Lateral root elongation in maize is related to auxin synthesis and transportation mediated by N metabolism under a mixed NO3– and NH4+ supply
Peng Wang ,Lan Yang ,Xichao Sun ,Wenjun ShiRui DongYuanhua WuGuohua Mi#
1 Key Laboratory of Tobacco Biology and Processing of Ministry of Agriculture and Rural Affairs, Tobacco Research Institute,Chinese Academy of Agricultural Sciences, Qingdao 266101, China
2 Department of Plant Nutrition, College of Resources and Environmental Sciences, China Agricultural University, Beijing 100193,China
3 College of Resources, Hunan Agricultural University, Changsha 410128, China
4 Agro-Environmental Protection Institute, Ministry of Agriculture and Rural Affairs, Tianjin 300191, China
Abstract A mixed nitrate (NO3–) and ammonium (NH4+) supply can promote root growth in maize (Zea mays),however,the changes in root morphology and the related physiological mechanism under different N forms are still unclear.Here,maize seedlings were grown hydroponically with three N supplied in three different forms (NO3– only,75/25 NO3–/NH4+ and NH4+ only). Compared with sole NO3– or NH4+,the mixed N supply increased the total root length of maize but did not affect the number of axial roots. The main reason was the increased total lateral root length,while the average lateral root (LR) length in each axle was only slightly increased. In addition,the average LR density of 2nd whorl crown root under mixed N was also increased. Compared with sole nitrate,mixed N could improve the N metabolism of roots (such as the N influx rate,nitrate reductase (NR) and glutamine synthase (GS)enzyme activities and total amino content of the roots). Experiments with exogenously added NR and GS inhibitors suggested that the increase in the average LR length under mixed N was related to the process of N assimilation,and whether the NR mediated NO synthesis participates in this process needs further exploration. Meanwhile,an investigation of the changes in root-shoot ratio and carbon (C) concentration showed that C transportation from shoots to roots may not be the key factor in mediating lateral root elongation,and the changes in the sugar concentration in roots further proved this conclusion. Furthermore,the synthesis and transportation of auxin in axial roots may play a key role in lateral root elongation,in which the expression of ZmPIN1B and ZmPIN9 may be involved in this pathway. This study preliminarily clarified the changes in root morphology and explored the possible physiological mechanism under a mixed N supply in maize,which may provide some theoretical basis for the cultivation of crop varieties with high N efficiency.
Keywords: maize,NO3–/NH4+ ratio,lateral root elongation,N assimilation,indole-3-acetic acid
1.Introduction
Currently,simultaneously achieving high-yield and highquality of crops and the efficient-utilization of resources in modern agricultural production is a great challenge. In the past 20 years,nitrogen (N) consumption in China has increased to 19 million tons,but the use efficiency of N fertilizer is still very low,with the partial factor productivity from applied N (PFPN) of the major crop species being only about 50% of that in the developed countries (Zhu and Zhang 2010). Therefore,further improving crop N efficiency is an important scientific problem which needs to be resolved in China (Zhuet al.2010;Jiaoet al.2016).Generally,N efficiency in plants can be decomposed into N uptake efficiency (NUpE) and N utilization efficiency(NUtE) (Miet al.2012,2016). Figuring out how to improve NUpE and NUtE may be an effective means to stimulate the N efficiency of crops under current conditions of highyield and intensive cultivation. Currently,optimizing the tillage model or N fertilization technology can effectively improve the N rate,while breeding new N efficient varieties is another an important approach.
The root is an important organ for the absorption of nutrients and water. Reasonable root architecture is one of important conditions for ensuing the high yield and nutrient absorption efficiency of crops (Miet al.2007,2010;Lynch 2013;Renet al.2022). Usually,the improvement of N uptake efficiency by roots can be carried out in two main aspects,increasing the N influx rate of the unit root surface or maximizing the contact area between root and soil (Miet al.2012,2016),and a strong root architecture is necessary for either of them(Miet al.2012,2016;Renet al.2022). Maize as an important multi-purpose crop of grain,feed,industrial materials and biomass energy,so the yield of maize has an important impact on China’s economy. Previous studies suggested that compared with a sole NO3–supply,a suitable NO3–and NH4+ratio could improve the N uptake of roots and increase maize biomass,thereby improving plant N efficiency (Georgeet al.2016;Wanget al.2019a,b). Several studies have shown that NO3–and NH4+have different effects on the root morphology of maize (Liuet al.2010;Miet al.2012;Guet al.2013;Wanget al.2019a,b),but the changes in root morphology and related physiological mechanism underlying the mixed N supply effect remain unclear.
