Prognostic value of ultrasound in early arterial complications post liver transplant
Ning-Bo Zhao,Yi Chen,Rui Xia,Jian-Bo Tang,Dong Zhao
Abstract Liver transplantation is the primary therapeutic intervention for end-stage liver disease.However,vascular complications,particularly those involving the hepatic artery,pose significant risks to patients.The clinical manifestations associated with early arterial complications following liver transplantation are often nonspecific.Without timely intervention,these complications can result in graft failure or patient mortality.Therefore,early diagnosis and the formulation of an optimal treatment plan are imperative.Ultrasound examination remains the predominant imaging modality for detecting complications post liver transplantation.This article comprehensively reviews common causes and clinical presentations of early hepatic artery complications in the post-transplantation period and delineates abnormal sonographic findings for accurate diagnosis of these conditions.Overall,ultrasound offers the advantages of convenience,safety,effectiveness,and non-invasiveness.It enables real-time,dynamic,and precise evaluation,making it the preferred diagnostic method for post-liver transplantation assessments.
Key Words: Liver transplantation;Vascular complication;Arterial complication;Ultrasound
lNTRODUCTlON
Liver transplantation stands as the primary therapeutic approach for end-stage liver disease.Continuous advancements in surgical techniques and the application of novel immunosuppressive agents contribute to ongoing improvements in the success rate and overall survival in patients undergoing liver transplantation procedures.Despite these advancements,vascular complications,particularly those involving the hepatic artery,pose significant risks to patients.During the early stages following liver transplantation (within the first 30 d),proper hepatic artery function is crucial for hepatic arterial blood flow.During later stages,collateral circulation,including arteries such as the phrenic artery,right gastric artery,and gastroduodenal artery,becomes important for maintaining hepatic blood supply.It is now understood that the establishment of effective collateral circulation is pivotal for determining the prognosis of hepatic artery complications.The clinical manifestations of these complications are closely linked to factors such as timing,severity,and the specific type of onset.Insufficient hepatic arterial blood flow can lead to abnormal liver function,hepatic infarction,and the formation of hepatic abscesses.Additionally,since the hepatic artery is the sole blood supply to the biliary tract,hepatic artery-related ischemia may result in biliary stricture,obstruction,and the formation of bile ducts.Ultrasound examination remains the primary imaging modality for diagnosing complications post liver transplantation.This article comprehensively reviews common causes and clinical presentations of early hepatic artery complications in the posttransplantation period and outlines abnormal sonographic findings for accurately diagnosing these conditions.
NORMAL HEPATlC ARTERY
During the intraoperative phase,an ultrasound examination is typically conducted to evaluate the hepatic artery anastomosis.The normal internal diameter of the hepatic artery typically ranges from 2 to 5 mm.Two strong echo points are typically identified near the anastomosis.To assess blood flow dynamics,peak systolic velocity,end-diastolic velocity,and resistance index are measured at the donor and recipient sides of the anastomosis following angle correction.Anastomotic stenosis presence and severity can be evaluated by comparing the velocity at the anastomotic site with that at the recipient side.Postoperatively,direct visualization of the anastomosis site through gray ultrasound scans is often challenging.The surgical approach has a significant impact on the proper hepatic artery’s position,resulting in a lower overall success rate of continuous visualization.Color Doppler ultrasound is primarily employed to trace the artery’s path,and spectral measurements are taken at the brightest position of the Color Doppler blood flow signal,primarily used to identify the presence of high-speed turbulence.Hepatic artery spectrum examination plays a crucial role,as a favorable arterial spectral waveform and appropriate hepatic artery flow velocity typically indicate a successful anastomosis,even in cases where the hepatic artery anastomosis cannot be directly visualized by ultrasound.The hepatic artery runs alongside the portal vein,often selected as a reference due to its larger inner diameter.A normal hepatic artery spectrum displays a regular pulsation pattern with a rapid rise in systole and a slow decline in diastole.Parameters for assessing hepatic artery resistance include a resistance index between 0.5 to 0.8 and an artery systolic acceleration of less than 80 ms.Instantaneous increases in the resistance index (RI > 0.8) often occur within 2 d after surgery,followed by a subsequent return to normal hepatic arterial parameters.It has been established that the maximum blood flow velocity during systole in the hepatic artery should not exceed 200 cm/s[1].
