ldentification,pathogenicity,and fungicide sensitivity of Eutiarosporella dactylidis associated with leaf blight on maize in China
Cheng Guo ,Xiaojie Zhang ,Baobao Wang ,Zhihuan Yang ,Jiping Li ,Shengjun Xu ,Chunming Wang,Zhijie Guo,Tianwang Zhou,Liu Hong,Xiaoming Wang#,Canxing Duan#
1 Institute of Plant Protection, Gansu Academy of Agricultural Sciences, Lanzhou 730070, China
2 Pratacultural College, Gansu Agricultural University, Lanzhou 730070, China
3 Shijiazhuang Academy of Agriculture and Forestry Sciences, Shijiazhuang 050050, China
4 Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China
Abstract Maize (Zea mays L.) is an economically vital grain crop that is cultivated worldwide. In 2011,a maize foliar disease was detected in Lingtai and Lintao counties in Gansu Province,China. The characteristic signs and symptoms of this disease include irregular chlorotic lesions on the tips and edges of infected leaves and black punctate fruiting bodies in dead leaf tissues. Given favourable environmental conditions,this disease spread to areas surrounding Gansu. In this study,infected leaves were collected from Gansu and Ningxia Hui Autonomous Region between 2018 and 2020 to identify the disease-causing pathogen. Based on morphological features,pathogenicity tests,and multilocus phylogenetic analysis involving internal transcribed spacer (ITS),18S small subunit rDNA (SSU),28S large subunit rDNA (LSU),translation elongation factor 1-alpha (TEF),and β-tubulin (TUB) sequences,Eutiarosporella dactylidis was identified as the causative pathogen of this newly discovered leaf blight. Furthermore,an in vitro bioassay was conducted on representative strains using six fungicides,and both fludioxonil and carbendazim were found to significantly inhibit the mycelial growth of E. dactylidis. The results of this study provide a reference for the detection and management of Eutiarosporella leaf blight.
Keywords: maize leaf blight,morphology,molecular phylogeny,Eutiarosporella dactylidis,fungicide sensitivity
1.Introduction
Maize (Zea maysL.) is an important commercial food crop that is also widely used as animal feed and as a raw material in various industries (Jong-Hwanet al.2014). It is grown in over 170 countries and regions worldwide.As the second largest maize producer worldwide,China produced 265 million tons of maize in 2020,accounting for 22.9% of the total global yield. Disease prevention and control are crucial factors in ensuring consistent maize production. From July to September 2011,Eutiarosporellaleaf blight was detected in Lingtai and Lintao counties in Gansu Province,China. Infected leaves displayed many black-spotted fruiting bodies scattered across the leaf surfaces,and they eventually withered and died. These symptoms differed notably from those of other maize foliar diseases,including grey leaf spot,northern corn leaf blight,and southern rust. From 2012 to 2016,the disease occurred to varying degrees in several ecoregions in Gansu outside of the Hexi Corridor,China. Following its initial outbreak in Gansu in 2017,Eutiarosporellaleaf blight was subsequently detected in several regions of Shaanxi Province and Inner Mongolia Autonomous Region,as well as in all counties in Ningxia Hui Autonomous Region,China. Furthermore,the disease onset shifted from the initial filling stage to heading stage,resulting in leaf death and severe yield losses.
Eutiarosporellaleaf blight,which is caused byEutiarosporella dactylidis(Botryosphaeriaceae),is becoming a major fungal disease in spring maize in Northwest China. The family Botryosphaeriaceae consists of 24 well-defined genera and more than 200 species(Burgesset al.2019),including saprobes,endophytes,and pathogens,which are detrimental to agriculture and forestry because of their diversity (e.g.,genera,species,and forms) and wide distribution (Slippers and Wingfield 2007;Slipperset al.2009;Lawrenceet al.2017). Early taxonomic research on Botryosphaeriaceae primarily involved analysis of morphological characteristics during the sexual stage. Unfortunately,relatively little information is available regarding sexual morphology under natural or artificial culture conditions. The lack of diverse teleomorph features makes it difficult to differentiate species (Denmanet al.2000). Therefore,the Botryosphaeriaceae fungi are primarily identified based on their asexual morphological features. Furthermore,the taxonomic classification ofEutiarosporellawas largely determined in earlier studies that used DNA sequence data to reveal the phylogenetic relationships among species (Crouset al.2015;Thynneet al.2015;Dissanayakeet al.2016;Liet al.2016).Integrated analyses of morphological characteristics and molecular data have enabled researchers to quickly and accurately identifyEutiarosporellaspecies.
