechanical and electrochemical stability of the flexible conductors [35].The Ag NW dispersion was firstly mixed with the alginic acid/poly(dopamine)complex (Aa-PDA) for spraying,which could significantly enhance the adhesion between the Ag NWs and the PET substrate and thus improve the mechanical stability.Furthermore,a diluted PEDOT:PSS solution was spray-coated onto the Ag NW network as a charge-balancing layer to enhance the electrochemical stability of the Ag NW/PET conductors.The morphology difference between pristine Ag NW films and composite films (i.e.,Ag NW/Aa-PDA,Ag NW/Aa-PDA-PEDOT:PSS) can be identified from the FE-SEM images (Fig.4a–c).The corresponding schematic diagrams are also shown in Fig.4d–f,which display the morphological difference between each of the samples.After carefully comparing the optical and electrical performance of the pristine Ag NW films and composite films (i.e.,Ag NW/Aa-PDA,Ag NW/Aa-PDA-PEDOT:PSS),the Ag NW/Aa-PDA-PEDOT:PSS/PET conductor is of significant promise for fabricating electrochromic devices (Fig.4g–i).This is due to the fact that such an Ag NW/Aa-PDA-PEDOT:PSS exhibited good mechanical stability and optical transmittance.

Fig.4.FE-SEM images of different types of Ag NW films a) Pristine Ag NW films,b) Ag NWs/Aa–PDA (AAFs) composite films and c) Ag NWs/Aa–PDA/PEDOT:PSS(AAPFs)composite films.Schematic diagrams(d,e,and f)of the different types of the Ag NW/PET conductors,which correspond to the SEM images shown in a,b and c,respectively.g)The transmittance spectra of pristine PET and AAFs have different sheet resistances.h)The mechanical stability test of AAFs and AAPFs.i)The sheet resistance evolution under different tape test times.Reproduced with permission [35].Copyright 2016,Royal Society Chemistry.
Besides the surface modification strategy for enhancing the adhesion between Ag NWs and PET substrate,the design of hierarchical Ag NWs provides another approach for fabricating mechanically stable Ag NW/PET conductors.T.Hao et al.developed a one-step hydrothermal method to fabricate Ag NWs having a core-shell structure[39].Such a core-shell structure is comprised of an Ag core and a layer of C shell.The hydroxyls on the surface of the Ag@C core-shell-structured NWs could significantly enhance their adhesion to the PET substrate,which leads to a great improvement in mechanical stability.Such an Ag@C NW/PET transparent conductor showed attractive stability when being subjected to the bending tests(ΔR/R0≈2.8% after 2000 bending times).
Besides the utilization of metal materials as the conductive component of a flexible conductor,carbon-based materials are another promising candidate for fabricating high-performance flexible conductors.Among carbon-based nanomaterials,graphene has emerged as a promising material for next-generation electronics because of its low sheet resistance,high transmittance,high strength,and stretchability [40].L.Gomez De Arco et al.reported that the chemical vapor deposition(CVD)method can be used to fabricate continuous,eminently flexible,and transparent graphene films [41].Then the transparent graphene films can be easily transferred onto a flexible and transparent PET substrate and thus forming a flexible conductor.Such a graphene/PET conductor delivered high electrical and optical performance (Fig.5),including a sheet resistance of~230 Ω/sq with a high transparency of 72%.

Fig.5.High transparency and toughness of the films are shown in panels a and b,respectively.c)The transmission spectra for CVD graphene,ITO,and SWNT films on glass.Reproduced with permission [41].Copyright 2010,American Chemical Society.
2.3.Nanocellulose film as substrate for foldable conductors
In addition to the above-mentioned two main polymers(i.e.,PI,PET)as a flexible transparent substrate for conductors,the exploration of other substrate materials has also attracted immense attention from the optoelectronic community.For example,nanocellulose materials have shown great potential for transparent substrates due to their strong mechanical strength,ubiquitous abundance,biocompatibility,tunable surface properties,etc.[42]W.Kang et al.reported that nanocellulose could be used as a promising foldable substrate with high transparency and low haze [43].Tempo-assisted oxidized cellulose nanofibrils were used as building blocks to fabricate nanocellulose substrates via a filtration method,which could produce a uniform composition of the transparent substrate with low surface roughness(RMS=6.3 nm)and low scattering angle,due to the presence of surface carboxylic group.Such transparent nanocellulose substrates can host the filtered single-walled carbon nanotubes (SWCNTs) and Ag NWs,and thus forming a foldable transparent conductor.Such transparent conductors showed outstanding mechanical deformation abilities that can fold to an extreme extent with a radius below 10 μm.Furthermore,the foldable conductor exhibited an impressive electro-optical performance,including a low sheet resistance of 10.5 Ω/sq and a high transparency of 94.6% in the visible range.Moreover,the SWCNT@Ag foldable conductor showed good bending performance,including the unaffected resistance and microscopic morphology change of the conductor during 360°bending cycles(Fig.6).

Fig.6.a)The membrane can undergo forward and reverse folding cycles.b)The change of sheet resistance under different folding cycles.The surface morphology of folded conductor at different angles: c) -180° and d) 180°.The SWCNT@Ag foldable conductor could light LEDs e) before and f) after crumpling,which showed excellent foldability.Reproduced with permission [43].Copyright 2016 Wiley-VCH.
2.4.Textile as substrate for wearable conductors
Besides the above-mentioned foldable transparent conductors,the development of wearable conductors has also attracted increasing attention due to their potential applications in modern healthcare and communication systems[21].The fabrication of the wearable conductors is comprised of the choice of textile substrate and the assembly of a conductive metal layer onto the textile substrates.Although the textile substrates are commercially available,the assembly of the conductive metal layer is still in its incipient state and considerably important for wearable electronics.The most commonly used method for fabricating conductive metal layers on textile substrates is the electroless plating approach,due to its excellent compatibility with different textile substrates[44–46].For example,the research group,led by C.Zhi from the City University of Hong Kong,has developed a universal electroless plating method for assembling conductive metal layers on different textile substrates(e.g.,cloth,paper,sponge)[47].This general approach of depositing a conductive metal layer onto textile substrates is shown in Fig.7,which includes the following three steps:i)The choice of a suitable soft substrate (cloth,paper,or sponge) according to the potential applications.ii) Then,the substrates were decorated with a sensitizer[SnCl4]2–and followed by loading the catalyst of [PdCl4]2–[48,49].iii)The final step is the electroless deposition of metal layers onto the modified substrates.By using this method,the metal-conductive cloths showed excellent high conductivity (4–200 kS m-1) and full-scale coverage of metal coatings on the textile fibers,without sacrificing the softness(~8 mm) of the cotton cloth.