HEPATlC ARTERY THROMBOSlS
Hepatic artery thrombosis (HAT) represents a serious complication following liver transplantation,with an incidence rate ranging from 3% to 5%[2,3].Early risk factors for HAT encompass ABO-blood type incompatibility,prolonged cold ischemia time for the donor’s liver,acute rejection reaction,hepatic artery spasm,intimal dissection,perianastomotic hematoma compression,arterial torsion,undersized donor hepatic artery diameter,and mismatched anastomotic size[4,5].HAT leads to a reduction in blood supply to the liver,particularly in the early postoperative period,and can result in severe complications,with mortality rates reaching 20%-60%.Clinical presentations of HAT include severe hepatic pain,fever,ascites,abrupt elevation of serum aminotransferases,decreased bile flow,altered bile properties,prolonged prothrombin time,and sepsis.Rapid progression of HAT can lead to biliary complications,graft necrosis,and mortality.Therefore,early diagnosis and timely treatment of HAT are crucial[6].
HAT is characterized by the absence of color Doppler and spectral Doppler signals in the region of the hepatic artery’s course.However,blood flow signals may still be detected in some HAT cases due to the development of hepatic arterial collateral circulation,which can occur as early as 2 wk postoperatively.Color Doppler ultrasound can detect blood flow signals in the hepatic artery after the formation of collateral circulation,showing tardus-parvus waveforms with specific features (RI < 0.5 and SAT > 80 ms).It is important to note that the inability to detect hepatic artery blood flow signals using Doppler ultrasound is not a definitive diagnostic criterion for hepatic artery occlusion.Studies indicate that the accuracy of using color Doppler in diagnosing HAT is only 64%,influenced by factors such as operator experience,equipment sensitivity,and the fine caliber of the hepatic artery,leading to a significant number of false-positive cases[7].Contrast-enhanced ultrasound (CEUS) has significantly improved the visualization of hepatic vessels.In specific imaging modes,CEUS can present hepatic artery images similar to digital subtraction angiography (DSA) in the arterial phase after intravenous ultrasound contrast combined with low mechanical index,thereby enhancing the sensitivity and specificity of HAT diagnosis[8].With a 100% diagnostic sensitivity for HAT,CEUS emerges as a highly reliable method for detecting this complication[9,10].Therefore,when color Doppler does not clearly show the hepatic artery after liver transplantation,an immediate CEUS examination is warranted,allowing for accurate diagnosis while avoiding unnecessary invasive angiography[12] (Figure 1).

Figure 1 A 32-year-old male patient underwent a piggyback liver transplant for decompensated cirrhosis and acute liver failure.A: This assessment occurred 15 d postoperatively.Routine ultrasound imaging had failed to reveal hepatic arteries within both the liver and extrahepatic regions.Contrastenhanced ultrasound showed that the portal vein was prematurely visible (indicated by the orange arrow),while neither intrahepatic nor extrahepatic arteries displayed any enhancement;B: Furthermore,multiple ischemic lesions within the liver were observed in the arterial (phase I),portal (phase II),and late phases (phase III),indicating a lack of enhancement (as indicated by the orange arrow).Surgical confirmation subsequently identified a diffuse formation of arterial thrombosis within the hepatic artery.
HEPATlC ARTERY STENOSlS
Hepatic artery stenosis (HAS) is a relatively common vascular complication after liver transplantation,with a high incidence ranging from 5% to 11%[13].While not as severe as HAT,significant HAS or progressive stenosis can lead to complications such as bile duct and liver ischemic necrosis and even liver failure.The gold standard for diagnosing HAS is hepatic artery angiography,and the degree of narrowing is classified as mild (< 50%),moderate (50% to 75%),or severe (> 75%).HAS often occurs at the anastomosis site,anastomotic acceptor segment,or donor segment.Various risk factors contribute to the development of HAS,including surgical technique,vascular endothelial damage,rejection,prolonged donor cold ischemia time,small donor artery size,and hypercoagulability.Clinical manifestations of HAS include elevated serum aminotransferase levels,increased bilirubin,liver abscess,ischemic cholangitis,bile duct stenosis,or necrosis.Severe HAS may even present with graft non-function[14].