The genusEutiarosporellawas first proposed by Crous(2015),based onEutiarosporella tritici(=Tiarosporella tritici) detected onTriticum aestivumplants in South Africa,and it was named based on its morphological similarity to the genusTiarosporella. However,Eutiarosporellahas conidiomata with long necks and undergoes holoblastic conidiogenesis,whereasTiarosporellahas globose to depressed and unilocular conidiomata as well as conidiogenous cells that undergo percurrent proliferation(Crouset al.2015). The genusEutiarosporellaconsists of seven species,namelyEutiarosporella africana,E.dactylidis,Eutiarosporella darliae,Eutiarosporella pseudodarliae,E.tritici,Eutiarosporella tritici-australis,andEutiarosporella urbis-rosarum,which are mainly distributed throughout South Africa,Australia,and Italy(Dissanayakeet al.2016).Eutiarosporellais associated with Poaceae and woody hosts (Jamiet al.2012,2014;Thambugalaet al.2014;Liet al.2016;Thynneet al.2015;Sutton and Marasas 1976). The pathogenicity of these fungi remains relatively unknown,but severalEutiarosporellaspecies have been associated with needle diseases in conifers,either as pathogens or endophytes (Sieber 1988;Müller and Hantula 1998).Eutiarosporella dactylidis(=Tiarosporella dactylidis) was first detected in the dead leaves ofDactylis glomerataandArrhenatherum eliatusplants in Italy (Thambugalaet al.2014). Subsequently,Liet al.(2016) and Dissanayakeet al.(2017) collected the dead stems ofAvenella fleuosaandA.elatius,respectively,from Italy and isolatedE.dactylidis. Barkat (2016) and Thynne (2017) isolated this pathogen from stored wheat grains and suggested that it might be responsible for grain storage diseases.
The pervasiveness of this pathogen worldwide and the recent outbreak in Gansu Province have necessitated further research on effective identification and control measures. Thus,the objectives of this study were the following: 1) identify the causal pathogen ofEutiarosporellaleaf blight using morphological characteristics,multi-locus phylogenetic analysis,and pathogenicity based on Koch’s postulates and 2) determine thein vitrosensitivity ofE.dactylidisto six fungicides. These results will provide a reference for maize growers to effectively identify and controlEutiarosporellaleaf blight.
2.Materials and methods
2.1.Sample collection and fungal isolation
From 2018 to 2020,maize leaves with typicalEutiarosporellaleaf blight symptoms were collected from 123 villages in Gansu Province and the Ningxia Hui Autonomous Region (Fig.1). Sample collection sites included the semi-arid region of the Loess Plateau (central area of Gansu),the semi-humid region of the Loess Plateau (eastern area of Gansu),the humid subtropical region (southeastern area of Gansu),the hilly loess region (southern hills of Ningxia),and the arid plain region(northern plain of Ningxia). Diseased leaves were placed in Kraft paper bags,transported to the laboratory,and stored at room temperature under dry conditions prior to pathogen isolation and identification. Culturable fungi were isolated from diseased leaf tissues that displayed fruiting bodies using a conventional tissue separation method (Fang 2007). Pure cultures of all fungal isolates were obtained using the single hyphal tip isolation method,and subsequently transferred to fresh potato dextrose agar (PDA),incubated at 25°C in darkness,and stored in tubes at 4°C.

Fig. 1 Diseased sample collection sites.