Fig.7.Different substrates: cloth,filter paper,and sponge could use similar methods to deposit metal.Reproduced with permission [47].Copyright 2021 Elsevier.
2.5.Metal mesh for free-standing conductors
In addition to the different substrates used to fabricate flexible conductors,the development of free-standing conductors may provide a new opportunity for future flexible electronics.The free-standing flexible conductors are always made of metal meshes,due to their good intrinsic mechanical strength and conductivity metals.H.Liu et al.developed a strategy that combined photolithography and electrochemical machining to fabricate the flexible ultrathin and ultralight free-standing Zn micromeshes [50].As shown in Fig.8a and b,a free-standing Zn micromesh was only 8 μm in thickness,while the areal density was 4.9 mg cm-2.Such ultralight character of the free-standing conductor enabled itself to be supported on a bubble (Fig.8c),which showed great promise in fabricating low-weight zinc ion batteries.Besides the ultrathin and ultralight properties,the free-standing Zn micromesh also exhibited excellent flexibility and enhanced mechanical strength.The Zn micromesh showed two times higher tensile strength in comparison to the Zn film(Fig.8d).Furthermore,the free-standing Zn micromesh also possesses good mechanical stability under bending tests.As shown in Fig.8e,the resistance of the Zn micromesh showed almost no change resistance after 10000 bending cycles,which points it a great candidate for flexible and wearable electronic devices in the coming future.To better guide the choice of conductive substrate,a detailed comparison of the state-of-the-art flexible conductors is shown in Table 2.

Fig.8.a)The optical photograph of the ultrathin Zn micromesh,which showed a thickness of 8 μm.b)The comparison of the conductors using different substrates.c)The optical photograph of the ultralight Zn micromesh placed on bubbles.d)The tensile strain tests of Zn micromesh and Zn film.e)Schematic diagram of mechanical stability tests.Reproduced with permission [50].Copyright 2021 Wiley-VCH.

Table 2Comparisons of the flexible conductors.
3.Design of FECDs
3.1.FECDs based on inorganic electrochromic materials
Inorganic electrochromic materials are the earliest type of electrochromic materials observed by the electrochromic community,which can be dated back to 1969 when S.K.Deb demonstrated the electrochromic coloration effect in WO3thin films[4].After decades of research and development,transition metal oxides and Prussian blue (iron hexacyanoferrate,Fe4[Fe(CN)6]3) analogues have developed rapidly and become the two major groups of inorganic electrochromic materials.This is due to their excellent photochemical stability,superior radiation resistance,and ease of device fabrication.The coloration mechanism of inorganic materials is according to the change of the valence state and the concentration evolution of transition metal ions under different applied voltage conditions.As such,the choice of different types of inorganic electrochromic materials for FECDs will result in different electrochromic characters,thus,enabling different applications.In this part,some recent researches on applying different inorganic electrochromic materials for FECDs are introduced and the corresponding performances are also discussed.
3.1.1.Metal oxides
It was a long history of metal oxides being utilized as electrochromic materials,due to their good chemical stability,durability,a wide range of working temperatures,low cost,and environmental friendliness.According to their different coloration mechanisms,electrochromic metal oxides can be divided into three categories:cathodic coloration materials with colorless oxidation state and colored reduction state(such as WO3,MoO3,TiO2,etc.),anodic coloration materials with colorless reduction state and colored oxidation state (such as NiO,MnO2,Cr2O5,etc.),and bipolar coloring materials with colored oxidation state and colored oxidation state (such as V2O5) [5].To develop FECDs having high performance,including mechanical stability and electrochromic functionalities,a list of recent achievements in the fabrication of promising electrochromic metal oxides is presented.It is promised that universal strategies for fabricating electrochromic metal oxides can be realized to advance the future development of the FECDs.
3.1.2.Tungsten oxide
Tungsten oxide is considered one of the most developed metal oxide electrochromic materials,due to its high chemical stability,rapid switching speed,and large optical modulation range.Many representative attempts had been reported to fabricate high-performance WO3-based FECDs.For example,D.W.Leitzke et al.prepared amorphous WO3films by a sol-gel method,which showed exciting electrochemical stability during 200 cycles[51].However,the transmittance variation(ΔT at 555 nm) between colored and bleached states of the flexible amorphous WO3film was only 42.6% while the coloration efficiency was 14.91 cm2C-1.Such an inferior electrochromic performance of the flexible amorphous WO3film is attributed to the weak binding force between the ITO/PET substrate and the WO3layer,due to the limitations of the sol-gel method.To improve the electrochromic performance of flexible WO3films,H.Li et al.fabricated stacked WO3/Ag/WO3(WAW)films on PET substrate by electron beam evaporation method at room temperature [52,53].These films displayed satisfactory optical contrast(53%) at 650 nm,short switching times (coloration time=11 s;bleaching time=10.5 s),and long-term cycling stability (3000 cycles).Furthermore,such films exhibited excellent mechanical stability,which showed almost unchanged square resistance after 1600 bending cycles.Magnetron sputtering technology can also be used to fabricate flexible WO3films with enhanced binding force between the substrate and WO3layer.C.Tang et al.prepared an all-solid-state inorganic large-scale(24 cm × 18 cm) flexible PET/ITO/WO3/Nb2O5/NiVOx/ITO monolithic electrochromic device via a reactive magnetron sputtering method[54].After the bending durability test (100 bending cycles),the optical transmittance only showed a slight increase for coloring and a negligible decrease for bleaching.Such a decay in electrochromic performance is attributed to the decrase in conductivity of flexible electrodes.The decrase in conductivity of flexible electrodes is caused by the formation of cracks in ITO layers,which results in the increase of resistance and thus hindering the transport of charges and cations.But,the solid-state electrochromic device could tolerate bending to a critical radius of curvature of 7.5 cm,due to its good deformability.More importantly,as shown in Fig.9,the device was capable of sustaining over 8000 coloring-bleaching cycles.
Besides the utilization of the sol-gel method,electron beam evaporation method,and magnetron sputtering approach for fabricating flexible electrochromic WO3films,the electrodeposition method is also considered a promising method due to its great advantages in terms of cost-effectiveness and the potential for synthesizing large-area samples[10].J.Chou et al.prepared WO1-Xthin films on ITO/PET by cyclic voltammetry (CV) via adjusting different scanning cycles [53].The results revealed that the WO1-Xfilms fabricated with 50 CV cycles showed better performance,which included a high optical contrast (79.54%) at 650 nm.Such an electrodeposition method is promising in fabricating WO3-based FECDs.
After carefully fabricating the flexible WO3films,the assembly of WO3-based FECDs requires the design of another important component(i.e.,electrolyte).P.Cossari et al.reported a flexible EC-organic lightemitting diode device(EC-OLED)via choosing a suitable Nafion polymer matrix [55].Such a device exhibited a strong enhancement in terms of interface properties,high transparency,and optical contrast(ΔT650nm=60%),with lower operating voltages(0.5–3 V),and long-term durability(1000 switching cycles).As such,this device shows potential and promising applications in smart glasses and fully transparent OLED displays.To further enhance the bending properties of electrochromic devices,a Li-doped ion gel electrolyte was investigated by T.Y.Yun et al.[56]It was shown that the FECDs having the Li-doped ion gel electrolyte exhibited low voltage operation (-0.9 V) and large transmittance contrast(~85%)between colored and bleached states.Most importantly,such an FECD exhibited excellent mechanical stability under bending tests as it had no electrolyte leakage during bending tests,which result in excellent electrochromic performance.The FECD maintained~90.3 and~84.5% of initial optical transmittance and coloration efficiency after 1000 bending tests.
In conclusion,the fabrication of WO3-based FECDs requires the investigation of flexible WO3films and gel/solid electrolytes.Future research work needs to focus on the assembly of large-area WO3-based FECDs and the devices’electrochromic performance.
3.1.3.Molybdenum oxide
Similar to tungsten oxide,molybdenum oxide is also a kind of cathodically coloring electrochromic material[57].As early as 2008,K.H.Krishna et al.fabricated molybdenum trioxide thin films on ITO-coated flexible Kapton substrates utilizing a plasma-assisted activated reactive evaporation technique [58].These molybdenum trioxide thin films showed nearly 80% of optical transmittance in the visible region when bleached and demonstrated 40% of optical modulation at the wavelength of 550 nm.To further enhance the flexibility of the MoO3--based flexible electrodes,Y.Liu et al.fabricated the stacked MoO3/Ag/MoO3(MAM)films for constructing high-performance flexible indium tin oxide (ITO)-free electrochromic devices [59].In comparison to the PET/ITO/MoO3films,the MAM films exhibited a faster response time (coloration time is 6.2 s/bleaching time is 10.9 s) and optical contrast of 27.7% at 528 nm,better bending and cycling stability(more than 2000 cycles).
Although it is reported that the reduced MoO3films can display a dark-blue color that is more sensitive to human eyes,the poor kinetics of lithium-ion diffusion in MoO3films and the extensive destruction of the structure caused by large-volume expansion during the lithiation and delithiation processes have hindered their practical applications in electrochromic devices.Further study on the design of nanostructured composite MoO3flexible films may overcome the aforementioned limitations and broaden the real-world applications of the MoO3flexible films.
3.1.4.Titanium oxide
Titanium oxide,a commonly-used cathodically coloring electrochromic material,has been investigated thoroughly due to its unique advantages of physical and chemical stability[11,61–66].A recent work utilizing titanium oxide to fabricate flexible titanium oxide was reported in 2021 by R.Li et al.[60].In this work,a liquid/liquid interfacial self-assembly (LLIA) technique was utilized to fabricate composite TiO2/Ti3C2TxMxene films,where the Ti3C2Tx((T=O,OH,or F))Mxene layer was serving as the transparent layer and the 2D TiO2as the electrochromic layer.As shown in Fig.10a,the Ti3C2Tx/PET flexible conductor showed less resistance increment than that of the commercial ITO/PET substrate after 1000 bending cycles,which is due to the 2D nature of Ti3C2Tx.Such a good flexibility of the Ti3C2Tx/PET substrate enabled the composite TiO2/Ti3C2Txelectrochromic films to show an enhanced electrochromic performance after bending tests(Fig.10b).As such,such composite TiO2/Ti3C2TxMxene films showed great promise in assembling large-area flexible electrochromic devices(Fig.10c–e).