Direct visualization of the HAS site by ultrasound is typically challenging,relying on the indirect diagnosis of intrahepatic artery changes.A tardus-parvus waveform in the hepatic artery spectrum is indicative of HAS[15],with the mechanism being the significant narrowing of the proximal artery leading to a drop in perfusion pressure in the distal segment.The specificity of the tardus-parvus waveform for diagnosing HAS is approximately 81.9%[16].However,it should be borne in mind that a tardus-parvus waveform does not necessarily confirm the presence of HAS,as it can be observed in various conditions such as severe aortic atherosclerosis,arteriovenous fistulas,artery-bile duct fistulas,extensive collateral circulation,large-area hepatic necrosis,or systemic hypotension[17].A more reliable diagnostic criterion for HAS is the measurement of high-velocity blood flow above 200 cm/s near the hepatic artery anastomosis (Figure 2).CEUS can provide direct visualization of the site of HAS,showing focal stenosis at the anastomosis site,bead-like or segmental intrahepatic or extrahepatic arterial stenosis,and diffuse stenosis.However,the limitations of CEUS,such as difficulty visualizing hepatic artery anastomosis due to gas interference and abdominal wall fat,should be considered[18,19],and it may have limited diagnostic value for HAS.
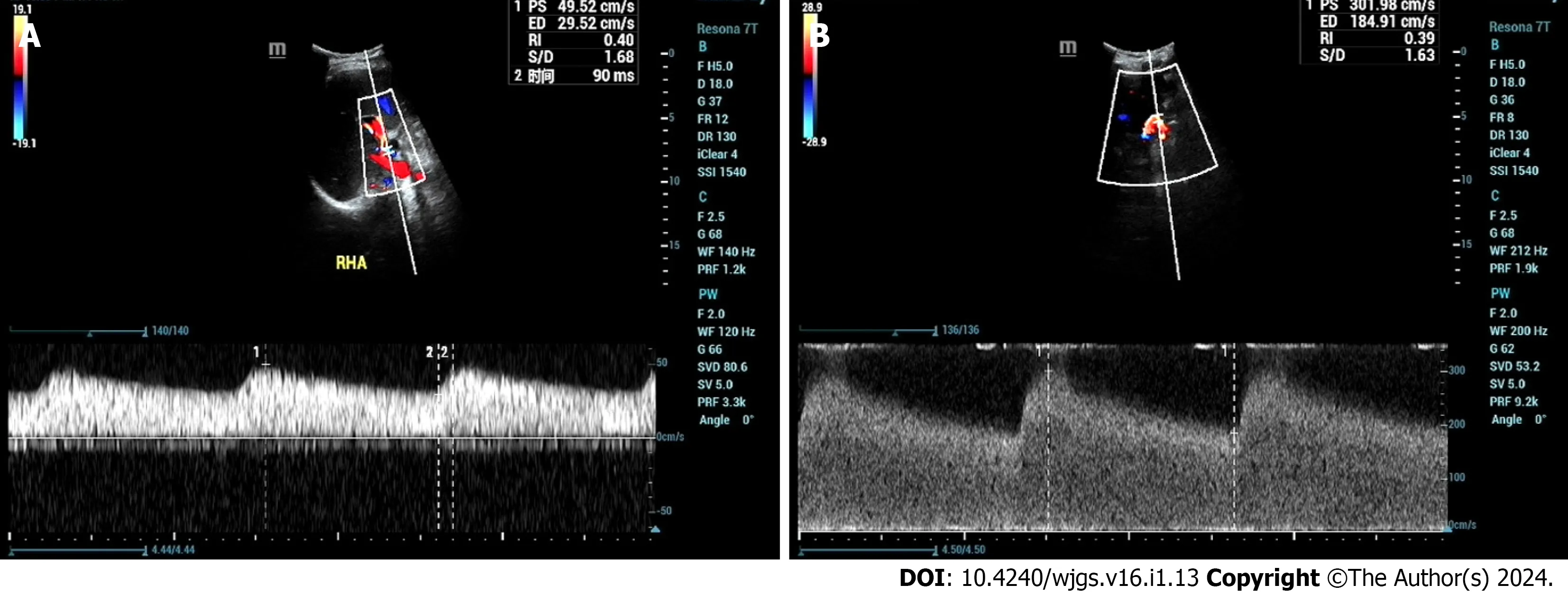
Figure 2 A 46-year-old male patient who underwent liver transplantation for liver cancer received an ultrasound examination at 1 year and 4 mo postoperatively,revealing hepatic artery stenosis.A: Ultrasound indicated an intrahepatic artery (right hepatic artery) resistive index of 0.40,with a systolic acceleration time of 90 ms;B: Blood flow near the hepatic artery anastomosis reached 302 cm/s.