In the present study,we further studied the changes in root morphology and its related physiological mechanism under a mixed N supply compared to either a sole NO3–or NH4+,which may provide a certain theoretical basis for improving the N uptake efficiency and increasing grain production in maize.
2.Materials and methods
2.1.Experimental procedures
Maize hybrid ZD958 was used in this study. A plastic container (55 cm×45 cm×35 cm) which could contain 90 L of solution was used to grow maize plants hydroponically in a climate chamber (60% relative humidity,average temperature of 28°C/22°C day/night,under 400 μmol m–2s–1light conditions). The seeds were sterilized with 10% (v/v) H2O2for 30 min,transferred to saturated CaSO4solution for 6 h,and then germinated on filter paper in dark conditions until the primary root was 1.5 cm long. The seeds were transferred to culture in rolled paper for 6–7 d until the seedlings had one expanded leaf. After removal of the endosperms from the seedlings,the seedlings were transferred to a container with modified Hoagland nutrient solution containing: 0.5 mmol L–1K2SO4,0.6 mmol L–1MgSO4·7H2O,0.3 mmol L–1KH2PO4,0.5 mmol L–1CaCl2·2H2O,1 μmol L–1H3BO3,0.5 μmol L–1MnSO4·H2O,0.5 μmol L–1ZnSO4·7H2O,0.2 μmol L–1CuSO4·5H2O,0.07 μmol L–1Na2MoO4·2H2O,and 0.1 mmol L–1Na-Fe-EDTA (Guet al.2013). Here,N was supplied at 1 mmol L–1with three different NO3–/NH4+ratios (NO3–only,75/25 NO3–/NH4+,and NH4+only) using KNO3and/or (NH4)2SO4in this experiment,which was based on our previous studies (Wanget al.2019a,b). In the present experiment,MgSO4and K2SO4were added to balance the potassium differences in the solutions. Solution pH was adjusted to 5.8 every 6–12 h. The containers were randomly placed and their positions were changed every three days. The nutrient solution was aerated continuously and renewed every three days.
For the experiments related to inhibitors of N assimilation,we used the same nutrient solution treatments as above,but the difference was that a 10 L gray box was used for the culturing of maize seedlings,and the concentrations of nitrate reductase (NR) inhibitor sodium tungstate (Na2WO4),NO synthase inhibitor(L-NAME),GS inhibitor methionine sulfoximine (MSO) and Gln were 500 μmol L–1(Gorskaet al.2008),100 μmol L–1(Tossiet al.2009),1 μmol L–1(Wanget al.2016) and 1 mmol L–1,respectively (Sivasankaret al.1997).
2.2.Root morphology
Five seedlings from each treatment were sampled at 0,3,6,9,12 and 15 d after transplanting. Axis root length was measured with a ruler (the accuracy was 0.1 cm). The total root length was determined by WinRHIZOTM2003b software (Regent Instruments,QC,Canada) after the root image was obtained with a scanner. Here,we used the following series of formulas to calculate the parameters:
Total lateral root length=Total root length–Total axis root length (Wanget al.2019b)
Average lateral root (LR) length=Total lateral root length/Number of root tips (Smitet al.2000)
Average lateral root (LR) density=Number of root tips/Length of axis root with growing lateral roots (Smitet al.2000)
Root/Shoot ratio=Root weight/Shoot weight (Smitet al.2000)
2.3.15N influx rate
The method for measuring the15N influx rate of maize roots under different forms of N referenced Guet al.(2013). At 12 d,after 5 h of lighting in the morning,seedling roots under different NO3–and NH4+ratios were washed softly with dd-H2O and transferred to 1 mmol L–1CaSO4solution for 1 min,and then transferred to nutrient solution (pH 5.8) with15N-labeled NO3–(10.16 atom%15N) or NH4+(99.14 atom%15N) for 5 min. After rinsing the roots in 1 mmol L–1CaSO4solution for 1 min to exchange the tracer from the apoplast,the roots were then harvested and freeze-dried. A 1.5 mg sample was used for15N analysis by isotope ratio mass spectrometry(DELTAplus XP,Thermo-Finnigan,Germany). There were four biological replicates for each treatment.