Fig.10.a) A comparison of the sheet resistance (Rs) and the Ti3C2Tx/PET film and the ITO/PET films during the 1000 bending cycles.b) The difference in electrochromic switching performance between the Ti3C2Tx/PET film and the ITO/PET film after 1000 bending cycles.c)Schematic illustration of the configuration of the FECD.d,e) Demonstration of large-area FECDs.Reproduced with permission [60].Copyright 2021 Springer Nature.
3.1.5.Nickel oxide
Besides the above-described cathodically coloring metal oxides(e.g.,WO3,MoO3),anodic electrochromic materials which can serve as counter layers have also shown great promise in FECDs.NiOx,the most important anodic electrochromic material,has been investigated comprehensively in complementary electrochromic devices due to its high phase stability [67–70].To fabricate flexible NiOx-based electrochromic electrodes,Z.Wu et al.utilized an electrophoretic deposition technique at room temperature[71].Such an electrophoretic deposition technique enabled a facile fabrication of NiO films directly on ITO/PET substrate.Such flexible NiO films showed high optical modulation(54.1% at 550 nm),fast switching speed (coloration time: 7.3 s,bleaching time: 3.9 s),and high coloration efficiency (25.7 cm2/C).To further demonstrate the potential applications of the flexible NiO films,a complementary electrochromic device was assembled using an electrodeposited WO3film as the working electrode.As shown in Fig.11,the as-assembled flexible complementary electrochromic device showed obvious color change between the colored and bleached states.The transmittance of the colored state and the bleached state was measured to be 3.17% and 46.05%,respectively.Furthermore,such a complementary device also showed good deformability as it can be bent by tweezers.

Fig.11.Optical photograph of flexible complementary ECD in the colored state (left) and bleached state (right) (±2.5 V,30 s).Reproduced with permission [71].Copyright 2019 IOP Publishing Group.
Although the electrodeposition method can be utilized to fabricate flexible NiO films,it still suffers poor adhesive force between the NiO layer and the flexible substrate.Therefore,it is of great interest to develop advanced solution-processed technologies (e.g.,ink-jet printed,spray-coating) for fabricating flexible NiO films having superior cyclic durability and mechanical stability.
3.1.6.Vanadium oxide
As bipolar coloring materials can provide more colors for electrochromic display applications,the exploration,and utilization of vanadium oxide (one of the most famous bipolar coloring electrochromic materials) have aroused great attention from both academia and industry.In our previous reports,we utilized multicolor characters of the vanadium oxide and the newly-born zinc anode-based electrochromic device configuration to fabricate transparent inorganic multicolor displays [7,13].However,the simple rod-coating method used in our previous reports currently are incompatible with flexible substrates due to the high solution-process temperature.The choice of a proper deposition method for fabricating flexible vanadium oxide films is an emerging research direction in this specific project.J.Wu et al.utilized the electrodeposition method to fabricate flexible V2O5thin films on double-layer graphene/PET substrates [72].Due to the multicolor characters of vanadium oxide,the electrodeposited flexible film has a variety of colors when being applied different voltages (Fig.12a).The flexible V2O5films showed a high transmittance modulation(68.94%)at 800 nm,due to the employment of graphene electrodes.In addition,the flexible V2O5films also showed good flexibility,which is shown in the inset photographs in Fig.12a.Such electrodeposited flexible V2O5films show great promise in future flexible optoelectronics but suffer poor mechanical stability due to the inferior adhesive force between the electrochromic layer and the substrate.To improve the mechanical stability of the flexible V2O5films,Y.Qi et al.fabricated V2O5nanosheets(NS)/graphene oxide (GO) composite films via a layer-by-layer (LBL)assembly method [73].The flexible V2O5NSs/GO films exhibited attractive bending stability along with excellent color change efficiency(2.7s/4.3s) and optical transmittance contrast (57.5% at 425 nm).As shown in Fig.12b,the flexible V2O5NSs/GO films displayed fairly high capacity retention (84.2%) after bending 200 bending cycles with a bending angle of 90°.