2.2.Morphological characterization
The maize leaf specimens with fruiting bodies were sliced. Thereafter,the sections with fungal structures were mounted in distilled water and examined using a microscope (Olympus DP73,Shenzhen Industrial Ltd.,China). Samples were photographed using an Axiocam 503 colour digital camera with differential interference contrast illumination and measured using the cellSens Standard software. The conidia were examined using an S-570 scanning electron microscope (Hitachi,Japan) and photographed and measured as described above. The maximum,minimum,and mean sizes were calculated using MS-Excel 2010. The morphological standards described by Barkat (2016),Sutton and Marasas (1976),and Thambugalaet al.(2014) were used as reference.
A total of 245 isolates were obtained from 258 strains according to the similarity in their colony morphology(colour,edge,texture,and growth rate) under identical incubation conditions. Twelve representative isolates (two to four isolates per ecoregion) were randomly selected and cultured on the following eight media: water agar (2%)(WA),PDA,potato sucrose agar (PSA),potato carrot agar(PCA),oatmeal agar (OA),cornmeal agar (CMA),malt extract agar (MEA),and maize leaf powder plus CaCO3agar (MLPCA;15 g maize leaf powder,1.5 g CaCO3,and 15 g agar). The mycelial diameter was measured after a 48-h incubation period at 25°C in darkness. Colonies were photographed after 7 d. All data were analysed using IBM SPSS Statistics for Windows,version 26.0 (IBM Corp). Significant differences (P<0.05) in the growth rate among the eight media were determined using one-way ANOVA followed by Tukey’s test. Multi-gene sequencing analysis was performed to validate the results of the morphological identification.
2.3.DNA extraction,PCR amplification,and sequencing
The strains were cultured for 7 to 10 d on PDA (at 25°C in darkness) in plates covered with a cellophane membrane.Mycelia were collected by gently scraping the cellophane sheet with a scalpel and transferred to a sterile 1.5-mL centrifuge tube. Genomic DNA was extracted from the mycelia using an All-In-One DNA/RNA Mini-Preps Kit(Sangon Biotech,China). Sequence data for the following genomic regions were used in the phylogenetic analyses:the internal transcribed spacer (ITS) region of the rRNA operon using primers ITS-1 and ITS-4 (Whiteet al.1990),part of the 5´ end of the 18S small subunit (SSU) nrRNA gene using primers NS1 and NS4 (Whiteet al.1990),the large subunit (LSU) rDNA gene region using primers LR0R and LR5 (Vilgalys and Hester 1990),part of the translation elongation factor 1-α (TEF-1α) gene using primers EF1-728F and EF1-986R (Carbone and Kohn 1999),and part of the β-tubulin (TUB) gene using primers Bt-2a and Bt-2b (Glass and Donaldson 1995). The PCR amplification was completed in 30 μL of reaction volume containing 2 μL of genomic DNA,1 μL of each primer(10 μmol L–1),16 μL of 2× Taq PCR Master Mix,and 10 μL of ddH2O. The following PCR program of the PTC-200 Peltier Thermal Cycler was used: 94°C for 4 min;35 cycles of 94°C for 45 s,56°C for 45 s,and 72°C for 1 min;and 72°C for 10 min. The PCR products were analysed using 1% agarose gel electrophoresis and sequenced by Shanghai Sangon Biotech Co.,Ltd.(Beijing,China).
2.4.Alignment and phylogenetic analysis
The ITS,SSU,LSU,TEF,andTUBgene fragment sequences from the isolates were aligned using the BLAST algorithm at NCBI to confirm their identities. One strain was randomly selected from each village to construct the phylogenetic tree. ClustalW in BioEdit software was used to manually correct the selected sequences to remove large gaps and ensure that the sequence lengths were consistent. The aligned nucleotide sequences at various loci were concatenated using PhyloSuite(v1.2.2) (Zhanget al.2020). Maximum likelihood phylogenies were inferred using IQ-TREE (Nguyenet al.2015). Specifically,the Edge-linked partition model for 10,000 ultrafast bootstraps (Minhet al.2013) and the Shimodaira–Hasegawa-like approximate likelihood–ratio test (Guindonet al.2010) were used. The constructed phylogenetic trees were visualised using iTOL (https://itol.embl.de/) and edited using Adobe Illustrator 2021. The DNA sequences of the 12 representative strains selected in this study have been deposited in GenBank.