HEPATlC ARTERY ANEURYSM
Hepatic artery aneurysm (HAA) is relatively rare following liver transplantation[20,21].There are three main types of HAA: Congenital or acquired true HAA caused by factors such as atherosclerosis,fungal infections,necrotizing vasculitis,and polyarteritis;traumatic or iatrogenic hepatic pseudoaneurysm (hepatic artery pseudoaneurysm,HAP)[22];and hepatic dissection aneurysm (hepatic dissecting aneurysm,HDA)[23] resulting from causes like surgical suture injury or percutaneous hepatic artery angiography.HAA typically remains clinically silent until rupture.When an aneurysm becomes too large and compresses the portal system,symptoms such as jaundice and portal hypertension may occur.Ruptured HAA can lead to life-threatening gastrointestinal bleeding and intra-abdominal bleeding.DSA is the gold standard for diagnosing HAA[24],revealing tumor-feeding arteries.Most HAAs present as sac-like or nodular structures with contrast stasis inside,while arterial damage and extravasation of contrast medium are direct signs for diagnosis.The purpose of ultrasound examination is to diagnose HAA earlier before rupture and provide a more accurate treatment plan.
Hepatic artery pseudoaneurysm
HAP often occurs at the arterial anastomosis site or at the site of intervention or puncture[25].The peripheral soft tissue wrapping hematoma forms due to the ruptured hemorrhage of the arterial wall[26,27].Gray-scale ultrasound shows the cystic anechoic area of the hepatic artery with a clear boundary.Color Doppler presents characteristic red and blue alternating blood flow signals,while spectral Doppler displays arterial blood flow signals.CEUS reveals the synchronous development of the tumor body with the hepatic artery during the arterial stage.
Hepatic dissecting aneurysm
HDA often results from the rupture of the intima and media layers due to various factors,leading to the entry of blood into the medial layer,causing the neoplastic structure to tear,resulting in the stratification of the vessel wall,and dividing the artery into true and false lumens.These lumens may or may not communicate through the rupture site.Ultrasound reveals the following characteristics: (1) Intimal stratification signs,where the separated intima may be fixed or float with the bloodstream.True and false cavities may have inlet and outlet connections or exist as separate structures (Figure 3A);(2) an increase in arterial diameter;(3) blood flow stratification phenomenon,where the separated intima divides arterial blood flow into two layers,representing the blood flow in the true lumen and the false lumen,each with distinct blood flow spectra (Figure 3B);(4) thrombosis in the false lumen;and (5) vascular narrowing in the true lumen.
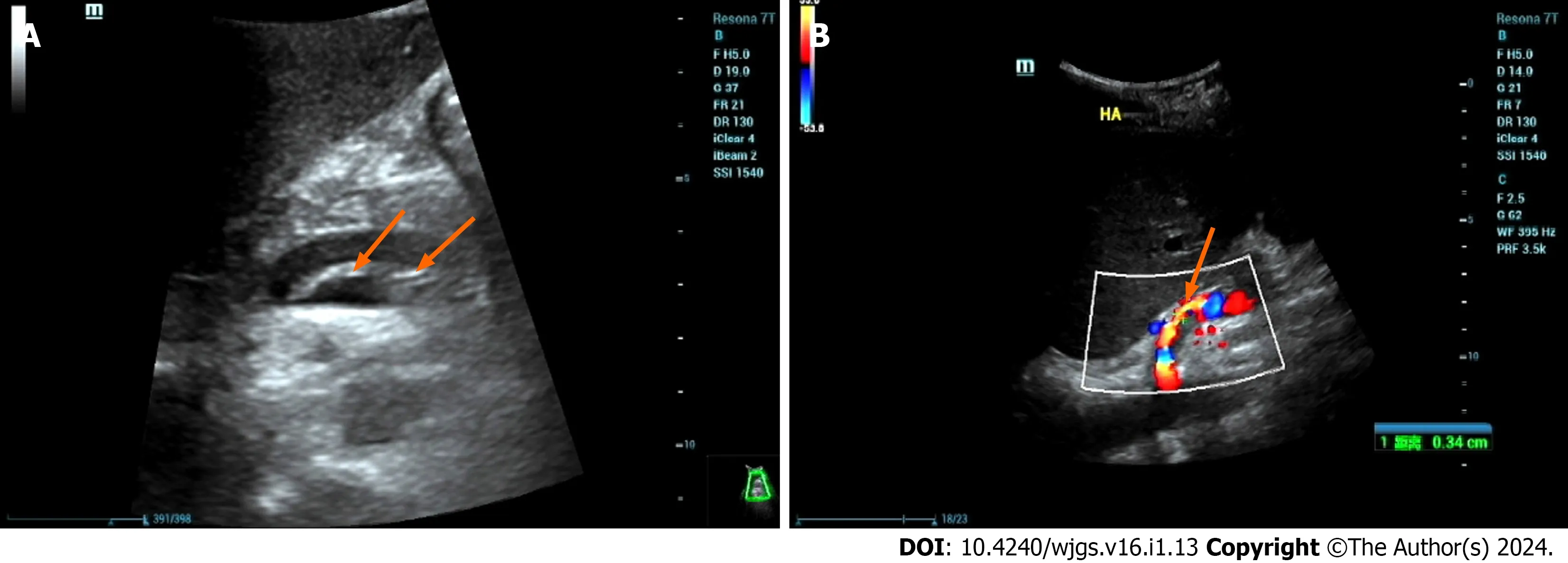
Figure 3 A 46-year-old male patient with decompensated cirrhosis due to hepatitis B underwent liver transplantation.On postoperative day 1,ultrasound showed no blood flow signals in the hepatic artery,followed by hepatic artery angiography and thrombosis.A: Ultrasound showed intramural-like echoes floating in the proper hepatic artery (arrow);B: Ultrasound showed blood flow in the true lumen of the proper hepatic artery (arrow),with no obvious blood flow signals in the false lumen.Then,the patient underwent hepatic artery stent graft implantation with satisfactory clinical results.