Fig.12.a) Transmittance spectra of the graphene-PET substrate,as-deposited V2O5 film,and the V2O5 film at different applied voltages.The inset photographs are flexible films under different color states.Reproduced with permission[72].Copyright 2018 IOP Publishing Group.b)Capacity retentions of the LBL-fabricated V2O5 NSs/GO films upon different bending angles and bending cycles.Reproduced with permission [73].Copyright 2020 Elsevier.
3.1.7.Others
To overcome the limitations of the mono-metal oxides,bimetallic/triple-metallic oxides have been developed for electrochromic applications due to their rapid kinetics,high optical modulation ability,and good cycling stability.Y.Lu et al.reported a triple-metallic oxide electrochromic material (i.e.,LixNa2-xW4O13) via a facile lithium-ion exchange method[74].Such a strategy could highly improve the dispersity of the Na2W4O13(LixNa2-xW4O13)in water(Fig.13),which therefore can be easily spray-coated onto a flexible ITO substrate and thus forming a flexible electrode having enhanced electrochromic performance.The assembled complementary devices delivered a large optical modulation(75% at 700 nm),fast switching times (less than 2 s in both coloration and bleaching processes),and outstanding cyclic stability.

Fig.13.Schematic illustration of Li-ion intercalated Na2W4O13 nanosheets.Reproduced with permission [74].Copyright 2021 Springer Nature.
Besides the oxides used for FECDs,Prussian blue and its analogues have also shown great promise in fabricating FECDs.As well known,Prussian blue (PB) (Fe4[Fe(CN)6]3),having a face-centered cubic structure,is an important electrochromic material with mixed-valence states(Fe3+and Fe2+) [75–77].The color switching mechanism of PB is attributed to the valence state change of Fe3+and Fe2+when being applied to an external voltage [78].As PB is colored when applying a positive voltage,it is typically used as a counter electrode for complementing the electrochromic effect of WO3-based electrochromic devices.For example,C.Y.Jeong and co-workers prepared complementary FECDs by wet coating WO3and PB thin films on ITO/PET substrates[79].These FECDs exhibited excellent electrochromic performance,including a large optical modulation (79% at 633 nm),high coloration efficiency(123.32 cm2C-1),and good cycling stability.In addition,A.Chaudhary et al.proposed the electrochromic effect of PB can be utilized to fabricate flexible glucose sensors (Fig.14) [80].The flexible PB electrode was fabricated via a facile electrodeposition method,and it delivered a fast switching speed (coloration time: 2.1 s,bleaching time: 2.4 s),high coloration efficiency (nearly 87 cm2C-1),and stability in absorbance switching cycles for nearly 390 s(Fig.14a and b).Moreover,the device does not need any counter electrode except electrolyte for its redox reaction.The electric current of the device could increase with the glucose concentration(Fig.14c and d).
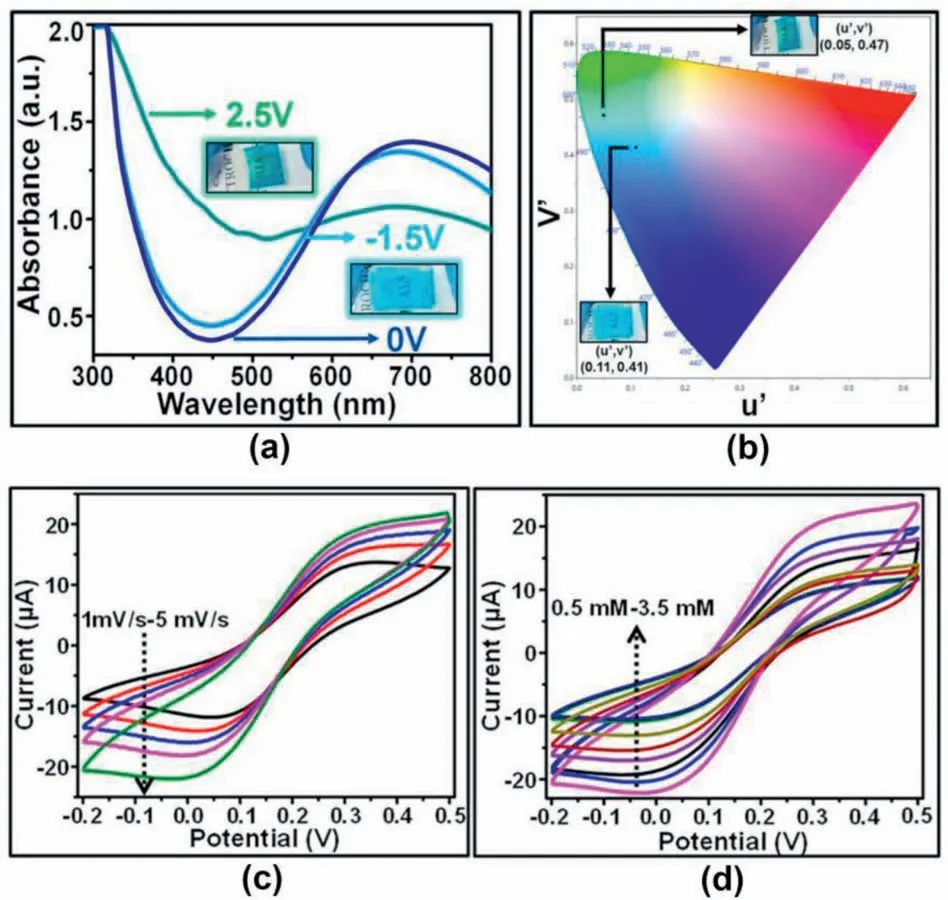
Fig.14.a) In-situ UV–Vis absorbance spectra under a different bias voltage.b)CIE diagram of the device.c)Cyclic voltammograms (CV)curves of the PB electrode under different scan rates and d)the change of current with the addition of glucose concentration for a given scan rate.Reproduced with permission[80].Copyright 2021 John Wiley and Sons.
3.2.FECDs based on organic electrochromic materials
Organic materials have shown various applications in flexible optoelectronics (e.g.,OLEDs,etc.),due to their advantages including lightweight,flexible nature and good compatibility with large-area,low-cost fabrication methods [81].Likewise,the research on organic electrochromic materials has attracted increasing attention due to the above-mentioned advantages[82,83].In general,organic electrochromic materials are divided into three categories according to the fundamental mechanisms,which include conjugated polymers,redox molecules,and metallo-supramolecular polymers [84].In this section,the recent researches on the above-mentioned polymers for fabricating FECDs is reviewed.The design of organic/inorganic composite electrochromic flexible films is also discussed in this section,as the organic/inorganic composite films may open new functionalities of the FECDs.
3.2.1.Conjugated polymers
Conjugated polymers,such as polyaniline(PANI),polypyrrole(PPy),and polythiophene,have shown great promise in fabricating FECDs due to their improved switching kinetics,better contrasts,lower power consumption,and higher coloration efficiencies when compared with the previously mentioned inorganic electrochromic materials.
PANI,the most commonly used conjugated polymer for electrochromic applications,exhibits reversible multicolors in the visible light region and tunable emittance in the infrared region.Therefore,flexible PANI films can be designed for different applications.For example,a selfdoped PANI(SPANI)film was electrodeposited on a flexible Au/nylon 66 electrode to fabricate flexible zinc ion electrochromic batteries [85].Such flexible zinc ion electrochromic batteries showed good mechanical stability,which presented significant capacity change under different bending angles.Furthermore,as zinc ion electrochromic batteries are a newly-born technology [15],the utilization of PANI for building the zinc-ion electrochromic batteries may accelerate the development of flexible intelligent energy storage devices.