2.4.Nitrate reductase (NR),glutamine synthase(GS),invertase activity,AGPase activity,and total free amino acid and Trp concentrations
20 min. The supernatant was used for the determinations of NR,sucrose invertase,and AGPase activities and tryptophan (Trp) concentration with an ELISA kit (Crowther 1995). For the ELISA kit,the corresponding substance antibody was provided by Shanghai Run Yu Biotechnology Co.,Ltd.,China. GS activity was determined with reference to Xue (1985). Four biological replicates were used for each treatment.
2.5.Carbon concentration
At 12 d,milled dry root samples (80 mg) were used for measuring the C concentration using an N/C analyzer(vario MACRO cube,Elementar,Germany). There were five biological replicates for each treatment (Wanget al.2019a).
2.6.Free nitrate and ammonium
For the free nitrate determination,100 mg fresh shoot or root samples were put in centrifuge tubes,1 mL dd-H2O was added,and they were kept in a 95°C water bath for 30 min.After centrifugation at 14,000 r min–1for 15 min at 4°C,the supernatant solution was collected. This experiment used a Walters H-Class UPLC and an Agilent strong anion exchange column (Agilent,USA) and detection used a UV detector at a wavelength of 200 nm. The mobile phase was 50 mmol L–1KH2PO4-H2PO4(pH=3.0).
For free ammonium,100 mg of the ground fresh shoot or root samples were put in centrifuge tube,1 mL precooled 10 mmol L–1formic acid was added,and the tube was centrifuged at 14,000 r min–1for 15 min. A 24 μL aliquot of supernatant was taken and mixed with 400 μL O-phthalaldehyde (OPA) buffer (50 mL,pH=6.8,100 mmol L–1KH2PO4/K2HPO4buffer with 0.201 g OPA and 35.2 μL β-mercaptoethanol). A column-free Agilent 1260 HPLC with a fluorescence detector was used to measure NH4+at an excitation wavelength of 410 nm and a wavelength of 470 nm.
2.7.Sucrose,glucose,fructose and starch
At 12 d,50 mg plant samples were used for measuring the sucrose,glucose,fructose and starch concentrations.The experimental method referenced Arnáizet al.(2012)and Wanget al.(2019a). There were six biological replicates per treatment.
2.8.lndole-3-acetic acid (lAA)
Samples of 100 mg of fresh root were put into centrifuge tubes,900 μL of 0.01 mol L–1PBS buffer (pH=7.3) was added and the mixture was centrifuged at 1,500×g for
For the assay of IAA,roots samples (250 mg) were placed in centrifuge tubes,500 μL N-propanol-ddH2O-HCl(2:1:0.002,v/v/v) was added with mixing and extraction for 30 min at 4°C. Then 1 mL dichloromethane was added for extraction for 30 min at 4°C,and after centrifugation at 5,000 r min–1for 20 min,1 mL of the lower phase was collected. The lower phase sample was concentrated by evaporation and then dissolved in 20 μL 80%methanol. After centrifugation,the sample was passed through a 0.22-μm filter. The chromatography and mass spectrometry conditions referred to previous research methods (Kojimaet al.2009).
2.9.RT-PCR analysis
Maize root was harvested after five hours of lighting at 12 d. The roots samples were excised,snap frozen in liquid N2and stored at–80°C. Total RNA was extracted and cDNA library generation was conducted as described in a previous study (Wanget al.2019a). A 7500 Real-Time PCR System (Applied Biosystems,USA) was used to carry out a two-step PCR procedure. The primers ofZmPIN1AandZmPIN1Breferred to Liet al.(2018),and the primers ofZmPIN9(GRMZM5G859099) referred to Yuet al.(2019) (F:5´-CACCGTCGCCTCGCTCTCCATGCTCC-3´;R:5´-CACCGTCGCCTCGCTCTCCATGCTCC-3´). The maizeZmUbiquitingene was used as an internal control for normalizing the gene expression levels in maize.
2.10.Statistical analysis
Data were subjected to variance analysis using the ANOVA procedure implemented in SPSS Statistics 19.0 (SPSS,Inc.,Chicage,IL,USA). Differences were compared using the least significant difference test at a 0.05 level of probability.