2.5.Pathogenicity test
The pathogenicity of the 12E.dactylidisstrains selected for phylogenetic analysis was assessed by inoculating the susceptible maize variety Xiongyu 587 with these strains. Maize seeds were sown in plastic pots (32 cm diameter) containing nutrient-rich soil. The pots were incubated at 20–25°C in a greenhouse. Four seedlings were retained in each pot after emergence. When the maize plants reached the 5–7-leaf stage,their leaves were evenly sprayed with a conidial suspension (1×105conidia mL–1) until they were completely drenched,after which they were covered with transparent plastic bags.Each treatment was repeated thrice. Plants sprayed with distilled water served as negative controls. The inoculated plants were incubated at 20–25°C in a greenhouse and monitored for the emergence of disease symptoms. After symptoms were detected in the infected plants,the fungal isolate associated with the leaf lesions was re-isolated and identified based on morphological features.
2.6.In vitro bioassay of six fungicides against E. dactylidis
A bioassay was conducted using technical grade pyraclostrobin (95%),tebuconazole (97%),azoxystrobin(98%),fludioxonil (98%),iprodione (97%),and carbendazim (98%) as the main materials. Tebuconazole,fludioxonil,and iprodione were each dissolved in dimethyl sulfoxide (DMSO) to prepare 1×104μg mL–1stock solutions,which were serially diluted using DMSO.Under aseptic conditions,300 μL of each solution was added to a conical flask containing 300 mL sterilized PDA at 50°C and shaken well. An equal amount of mixture was poured into 9-cm diameter Petri dishes to make the corresponding concentration of fungicide-containing PDA plates (Table 1). Since the fungicides were dissolved in DMSO,solvent concentrations were adjusted to 0.1%(v/v) in all reactions,and DMSO (0.1%,v/v) alone was used as a control. Pyraclostrobin and azoxystrobin were each dissolved in DMSO to prepare 2×105μg mL–1stock solutions,which were serially diluted using DMSO.Next,100 μg mL–1salicylhydroxamic acid (SHAM,97%;Shanghai Yuanye Bio-Technology Co.,Ltd.,China)was added to media amended with pyraclostrobin and azoxystrobin to inhibit alternative oxidase (Zhanget al.2017;Seyranet al.2010). The concentration of SHAM(100 μg mL–1) had a moderate (<30%) inhibitory effect(Rebollar-Alviter et al.2007) on the mycelial growth ofE.dactylidisbased on our pre-experiment results. Control Petri dishes containing equal amounts of DMSO (0.1%,v/v)and 100 μg mL–1SHAM were prepared on PDA without fungicide. Carbendazim was dissolved in the 0.5 mol L–1hydrochloric acid to prepare 5×103μg mL–1stock solutions,which were serially diluted using sterile pure water to obtain the final concentrations of carbendazim in each media (0.25,0.075,0.05,0.025,0.02,and 0.015 μg mL–1). In the carbendazim test group,medium containing 0.1% (v/v) sterile pure water was used as the control.Each treatment was prepared in triplicate.

Table 1 EC50 values of Eutiarosporella dactylidis isolates to six fungicides
The antifungal activities of the six fungicides againstE.dactylidiswere determined using the mycelial growth rate method. Twelve randomly chosenE.dactylidismonoconidial strains were grown on PDA at 25°C in dark conditions for 3 d. Agar plugs (0.5 cm diameter) from the actively growing colony margin were placed at the centre of the fungicide-containing media. After culturing for 48 h at 25°C in darkness,the diameters of the colony was measured using the cross method (i.e.,two perpendicular directions). The mycelial growth inhibition rate was calculated using the following formula:
Inhibition (%)=(Control colony diameter–0.5)–(Treated colony diameter–0.5)/(Control colony diameter–0.5)×100
This experiment was repeated once under the same conditions. Data analyses were performed using MSExcel 2010 and SPSS version 26.0. The logarithm of the fungicide mass concentration was set as the independent variable (x) and the mycelial growth inhibition rate was set as the dependent variable (y) to obtain the toxicity regression equation. Subsequently,the EC50values of the fungicides were calculated. One-way ANOVA was used to resolve the differences in the susceptibility ofE.dactylidisto different fungicides.