Besides the design of PANI-based FECDs for regulating the colors in the visible light region,the development of infrared FECDs based on polyaniline has also attracted immense attention due to their potential military applications.H.Gong et al.fabricated a flexible self-driven IRECD along with variable optical and thermal management in a lowenergy mode [86].Within such an electrochromic device,an obvious increase in the IR reflectance could be driven by the built-in potential difference between the EC PANI cathode and the aluminum foil anode.Inversely,the device could recover through charging by a solar cell.Furthermore,this IR-ECD delivered a huge reflectance contrast(21.2%),a high coloration efficiency (93.6 cm2C-1at 1500 nm) and a fast switching speed from the high-to low-IR reflectance states (~6s).Likewise,S.Song et al.designed a monolithic integrated flexible infrared electrochromic device based on PANI conducting polymer,which demonstrated ultra-high flexibility and excellent structural stability[87].The monolithic integrated flexible infrared electrochromic device can reversibly convert between a colored state (dark green) and a bleached state (golden yellow) upon the imposed external voltages,and the infrared emission modulations of 0.43 and 0.40 were achieved at the wavelength ranges of 8–14 μm and 2.5–25 μm,respectively.With the exploration of the infrared FECDs,a visible-to-infrared broadband FECD based on H2SO4-doped polyaniline films was designed for simultaneously variable optical and thermal management by the same group [88].The visible color and infrared emissivity of the device can be controlled reversibly between-0.05 to 0.3 V.The visible color could change from green to yellow,while the infrared emissivity changed from high to low values.These exciting results made it appealing for both camouflage and thermal regulation applications.In addition,such a device exhibited robust durability and structural stability,which retained good electrochromic performance after 100 bending cycles.
Polythiophene and its derivatives,another conjugated electrochromic polymer,have also been extensively developed for FECDs due to their good electrochemical and electrochromic properties.Among them,poly(3,4-ethylenedioxythiophene) (PEDOT) is one of the most studied and commercially used conjugated polymers for fabricating FECDs,due to its stability in both doped and undoped states [89].A recent demonstration of PEDOT-based FECDs that comprised of PEDOT electrochromic layer and nickel grid conductive layer was fabricated by C.Chen et al.[90]Such devices delivered rapid coloration and bleaching times(~1 s for 90% optical contrast),a high coloration efficiency(325 cm2/C),and excellent mechanical reliability (optical contrast retention~80% after 1000 bending cycles).As the doping states of the PEDOT would greatly affect its electrochromic performance,the recent researches on PEDOT-based FECDs are mainly focused on the treatments of PEDOT electrodes.For instance,D.A.Barus et al.investigated the effect of chemical treatment on the performance of PEDOT for FECDs[91].After carefully comparing the performance of PEDOT electrodes being treated with various solvents,the benzenesulfonic acid (BSA) treated PEDOT electrode showed the highest optical transmittance and conductivity.Thus,utilizing BSA-treated PEDOT as electrochromic layers provided a new strategy for designing high-performance PEDOT-based FECDs.
3.2.2.Redox molecules
Viologen (i.e.,4,4′-Bipyridinium salts) and its derivatives represent the most well-studied redox molecules in fabricating electrochromic devices.The electrochromic effect of viologen is attributed to its three reversible redox states,that is,V2+(dication,pale yellow colored/colorless) ↔V+⋅(radical cation,violet/blue/green) ↔V0(neutral,colorless)[92].Such molecules are typically dissolved in a gel electrolyte that is sandwiched between two conductive electrodes.As the viologen and its derivatives have shown high optical contrast,rapid switching speed,and good durability [93],the utilization of viologen and its derivatives for FECDs gained increasing attention.V.Yekefallah et al.proposed a water-soluble viologen-functionalized dendrimer electrochromic material by attaching a viologen chromophore to the end groups of the polyamidoamine dendrimer branches [94].The water-based electrochromic materials were ink-jet printed onto different flexible substrates,which was friendly to the environment.Moreover,the FECD showed an excellent optical transmittance(79.2% in bleached state and 32.2% in colored state)and a high coloration efficiency(311.5 cm2C-1at 600 nm).A.Chaudhary et al.reported an all-organic FECD by the combination of viologen and poly-3-hexylthiophene(P3HT)[95].This FECD could change colors between maroon and blue with a small voltage(1.4 V) and showed a high optical modulation (80%).Furthermore,this all-organic device possessed a fast switching speed (~1 s) without the requirement for an electrolyte.G.Balamurugan et al.reported a terpyridine-attached asymmetric viologen (TpyV),which showed better electrochromic performance than previously reported viologen-based ECDs [96].The TpyV-based ECD exhibited a maximum transmittance of >91%,fast switching speeds (6.3 s for coloration and 5.8 s for bleaching),and a high coloration efficiency(232.87 cm2C-1).In addition,the TpyV-based FECD showed a good stability after 20 bending cycles,which demonstrated the practicability of the material.
To unblock new functionalities of the viologen-based ECDs,the investigation of multifunctional viologen-based FECDs have attracted immense attention.For example,C.Wang et al.proposed a flexible electrochromic mirror(EM)based on Zn/viologen hybrid batteries[97].The flexible EMs showed energy storage functionality along with reversible electrochemical mirror(REM)electrochromic function via the electrodeposition and dissolution of Zn.The principle of such devices is shown in Fig.15.Such devices showed a high reflectance value(84.9%)and excellent memory effect(the reflectance is stable after one week).