3.Results
3.1.Changes in root morphology under different NO3–/NH4+ratios
Compared with a sole NO3–supply,mixed N increased the total root length. The increase began at 3–6 d(Fig.1-A),and the total root length under mixed N was 1.56–2.39 times that under the sole NO3–supply at 6–15 d.Meanwhile,there was no significant difference in total root length under the sole NO3–and NH4+treatments at 0–15 d. In addition,compared with the sole NO3–supply,total axial root length under mixed N was not changed,but the total lateral root length was significantly increased at 3–15 d (Fig.1-B and C),in which the total lateral root length under mixed N was 1.62–2.79 times that under the sole NO3–supply. These results suggested that the improvement in total root length under a mixed N supply was mainly due to the increase in the total lateral root length.
There was no significant difference in the numbers of seminal roots,1st whorl crown roots or 2nd whorl crown roots under the three N form treatments (Appendix A).Meanwhile,compared with the sole NO3–supply,the average lateral root lengths and average lateral root densities of primary roots,seminal roots,1st whorl crown roots and 2nd whorl crown roots were all increased significantly under mixed N at 15 d. Furthermore,compared with the sole NH4+supply,the average lateral root lengths of primary roots,seminal roots,1st whorl crown roots and 2nd whorl crown roots under mixed N were all increased,while the average lateral root density was only increased in 2nd whorl crown roots at 15 d(Fig.2). These results indicated that compared to a sole NO3–or NH4+supply,the increase in total root length under mixed N may be caused by the further slight increasing of the average lateral root length in each axial root.
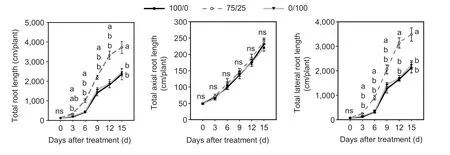
Fig. 1 Effects of a mixed N,sole NO3– or sole NH4+ supply on total lateral root length of maize. A,total root length. B,total axial root length. C,total lateral root length. Treatment notations of 100/0,75/25 and 0/100 represent sole NO3– supply,75/25 NO3–/NH4+ and sole ammonium supply,respectively. Values are mean±SE (n=4). At the same day,significant differences at P<0.05 are shown with different letters;ns,no significant difference.
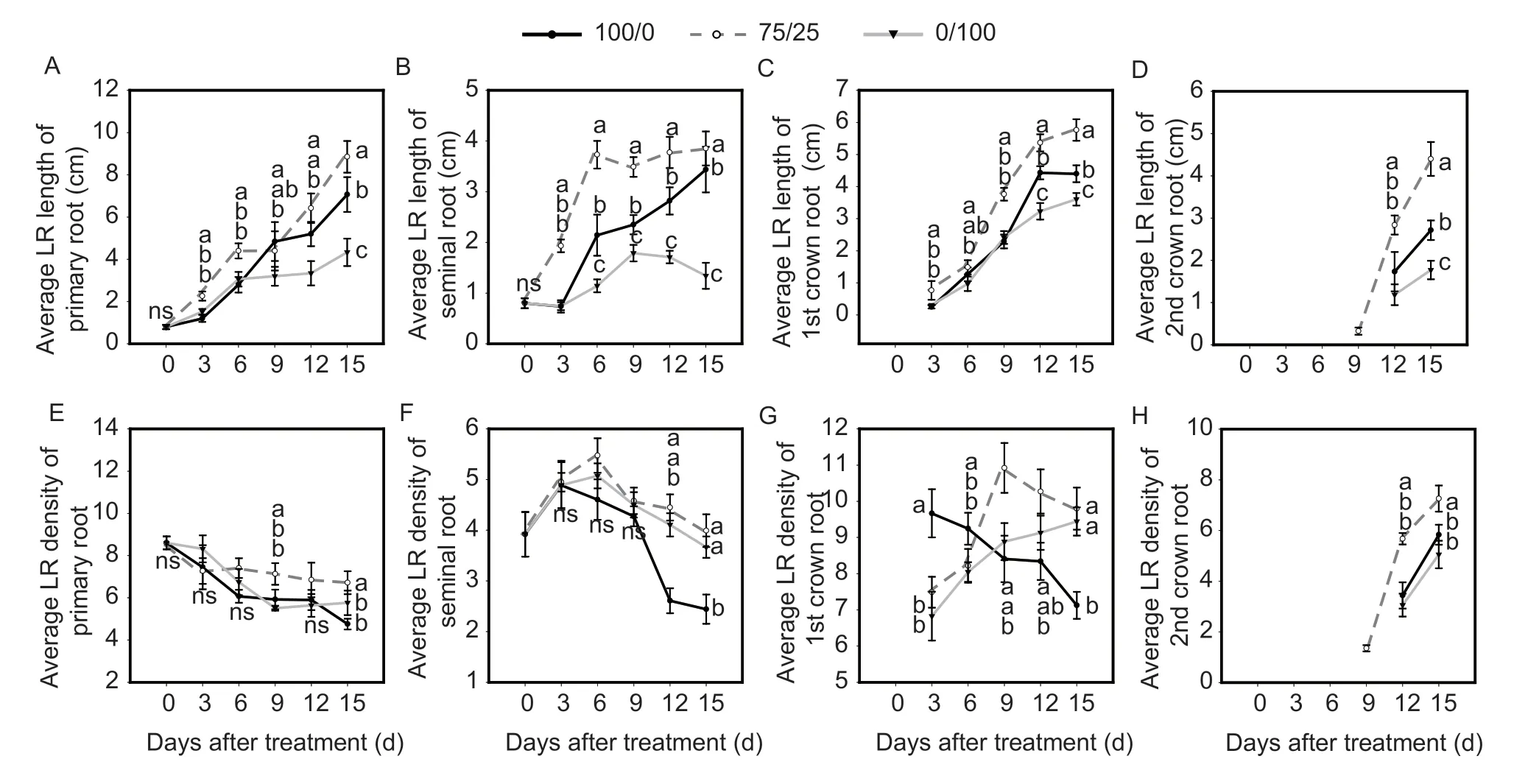