3.Results
3.1.Disease symptoms and field survey
Eutiarosporellaleaf blight symptoms were measured regularly as the infection progressed. During the initial stage of the disease,scattered small fusiform spots on the leaves of the susceptible varieties gradually led to leaf-tip chlorosis and greying (Fig.2-A). In the later stages of the disease,lesions at the leaf tip or edge expanded internally to form irregular strips (Fig.2-B and C). In severe cases,a large necrotic area formed,resulting in cell death in either half of the leaf or the entire leaf. The diseased leaves were dry and fragile,with many black punctate fruiting bodies scattered among dead tissues (Fig.2-D).
The occurrence ofEutiarosporellaleaf blight in Gansu and Ningxia was monitored from 2018 to 2020. The average disease incidence across all regions varied from 20.4 to 91.2%. The incidence ofEutiarosporellaleaf blight in Gansu increased yearly during the study period,with the largest increase observed in the semiarid region of the Loess Plateau (Fig.2-E),followed by that in the semi-humid region of the Loess Plateau and the humid subtropical region. In contrast,the disease incidence was generally low in Ningxia.

Fig. 2 Maize leaf blight symptoms (caused by Eutiarosporella dactylidis). A,B and C,typical symptoms at the leaf tip and edge. D,fruiting bodies on the leaf surface as observed using the Olympus DP73 microscope. Scale bars=200 μm. E,infected maize plants in a field in Anding County,China (i.e.,semi-arid region of the Loess Plateau).
3.2.Pathogenicity test
AllE.dactylidisstrains tested in this study were determined to be pathogenic based on Koch’s postulates.At 6 d post-inoculation (dpi),the plants were relatively healthy but had chlorotic spots at the leaf tip (Fig.3-A).However,at 14 dpi,necrotic lesions containing pycnidia were detected at the leaf tips and edges (Fig.3-B–D).These symptoms were similar to those observed in the field. Disease symptoms and fungal colonisation were not detected in negative controls. The pathogens were re-isolated from the inoculated leaves and identified asE.dactylidisusing morphological analysis. Thus,Koch’s postulates were fulfilled. At 30 dpi,the average diseased plant rate was 100%,whereas the average diseased leaf rate was 38.1–55.6%.
3.3.Morphological characteristics of E. dactylidis isolates
Examination of naturally occurringEutiarosporellaleaf blight sections revealed fruiting bodies (130–240 μm in diameter) in the host tissue (Fig.2-D). Analysis of the sexual morph indicated that the ascomata were papillary,hollow,and round,whereas the peridium,which was relatively broad at the base,comprised a dark brown,thick-walled textura angularis (Fig.4-A). The asci were clavate to cylindro-clavate,fissured,pedicellate,hyaline,apically rounded,bitunicate,with an average size of 59.8 μm×18.9 μm (n=27;range: 51.1–68.3 μm×14.3–22.6 μm) (Fig.4-B). Ascospores were oval or fusiform,smooth,and transparent,with an average size of 5.2 μm×2.7 μm (n=20;range: 3.1–7.1 μm×2.0–3.6 μm).Analysis of the asexual morph revealed that the pycnidia were spherical to oblate and had brown walls. Hyaline and rod-shaped conidiogenous cells formed from the cells lining the inner wall of the conidiomata (Fig.4-C).The conidia were long,oval,slightly pointed at the base,solitary,smooth or rough,mostly colourless,and partly brown,with an average size of 26.6 μm×11.4 μm (n=50;range: 17.8–33.0 μm×9.5–13.4 μm) (Fig.4-D–G).

Fig. 3 Eutiarosporella dactylidis pathogenicity determined using leaves from maize cultivar Xiongyu 587. A,chlorotic spots were detected at the leaf tip at 6 d post-inoculation (dpi). B,C,and D,pycnidia first appeared at the leaf tip and edges at 14 dpi.