Fig.15.Schematic diagram of the structure and mechanism of Zn-Ems.Reproduced with permission [97].Copyright 2020,American Chemical Society.
3.2.3.Metallo-supramolecular polymers
Metallo-supramolecular polymers,a new type of cathodically coloring EC materials,have attracted huge attention due to their outstanding optoelectronic performance in recent years [98,99].Such metallo-supramolecular polymers are synthesized by the coordination polymerization of organic small molecules or polymers with metal ions,leading to a strong metal-to-ligand charge-transfer transition within the polymer,which brings strong optical absorption in the visible range.
Due to the excellent optoelectronic performance,the metallosupramolecular polymers have shown great promise in fabricating FECDs.For example,G.Cai et al.reported a large-area(810 cm2) FECD via utilizing Fe(II)-based metallo-supramolecular polymer (polyFe)[100].The FECD exhibited extraordinary electrochromic properties,including a high coloration efficiency (750.3 cm2C-1),a fast switching speed(less than 1 s),and a good electrochemical durability(no obvious degradation after 10000 cycles).N.Malik et al.demonstrated an ultrasonic spray coating method to prepare homogeneous and uniform electrochromic metallo-supramolecular films[101].The films were prepared by alternatingly spray-coating dilute solutions of structurally well-defined iron polypyridyl ([Fe(mbpy-py)3]2+) complexes and bis(-benzonitrile)palladium dichloride (Pd(PhCN)2Cl2) onto a flexible substrate (i.e.,PET),where the latter palladium salt was used as the inorganic cross-linker.Such films were further utilized for ECDs,which showed high optical contrast and good switching stability.J.Arockiam et al.reported that an iron phthalocyanine incorporated iron metallo-supramolecular polymer (PcFe-polymer) could cover a broad wavelength range from visible light to near-infrared light regions[102].The solid-state ECDs,utilizing PcFe-polymer as active material,exhibited admirable switching stability (>1000 times),high coloration efficiency(211.4 cm2C-1),and high transmittance contrast (>40%).Furthermore,such a PcFe-polymer is compatible with the fabrication of FECDs,which also exhibited high stability and efficient switches.
3.2.4.Organic/inorganic composite-based FECDs
Recently,the incorporation of organic materials and inorganic materials for developing composite FECDs has attracted immense attention due to the synergic effect of the hybrid materials,which combines the advantages of inorganic materials and organic materials.K.C.Lee et al.designed the Ni(OH)2/PEDOT (P–Ni(OH)2) composite material for FECDs and asymmetric supercapacitors [103].The P–Ni(OH)2nanocomposite materials addressed critical shortcomings of bare Ni(OH)2such as the poor electrical conductivity and the reaggregation during cycling.As such,the P–Ni(OH)2-based FECD showed greater electrochemical activity and kinetics,resulting in a more rapid switching(9.0 s),a huge optical contrast (52.7%),a high coloration efficiency (90.2 cm2C-1),and the degradation of electrochemical performance after 500 bending cycles is negligible.Similarly,Y.Sui et al.reported MoO3-x@-PANI core-shell composite for FECDs [104].In such a core-shell structure,the MoO3-xnanobelt core provided enhanced cycling stability while the PANI shell endowed high specific capacitance and electrochromic performance.Therefore,the MoO3-x@PANI-based FECDs exhibited a high optical contrast(33% at 732 nm)and fast switching times(4 s and 3.9 s for bleaching and coloration,respectively).As shown in Fig.16,S.Zhang et al.reported a flexible and patterned electrochromic device,which was fabricated by spray-coating monodisperse SiO2@PANI core-shell nanospheres onto flexible ITO/PET substrates[105].The FECD showed a large optical contrast(34.1% at 510 nm),rapid response speed(8.5s for bleaching and 9.4s for coloring),and high coloration efficiency(86.7 cm2C-1),and outstanding mechanical stability (inconspicuous attenuation of the optical contrast after 500 cycles of bending).

Fig.16.a-b)Photographs of the flexible electrode with customized patterns under different bias voltages.c)Photographs of the FECD at bleached and colored states.Reproduced with permission [105].Copyright 2019,IOP Publishing Group.
Besides the PANI/inorganic compound composite used for FECDs,the WO3/PEDOT:PSS composites have also shown great promise in fabricating FECDs.For example,E.Eren et al.reported an rf rotating plasma polymerization method for fabricating WO3-PEDOT and WO3-polyfuran(WO3-PFu)electrodes.The WO3-PEDOT-based FECD showed low voltage operation(from-1 V to 1 V),admirable optical modulation(61.5%)and high coloration efficiency(527 cm2C-1).The same group also successfully produced single-layer FECDs composed of PEDOT and WO3via a simple in-situ UV-curing method [106].The FECD exhibited an optical transmittance modulation of 38.7% via a proper choice of LiClO4electrolyte,while a high mechanical bendability can be achieved by utilizing lithium trifluoromethanesulfonate(LiTRIF)electrolyte.
To better conclude the electrochromic performance of FECDs using different active materials,a detailed comparison of representative FECDs are listed in Table 3.
3.3.FECDs based on EC-hydrogels
Besides the above-mentioned FECDs based on inorganic and organic electrochromic materials,FECDs based on EC-hydrogels have also attracted immense attention due to their advantages including the ease of device fabrication,excellent flexibility and good durability [111,112].More importantly,the design of EC hydrogels could broaden the functionality of the ECDs.For example,a triple functional ECD was realized by the Walter group [113],which was prepared by dissolving a water-soluble,chromogenic thiazolo(5,4-d)thiazole (TTz) dye into a polyvinyl alcohol (PVA)/borax hydrogel.The utilization of the water-soluble dye enabled the integration of electrochromism,electrofluorochromism,and photochromism functionalities within a single ECD.
In addition,the highly stretchability of the hydrogel enables it being one of the most promise candidate for fabricating FECDs.G.Yang et al.reported a FECD using an EC hydrogel that composed of transparent,elastic and conductive polyacrylamide (PAAM) hydrogel and watersoluble EC molecules [114].Such FECDs,utilizing stretchable Ag/PDMS and Au/PDMS electrodes,showed good stretchability and electrochromic performance.The devices showed no obvious decay in electrochromic performance after 100 stretching cycles,which exhibited a highly reversible color switch between bright red and dark brown(Fig.17a and b).While the EC molecules dissolved in hydrogels bring poor bistability of ECDs,J.Li et al.recently designed the hydrogen-bonded ionic gels to improve the bistability of the FECDs[115].Due to the existence of hydrogen bonds,the gel film can exhibited better elasticity than PMMA film.Moreover,the weak polarity-inducing ability of the hydroxyl group enabled the EC ionic gel exhibited an admirable bistability(54 h,Fig.17c).