Fig. 2 Effects of a mixed N,sole NO3– or sole NH4+ supply on average lateral root length of maize. A–D,average lateral root (LR)length. E–H,average lateral root (LR) density. Treatment notations of 100/0,75/25 and 0/100 represent sole NO3– supply,75/25 NO3–/NH4+ and sole ammonium supply,respectively. Values are mean±SE (n=4). At the same plant day,significant differences at P<0.05 are shown with different letters;ns,no significant difference.
3.2.Relationship between average LR length and N uptake or assimilation ability of roots under different NO3–/NH4+ ratios
To demonstrate whether the changes in root morphology under a mixed N supply were influenced by the changes of N uptake and assimilation processes,the15N influx rate and the activities of two N-assimilation enzymes(NR and GS) of roots under the different treatments were measured. Compared with the sole NO3–supply,mixed N significantly improved the total N influx rate (Fig.3-A).Meanwhile,the NR activity of roots under mixed N showed the highest value among the three treatments,while the GS activity of roots increased significantly with the increasing proportion of NH4+(Fig.3-B and C).Furthermore,the total amino acid concentration in roots under mixed N and sole NH4+were significantly increased compared to the sole NO3–supply (Fig.3-D).
To further elucidate whether N assimilation and metabolic processes were involved in the regulation of lateral root elongation,we invested the changes in root morphology under the different N form treatments combined with N-assimilation inhibitors (Fig.3-E). In the control (CK) treatment,compared with the sole NO3–supply,the average LR lengths of primary roots,seminal roots and 1st whorl crown roots under a mixed N supply were significantly increased,respectively,by 15,33 and 25%. However,after the addition of Na2WO4(an NR enzyme inhibitor),the average lateral root length in primary roots,seminal roots and 1st whorl crown roots under the different N forms all decreased,while there was no significant difference among the three N form treatments (Fig.3-F–H). Aside from the NO3–assimilation process,the NR pathway is another main pathway for NO synthesis. In order to further investigate whether NO synthesis in the NR pathway could regulate lateral root elongation under a mixed N supply,aside from the changes in root morphology combined with the NR inhibitor,we also explored the changes of root morphology under different N forms combined with an NOS synthesis inhibitor (L-NAME),which is another main pathway for NO synthesis. The average lateral root length in each axial root under mixed N was higher than other two treatments(Fig.3-F and H).