After incubating the selected strains for 48 h at 25°C in darkness,the surface of the medium in plates was covered with white and sparse aerial hyphae. Notably,the mycelial growth rate was higher on PDA and PSA than on WA,PCA,OA,MA,MEA,and MLPCA (Fig.5-A).At 7 dpi,the colonies were either grey or white,except for the colonies on WA (Fig.5-B). The colonies on PDA,PSA,PCA,OA,and MA flattened over time and became increasingly dense with white mycelial branches on their surfaces. The morphological changes observed in the colonies grown on MEA were similar but with sparse to moderate hyphae. The aerial hyphae on MLPCA became abundant and formed a tangled mass. After a 7-d incubation period under ultraviolet light at 25°C,the PDA medium contained pycnidia,which were either solitary or in groups,globular,black,and buried or semiburied. The morphological characteristics of the colonies varied among media;however,there were relatively few differences among the tested strains on the same medium.

Fig. 4 Eutiarosporella dactylidis morphological characteristics.A,ascostroma. B,asci. C,pycnidium. D,conidia and mature ascospores,which are indicated by arrows. E,F and G,conidia as observed using the Hitachi S-570 scanning electron microscope. Scale bars=20 μm in A,B,C,and D.
3.4.Phylogenetic analysis
The PCR-amplified products for the ITS,SSU,LSU,TEF,andTUBloci in theE.dactylidisstrains were approximately 540,1,030,910,180,and 420 bp,respectively,which was consistent with the expected sizes of the target fragments. The ITS,SSU,LSU,TEF,andTUBnucleotide sequences of the 12 strains were deposited in NCBI’s GenBank database(accession numbers are listed in Table 2). Sequence data for all five loci were concatenated to create a multi-locus sequence of 3,027 bp in length. Using the genusDarkera(Phacidiaceae) as the outgroup taxa,and other Botryosphaeriaceae members (e.g.,Diplodia,Lasiodiplodia,Neodeightonia,Neofusicoccum,Sphaeropsis,Tiarosporella) as the ingroup taxa (Table 3),a phylogenetic tree was constructed using the maximum likelihood method (Fig.6). The 123 strains were clustered into one branch withE.dactylidis,which was consistent with the morphological identification results.Details regarding one clade consisting of 123 isolates are shown in Fig.7 whileE.dactylidisis presented in Fig.6. The results of phylogenetic analysis supported the differentiation ofE.dactylidisand the following related fungi:T.africana(=E.africana),E.darliae,E.pseudodarliae,E.tritici,E.tritici-australis,andT.urbisrosarum(=E.urbis-rosarum). Among these species,E.dactylidisandE.pseudodarliaeexhibited the closest phylogenetic relationship. Thorough phylogenetic analysis has clarified the taxonomy ofEutiarosporellaand their positioning relative to other genera of the Botryosphaeriaceae family.

Table 2 Origin and GenBank ID of the sequences for 12 Eutiarosporella dactylidis isolates used in this study

Table 3 Reference sequences used in phylogenetic analysis for species placement of Eutiarosporella dactylidis
3.5.In vitro bioassay of six fungicides against E. dactylidis

Fig. 5 Eutiarosporella dactylidis colony morphology. A,average colony growth rates for the E. dactylidis isolates on eight media.Each column is the mean of three repeated experiments,and error bars represent standard errors. Values on the bars followed by the same letters are not significantly different at P=0.05. B,appearance of the E. dactylidis colonies on eight media after a 7-day incubation at 25°C.
Compared with their respective controls,the six fungicides tested in this study showed different inhibition activities againstE.dactylidis. These inhibitory effects increased with increasing fungicide concentration (Fig.8). The EC50values for pyraclostrobin,tebuconazole,fludioxonil,iprodione,and carbendazim were 0.7945±0.1633,0.1158±0.0129,0.0063±0.0013,0.2890±0.0569,and 0.0260±0.0035 μg mL–1,respectively.Moreover,E.dactylidiswas significantly more sensitive to the five fungicides listed,except azoxystrobin(P<0.05). Fludioxonil and carbendazim showed higher inhibitory effects on theE.dactylidishyphae,with EC50values<0.05 μg mL–1,followed by tebuconazole,iprodione,and pyraclostrobin (Table 1).