Fig.17.a) Photographs of FECD in different colors at stretched and normal states.b) Photographs of FECD bending cycle.Reproduced with permission [114].Copyright 2019,The Royal Society of Chemistry.c) Steady-state display photos and application directions of patterned electrochromic devices.Reproduced with permission [115].Copyright 2021,American Chemical Society.
3.4.FECDs using gel electrolyte
As the design of FECDs will bring significant challenges including poor flexibility,performance decay upon deformation,and the leakage of liquid electrolyte during mechanical strain/stress,it is of great importance to design deformable electrolytes.Therefore,gel electrolytes is promising for fabricating FECDs due to their excellent deformability[116–118].For example,J.Zeng et al.prepared a transparent solid-state electrolyte (T-SE),which is consist of polymethacrylate (PMMA),polyoxypropylene glycol (PPG),and lithium perchlorate (LiClO4) (Fig.18)[119].The utilization of such a gel electrolyte not only enables the good flexibility of the polymer-based FECDs (no obvious decay in electrochromic performance under bending conditions),but also delivers fast switching speed (5.2 s for coloration,2.6 s for bleaching),high optical modulation (61.0% at 700 nm),and excellent bistability.In another pioneer example,R.Alves et al.used gellan gum and ionic liquid (IL)1-ethyl-3-methyl-imidazolium-thiocyanate [Emim][SCN]to synthesize an environmental friendly solid polymer electrolyte [120].The electrolytes exhibited acceptable mechanical properties (Young modulus: 23 MPa) and good thermal stability (the ionic conductivity value is 1.8 ×10-2S cm-1at 90°C).

Fig.18.Schematic diagram of the preparation process of electrolytes and ECDs.Reproduced with permission[119].Copyright 2019,The Royal Society of Chemistry.
Although the above-mentioned gel electrolytes eliminate the leakage of electrolytes during mechanical strain/stress,they still suffer the ease formation of cracks during mechanical strain/stress.Therefore,it is of significant importance to design self-healing gel electrolytes for FECDs.Y.Wang et al.synthesized a self-healing polymeric electrolyte P(AA-VIm-VSN) by copolymerizing acrylic acid (AA),1-vinylimidazole (VIm) and vinyl hybrid silica nanoparticles (VSN) [121].The dynamically cross-linked hydrogen bonds could effectively recover the cracked polymeric network,thus enabling admirable mechanical stability(tensile strength 11 kPa) and outstanding slef-healing properties (healing efficiency of 90.5% within 20 min).To improve the self-healing efficiency,Q.Chen et al.designed a dynamically cross-linked hydrogel electrolyte(i.e.,P(GMA2-AAm8)-borate hydrogel electrolyte)by copolymerizing glycerol monomethacrylate (GMA) and acrylamide (AAm),which showed remarkable stretchability and self-healing capability for FECD[122].Such a hydrogel electrolyte,possessing a fracture strain of 115% and a high ionic conductivity (4.5 mS cm-1),which enabled almost unchanged optical modulation of FECDs after 1000 bending cycles(Fig.19a).Most importantly,the hydrogel electrolyte in FECD could heal the cracks that formed by physical damages (Fig.19 b).As shown in Fig.19c,the cutted FECD was still able to operate after the hydrogel electrolyte healed.In conclusion,the design of self-healing gel electrolyte may accelerate the real-world applications of FECDs.More attention should be focused on the design of novel self-healing gel electrolytes.

Fig.19.a) Transmittance change of the FECDs during 1000 bending cycles at 582 nm.b) Photograph of the self-healing process of the FECDs.c) The ECD can still work after being cutted due to the self-healing property.Reproduced with permission [122].Copyright 2021,American Chemical Society.
3.5.FECDs toward wearable electronics
As FECDs are always twistable and foldable,such devices are also promising for wearable electronics due to their broadened applications[123].To fabricate wearable FECDs,the fabric is one of the most ideal choices for device substrate.S.Sinha et al.designed an electrochromic fabric display by utilizing the poly (ethylene terephthalate) (PET) synthetic leather substrate [124].Such electrochromic fabric displays showed a switching speed of ≈30 s between red to blue colors,along with a good “fabric” feel for wearable applications.Although the electrochromic fabric displays showed great promise for flexible textile display applications,the limited stretchability of the textile substrate blocked futuristic applications such as implantable smart displays having curvilinear surfaces.To develop stretchable ECDs,C.Y.Yan et al.employed AgNW networks embedded in polydimethylsiloxane (PDMS) elastomer matrix as stretchable conductors [20].As shown in Fig.20,such PDMS conductors enabled excellent stretchability(50% strain)along with good electrochromic performance (1 s and 4 s for coloration and bleaching,respectively).

Fig.20.a) Schematic diagram of the stretchable EC device structure.b-c) The stretchable EC device could endure the states of the twisted and crumpled.d-e)Patterned EC device showed bleached/colored states under 0 and 50% strain states respectively.Reproduced with permission [20].Copyright 2014,American Chemical Society.
With the development of the research on stretchable and wearable electrochromic devices,H.Park et al.developed a skin-integrated transparent and stretchable electrochromic device for an interactive color-changing system[107].Such an interactive color-changing system is comprised of a skin-attachable strain sensor and a wearable electrochromic device.The skin-attachable strain sensor exhibited excellent performance including high sensitivity,high durability,fast response(20 ms),and high transparency (77%).By changing the strain,the applied voltage on the FECD would be varied and thus leading to different colors and transmittance (Fig.21a,b,c).Such an integrated system enabled a real-time visual display of body motion (Fig.21d),which showed great promise for future use in interactive wearable devices,military applications,and smart robots.To eliminate the requirement of external voltage for powering the wearable electrochromic devices,Sun et al.designed a self-powered wearable Sichuan Opera mask by integrating a transparent flexible triboelectric nanogenerator (TENG) with FECD in a single platform [125].The TENG generated power by body motions,which was used for operating the PB-based FECDs.Therefore,such an integrated system eliminated the requirement for external power,while providing additional opportunities for wearable electronics.

Fig.21.a)Transmittance change under the infliction of different strains.b)The real-time monitoring of transmittance along with the infliction of different strains.d)Photograph of the color changed together with finger motions.Reproduced with permission [107].Copyright 2017,The Royal Society of Chemistry.
Besides the utilization of stretchable substrates for wearable electrochromic devices,the development of fiber-shaped electrochromic devices has attracted immense attention due to their desired designability and integration features[21,126].For example,T.Zhu et al.reported a novel kind of flexible electrochromic nylon fiber that was prepared by dip-coating AgNWs,PEDOT:PSS,and WO3layers orderly [108].The electrochromic fibers showed excellent electrochromic properties including a rapid color switching speed (2.5 s for bleaching and 9 s for coloration),a large optical modulation (65.5% at 633 nm) and an outstanding electrochemical stability (70% retainment of capacitance after 5000 operating cycles).To develop electrochromic fibers which are suitable for industrial weaving,H.Fan et al.reported a continuous method to fabricate hundreds-meter-long electrochromic fibers[127].As shown in Fig.22(a,b),the EC fibers can be knitted and implantable for large-area smart color-changing textiles,which can also be applied to adaptive camouflage.In addition,the EC fibers with various color changes can be implanted/woven into textiles and used for wearable displays(Fig.22(c,d)).