The following features indicate that urgent intervention is necessary for a dissecting aneurysm: (1) Tardus-parvus waveform observed in the hepatic artery of the liver;(2) an increase in peak contraction velocity at the tumor body (> 200 cm/s);and (3) absence of blood flow signal in the liver[28].
HEPATlC ARTERY VASOSPASM
Hepatic artery vasospasm (HAV) is characterized by intense constriction of the arterial vessel wall,resulting in rigidity,narrowing,or occlusion of the affected portion or the entire vessel[29].This common complication during the recipient’s surgical process is often secondary to excessive manipulation and suturing.Despite a lack of sufficient data and widely recognized diagnostic criteria,animal and clinical studies suggest that elevated serum norepinephrine levels may influence HAV[30].High-risk factors include surgical compression,increased hepatic perfusion resistance,exposure of the hepatic artery to bile,and liver resection[31,32].The impact of HAV on the transplanted liver remains uncertain,as vascular spasms may reduce hepatic artery flow and potentially induce thrombus formation.Richardset al[33] emphasize that vascular spasms in vascular reconstruction tissue can compromise tissue perfusion even with a patent hepatic artery anastomosis.
Doppler ultrasound lacks uniform diagnostic criteria for HAV,with manifestations such as arterial shortening,narrowing,absence of focal anastomotic stenosis (resulting in an increased resistance index),and,at times,diastolic flow loss or reversal.Ruling out other causes of increased resistance index is essential.The use of vasodilators can lead to a reduction in the resistance index,and postoperative ultrasound follow-up can support the diagnosis of HAV[31].
HEPATlC ARTERY DlSTORTlON
Hepatic artery distortion (HAD) is a common condition in liver transplant surgery,where the recipient’s hepatic artery is lengthened to reduce tension at the anastomosis site,resulting in a twisted appearance near the liver hilum.This twisting generally does not affect hepatic blood supply,and color Doppler shows a bright blood flow signal in the distorted segment.Spectral Doppler detects high-velocity blood flow in the region,and there is usually no abnormality in the blood flow velocity in the hepatic artery affected by HAD,with liver function remaining generally normal.In traditional twodimensional imaging modes,the twisted hepatic arteries can overlap and form angular shapes in images,resembling HAS,making it challenging to distinguish between HAS and HAD.Three-dimensional (3D) CEUS overcomes this limitation by displaying vascular structures in three spatial dimensions.It provides a clear representation of the 3D architecture of the hepatic artery without being restricted by changes in vascular curvature.Dynamic 3D reconstruction of Doppler blood flow combines 3D flow data with anatomical structures,offering a more complete understanding of the hemodynamic characteristics of the examined organs and their relationship to anatomy.