4.Discussion
Compared to other genera in the Botryosphaeriaceae family,Eutiarosporelladiffers in terms of cultural characteristics and conidial morphology (Jamiet al.2012;Thambugalaet al.2014;Crouset al.2015).However,closely related or recently diverged fungal species may remain morphologically similar (Slipperset al.2009). Therefore,distinguishingEutiarosporellaspecies from related species based solely on colony morphology is challenging. In addition,some asexual morphological characteristics may overlap among certain species,and conidia may undergo morphological changes as they develop,which increases the difficulty of classifying and identifying species (Shoemaker 1964;Phillips 2002;Slippers and Wingfield 2007).For example,Thambugalaet al.(2014) reported thatT.dactylidis(=E.dactylidis) conidia (2.7 μm×1.4 μm) are smooth,whereas Barket (2016) described the conidia((22.9±0.77) μm×(6.7±0.21) μm) as having a rough outer surface. Based on classical taxonomy,the isolates in these earlier studies could not be regarded as belonging to the same species. The conidia ((17.8–33.0) μm×(9.5–13.4) μm) in the current study were smooth to rough.

Fig. 6 Maximum likelihood analysis of Eutiarosporella dactylidis and related taxa conducted using concatenated ITS,SSU,LSU,TEF,and TUB sequences. Darkera was selected as an outgroup. Maximum likelihood bootstrap values (>50) are provided at each node.
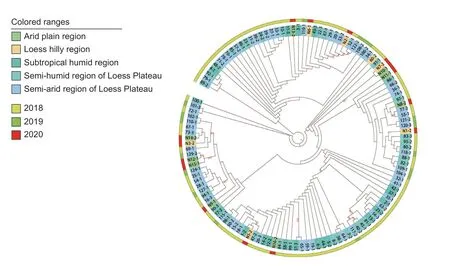
Fig. 7 Circular cladogram representing the maximum likelihood tree inferred from the concatenated ITS,SSU,LSU,TEF,and TUB sequences for the 123 Eutiarosporella dactylidis strains included in this study. The cladogram provides details for one clade presented in Fig.6. Branch lengths are not drawn to scale. Colors correspond to different sampling locations and years,as shown in the legend.

Fig. 8 Inhibition effect of six different fungicides on the mycelial growth of Eutiarosporella dactylidis.
Multi-locus phylogenetic analyses are more effective than single-locus phylogenetic analyses for elucidating interspecific classifications (Qin 2011). Tayloret al.(1999) proposed that relationships among species should be determined by constructing phylogenetic trees using five or more loci. Earlier research on the family Botryosphaeriaceae revealed the power of a combined analysis of multiple loci and morphological traits to differentiate species (Jamiet al.2014;Slipperset al.2004). For example,Thambugalaet al.(2014)combined phylogenetic analyses using ITS,LSU,and EF1-α sequence data and examined morphological characteristics to identify a new species,which was namedT.dactylidis(=E.dactylidis). Furthermore,Thynneet al.(2015) confirmed the presence ofE.tritici-australis,E.darliae,andE.pseudodarliaein infected grains using ITS,EF1-α,and β-tubulin sequences. In this study,phylogenetic analyses of ITS,SSU,LSU,TEF,andTUBgenomic loci were performed to efficiently identify and classify species at the family and genus levels. TheE.dactylidis TUBgene was deposited in GenBank for the first time. Conserved exons and numerous introns makeTUBgenes useful for the phylogenetic investigation of low-order taxa (Baldauf and Doolittle 1997;Edlindet al.1996) as well as complex species (O’Donnellet al.1998).Additionally,these genes have been used to elucidate the phylogenetic relationships among species strains from different regions. Moreover,many studies have used partialTUBgene sequences for phylogenetic analyses of Botryosphaeriaceaespecies (O’Donnellet al.1998;Slipperset al.2004).