Fig.22.a) Digital photographs of large-area smart textile knitted using long EC fibers(scale bar:20 cm).b) Digital photographs of smart EC textile in the colored/bleached states for proof-of-concept military camouflage(scale bar:15 cm).c)Digital photographs of smart textile implanted with different EC fibers for wearable display (scale bar: 2 cm).d) Digital photographs of large-area embroidery with smart colorchanging flowers composed of multiple EC petals(scale bar: 20 cm).Reproduced with permission[127].Copyright 2014,American Chemical Society.
3.6.FECDs having energy storage functionalities
While ECDs are considered as thin film supercapacitors,the FECDs can also integrate energy storage and electrochromic functionalities in a single platform.Such an integration endows the device to store electrical energy along with the reversible changing of the colors [128],which is supposed to significantly broaden the applications of FECDs.For example,the development of WO3-based electrochromic supercapacitors enable the visually displaying of energy storage level in real time,as well as a rapid and reversible color switching [34].T.Yun et al.reported an all-transparent stretchable electrochromic supercapacitor,utilizing WO3nanotube and PEDOT:PSS as active materials,has shown exciting electrochromic and electrochemical performance [129].Such a multifunctional FECD was comprised of Au/Ag core-shell nanowire-embedded PDMS stretchable electrodes,a hydrogel electrolyte,and WO3/PEDOT:PSS electrochromic layer(Fig.23a),which showed reversible color switching along with good stretchability (Fig.23b and c).In addition,such a device exhibited excellent energy storage performance,which realized a specific capacitance of 471.0 F g-1and good stretchability(98.6% retention of capacitance under 20% stretchment)(Fig.23(d–f)).In this regard,such an integration was proved to be highly suitable for multifunctional applications.

Fig.23.a)Schematic diagram of the wearable FECD.b)Digital photographs of the colored and bleached state of the wearable FECD.c)Demonstration of the device on the wrist.d) Normalized capacitance of the FECD under different applied stretches.Reproduced with permission [129].Copyright 2019,American Chemical Society.
With the development of multifunctional FECDs and the emerging demands of high-capacitance FECDs,the exploration of composite electrochromic materials are of great interest in the electrochromic community.M.Hassan et al.fabricated a W18O49NWs/rGO composite electrode,which exhibited a higher specific capacitance (92 mF cm-2)and good electrochromic performance due to the addition of conductive rGO[109].The composite electrode showed a high coloration efficiency(46 cm2C-1),a fast switching speed (8 s for coloration and 8.45 s for bleaching),a high optical modulation (~50% at 633 nm),and a remarkable electrochemical durability (maintaining 96.41% of its original optical modulation after 100 cycles).In another pioneer work,T.Yun et al.developed an electrochromic supercapacitor that used cellulose-nanofiber/Ag NWs/rGO/WO3-composite electrode,which exhibited a high coloration efficiency of 64.8 cm2C-1and electrochemical performance with specific capacitance of 406.0 F g-1,energy/power densities of 40.6–47.8 Wh kg-1and 6.8–16.9 kW kg-1[110].Besides the above-mentioned pioneer reports of electrochromic supercapacitors using WO3-based materials,some other electrochromic materials also showed great promised for electrochromic supercapacitors(e.g.,polypyrrole (PPy) [130],PANI [131],and rGO–Co(1-x)Nix(OH)2[132]).The development of FECDs having energy storage functionalities will certainly advance the real-world applications of FECDs.
4.Outlook and conclusion
Recently,the research on FECDs has rapidly evolved due to the development of flexible and wearable electronics.However,real-world applications of the FECDs are still in their infancy and require more investigation and development.In the next few years,researchers are expected to work closely with the industry and focus their attention on device assembly,electrodes,and materials design to develop robust FECDs.It is envisioned that FECDs will provide new opportunities for electrochromism as they provide multifunctionalities compared to rigid ones.
As the performance of FECDs is significantly affected by the device assembly,practical customization of a standardized production line of FECDs that are compatible with different types of substrates,electrochromic materials,and electrolytes should be developed.The design of the production line of FECDs requires a close collaboration between academia and industry,which is the lack of the current electrochromic community.
In terms of the electrodes and materials design,which requires indepth research by materials scientists.The electrode design includes the fabrication of conductor substrates as well as deformable conductive layers.The conductor substrates should possess excellent mechanical and electrochemical stability,along with good optical transparency.The deformable conductive layers should be comprised of low-cost materials for practical application.Moreover,a good candidate conductive layer material should also have excellent resistance to the electrochemical redox reactions,which will greatly improve the practicality of the FECDs.The materials design needs to focus on the design of nanostructured electrochromic materials as the nanostructured electrochromic materials could significantly shorten the cations transport pathway and thus leading to outstanding electrochromic performance[14].
With the development of FECDs,another research goal should be the design of multifunctional FECDs that incorporate flexible electrochromism and other additional functions into a single platform,due to the increasing demands for multifunctional electronic devices.For example,the energy storage function of FECDs can be realized by integrating electrochromic devices and zinc ion batteries[10,15,16].
In summary,this review summarized the recent advances in FECDs,which we hope could complement the lacking of a comprehensive review of recent achievements in FECDs.We systematically discussed recent progress in designing flexible conductors,electrochromic materials,and the assembly for FECDs.In addition,the latest advances in the fields of wearable ECDs are introduced.By extensively reviewing the recent achievements in FECDs,we hope this review article could be helpful to those scientists who have recently entered this specific field.Also,it is expected that this review article would broaden the impact and development of the FECDs.
Declaration of competing interest
The authors have no conflict of interest to declare.
Acknowledgements
The authors acknowledge the support from the “Qilu Young Scholar”program (62460082163097) of Shandong University,the National Natural Science Foundation of China (62105185),Shandong Excellent Young Scientists Fund Program (Overseas) (2022HWYQ-021),and Guangdong Basic and Applied Basic Research Foundation(2022A1515011516).
- Namo Materials Science的其它文章
- Advancing the pressure sensing performance of conductive CNT/PDMS composite film by constructing a hierarchical-structured surface
- In situ confined vertical growth of Co2.5Ni0.5Si2O5(OH)4 nanoarrays on rGO for an efficient oxygen evolution reaction
- Water-based synthesis of nanoscale hierarchical metal-organic frameworks:Boosting adsorption and catalytic performance
- Wearable and stretchable conductive polymer composites for strain sensors:How to design a superior one?
- Addressing cation mixing in layered structured cathodes for lithium-ion batteries: A critical review
- Synergistic coupling of 0D–2D heterostructure from ZnO and Ti3C2Tx MXene-derived TiO2 for boosted NO2 detection at room temperature