HEPATlC ARTERY RUPTURE
Hepatic artery rupture (HAR) carries severe consequences,potentially leading to hemorrhagic shock or even death[34].Immediate intervention through surgical or vascular procedures is essential once the diagnosis is confirmed.Ruptures typically occur near the anastomosis site and can result from various factors,including suture line failure,avascular necrosis of the arterial wall at the anastomosis,arterial wall infection,or aneurysms[35].Ultrasound findings in cases of HAR include reduced or normal blood flow velocity within the hepatic artery in the liver and a significant accumulation of fluid near the hepatic artery anastomosis site.CEUS is valuable in the diagnosis of HAR,revealing a concentrated area of contrast agent accumulation next to the hepatic artery with consistently higher enhancement levels compared to the surrounding normal liver tissue.This demonstrates continuous extravasation of contrast agents with strong enhancement characteristics (Figure 4).

Figure 4 A 48-year-old female patient underwent a piggyback liver transplantation procedure due to decompensated cirrhosis resulting from hepatitis B. A: Evaluated 13 d postoperatively,the patient’s clinical history revealed a progressive decline in blood pressure and red blood cell count,accompanied by the accumulation of a significant volume of intraperitoneal fluid.Conventional ultrasound examination demonstrated a substantial accumulation of fluid in the porta hepatis region,characterized by hypoechoic features and the presence of numerous fine,low-level echogenic particles suspended within.Contrastenhanced ultrasound revealed enhanced contrast agent pooling within the intraperitoneal fluid of the porta hepatis,exhibiting a morphology resembling a mushroom cloud (highlighted by the orange arrow);B: Notably,further tracking of the contrast agent leakage within the porta hepatis area (indicated by the orange arrow) indicated that it originated from the hepatic artery anastomosis site.
CONCLUSlON
In conclusion,the clinical presentations associated with early arterial complications after liver transplantation are often nonspecific.Without timely intervention,these complications can lead to graft failure or patient mortality.Early diagnosis and the establishment of the best treatment plan are crucial.Ultrasound,with its advantages of convenience,safety,effectiveness,and non-invasiveness,can be evaluated in real time,providing dynamic and accurate information.As a result,it has become the preferred diagnostic method for post-liver transplantation evaluation.
FOOTNOTES
Author contributions:Zhao D and Zhao NB contributed to study conception and design;Zhao D contributed to administrative support;Zhao D and Zhao NB contributed to provision of the study materials,and collection and assembly of the data;Chen Y,Xia R,and Tang JB contributed to data analysis and interpretation;all authors contributed to the writing and final approval of the manuscript.
Supported bythe Shenzhen Science and Technology R&D Fund,No.JCYJ20220530163 011026;and Shenzhen Third People’s Hospital,No.G2022008 and No.G2021008.
Conflict-of-interest statement:The authors have no conflicts of interest to declare.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Dong Zhao 0000-0003-3773-721X.
S-Editor:Chen YL
L-Editor:Wang TQ
P-Editor:Xu ZH
 World Journal of Gastrointestinal Surgery2024年1期
World Journal of Gastrointestinal Surgery2024年1期
- World Journal of Gastrointestinal Surgery的其它文章
- Prospects in the application of ultrasensitive chromosomal aneuploidy detection in precancerous lesions of gastric cancer
- Added value of ratio of cross diameters of the appendix in ultrasound diagnosis of acute appendicitis
- Single-incision laparoscopic transabdominal preperitoneal repair in the treatment of adult female patients with inguinal hernia
- Predictive value of machine learning models for lymph node metastasis in gastric cancer: A two-center study
- Micro-power negative pressure wound technique reduces risk of incision infection following loop ileostomy closure
- Unraveling the efficacy network: A network meta-analysis of adjuvant external beam radiation therapy methods after hepatectomy