In our study,the incidence ofEutiarosporellaleaf blight ranged from 5 to 100% in Gansu,Ningxia,Inner Mongolia,and other regions. Local farmers lacked the ability to distinguish between various foliage diseases in maize and had insufficient knowledge of prevention and control measures. These factors likely contributed to the increasing spread ofE.dactylidisin recent years.Severe cases ofEutiarosporellaleaf blight necessitate the application of fungicides. Among the evaluated fungicides,fludioxonil and carbendazim showed the greatest inhibitory effects on mycelial growth. However,the efficacy of fungicides may differ between laboratory and field trials. Further research should be conducted to evaluate the efficiency of fludioxonil and carbendazim againstEutiarosporellaleaf blightin situ. Fludioxonil is a phenylpyrrole fungicide that is broad-spectrum,highly efficient,and relatively nontoxic (Chenet al.2021). It is widely used in agricultural fields to protect crops from diseases caused by diverse fungal species (Schirraet al.2005). The speculative mechanism of fludioxonil is interference with high osmolarity glycerol (Hog1) cascade of the mitogen-activated protein kinase signalling pathway(Lew 2010;Yunet al,2014),which eventually leads to the death of pathogens. Due to this unique mode of action,fludioxonil shows no cross-resistance with other known fungicides (Heet al.2018),and recent studies have indicated that resistance to fludioxonil is sporadic (Liet al.2023). Therefore,it represents a valuable choice in fungicide rotation programmes inEutiarosporellaleaf blight management. Carbendazim,belonging to the group of benzimidazole fungicides,is first taken up by plants through the roots,seeds,or leaves and then transferred to the whole plant (Gonzálezet al.2000),providing both therapeutic and protective effects. It restricts the growth of mycelia and spore germ tubes by binding tothe β-tubulin protein in fungi (Suet al.2019). This single-site mode of action implies an inherent high risk of resistance development (Brent and Hollomon 1998). Repetitive and extensive applications of carbendazim have led to the emergence and prevalence of carbendazim-resistance in target pathogens,eventually reducing its utility in disease control.
Eutiarosporellaleaf blight is a common disease in Gansu,leading to considerable decreases in the crop yield and quality of maize in this region. Gansu is the largest maize seed-production base in China. Hence,it is imperative that growers and phytopathologists correctly identify the pathogens that causeEutiarosporellaleaf blight. Furthermore,maize growers must protect plants from this disease by alternating and combining fungicides with different mechanisms,improving planting conditions,and screening for resistant varieties to ensure sustainable maize production.
5.Conclusion
Leaf blight in maize is caused byE.dactylidisand may be controlled by fludioxonil and carbendazim. As far as we know,this is the first reliable report ofE.dactylidison maize at home and abroad. The results of this study will provide a basis for the diagnosis and treatment ofEutiarosporellaleaf blight.
Acknowledgements
The research was supported by the Doctor Foundation of Gansu Academy of Agricultural Sciences,China(2020GAAS33),the Young Science and Technology Lifting Engineering Talents in Gansu Province,China (2020-18),and the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences(CAAS-ASTIP-2017-ICS). We would like to thank Editage(www.editage.cn) for English language editing.
Declaration of competing interest
The authors declare that they have no conflict of interest.
 Journal of Integrative Agriculture2024年3期
Journal of Integrative Agriculture2024年3期
- Journal of Integrative Agriculture的其它文章
- Molecular mechanisms of stress resistance in sorghum: lmplications for crop improvement strategies
- Artificial selection of the Green Revolution gene Semidwarf 1 is implicated in upland rice breeding
- Dynamics and genetic regulation of macronutrient concentrations during grain development in maize
- The NAC transcription factor LuNAC61 negatively regulates fiber development in flax (Linum usitatissimum L.)
- The underlying mechanism of variety–water–nitrogen–stubble damage interactions on yield formation in ratoon rice with low stubble height under mechanized harvesting
- Rice canopy temperature is affected by nitrogen fertilizer