Fig. 3 Effects of N supply form and N assimilation inhibitors on the assimilation of NO3– and NH4+. A,15N influx rate. B,nitrate reductase (NR) activity. C,glutamine synthase (GS) activity. D,total amino acid concentration of roots under different N forms at 12 d. E,a regulatory model diagram of NO3– and NH4+ assimilation and related enzyme inhibitors. Treatment notations of 100/0,75/25 and 0/100 represent sole NO3– supply,75/25 NO3–/NH4+ and sole ammonium supply,respectively. Sodium tungstate(Na2WO4),L-NAME and methionine sulfoximine (MSX) are respectively nitrate reductase,NO synthase (NOS) and glutamine synthetase inhibitors. The solid arrows represent an increasing effect,while the red dotted lines represent inhibiting effects. F–H,average lateral root (LR) length of primary roots,seminal roots and 1st whorl crown roots,respectively,under the different N forms combined with NR,GS,or NOS inhibitors or Gln at 12 d. Values are mean±SE (A–D: n=4;H–G: n=10). Significant differences at P<0.05 are shown with different letters.
Furthermore,after adding a GS enzyme inhibitor(methionine sulfoximine),the average lateral root lengths of primary roots,seminal roots and 1st whorl crown roots were all inhibited under each N form treatment. Among them,the average lateral root length under mixed N and sole NH4+were significantly lower than that under the sole NO3–treatment (Fig.3-F and H). After the addition of Gln,the average lateral root lengths of primary roots,seminal roots and 1st whorl crown roots were not significantly different from that under the mixed N or sole NO3–treatment,but both were significantly higher than under the sole NH4+treatment. These results suggest that N assimilation mediated by NR and GS may be involved in the regulation of lateral root elongation.
In addition,we measured the concentrations of free NO3–and NH4+in roots under the conditions of different N forms combined with the N-assimilation inhibitors.The results showed that the concentration of free NO3–in roots was significantly reduced with an increase in the NH4+amount under each inhibitor treatment,while the concentration of free NH4+in roots was significantly increased with an increase in the NH4+under each inhibitor treatment (Fig.4-A and B). The concentration of NH4+in roots was increased when added GS inhibitor under three N form supply,especially under mixed N and sole NH4+treatments (Fig.4-B). Furthermore,exogenously added Gln also resulted in an increased NH4+concentration in roots,especially under the NH4+treatment. These results illustrated the importance of NH4+conversion into amino acids in regulating the average LR length of axial roots under different N form treatments;and among them,an appropriate dynamic balance of the NH4+concentration in roots may be the main factor which mediates lateral root elongation (Fig.4-B).
3.3.Translocation of C from shoot to root may not be the main factor that determines lateral root elongation
The next step was to further explore whether the increased average LR length of maize under a mixed N treatment was caused by C metabolism. Generally,the root/shoot ratio represents the distribution ratio of C in the shoot and the root (Wanget al.2019b). In the present study,there was no significant difference in root/shoot ratios between the mixed N and the sole NO3–treatment,while both were lower than under the sole NH4+supply,regardless of whether they were combined with N assimilation inhibitors(Fig.4-C). A similar trend was also found in the change of the C concentration in roots (Fig.4-D). There were no significant differences in C concentrations of roots under a mixed N or sole NO3–supply,but both were lower than that under the sole NH4+supply,regardless of the inhibitor treatments. These results preliminarily indicated that the changes in N assimilation in roots did not cause the change of C transportation from shoots to roots under the treatments with different N forms.

Fig. 4 Distribution ratios of C from shoot to root and concentrations of nitrate,ammonium and C in roots. A and B,free NO3– and NH4+ concentrations of roots. C,root/shoot ratio. D,C concentration of roots in maize under different N forms combined with nitrate reductase (NR),glutamine synthase (GS) or NO synthase (NOS) inhibitors or Gln treatments at 12 d. Sodium tungstate (Na2WO4),L-NAME and methionine sulfoximine (MSX) are respectively nitrate reductase,NOS and glutamine synthetase inhibitors. Treatment notations of 100/0,75/25 and 0/100 represent sole NO3– supply,75/25 NO3–/NH4+ and sole ammonium supply,respectively. Values are mean±SE (A and B: n=4;C and D: n=5). Significant differences at P<0.05 are shown with different letters.
Glucose,fructose,sucrose,starch and related key enzyme activities in roots were also measured in this study. The changes in each sugar concentration in roots were consistent with the change in the C concentration.Among them,the concentrations of glucose,fructose,sucrose and starch in roots had no differences under the mixed N or sole NO3–supply,but both were significantly lower than under the sole NH4+treatment (Fig.5-A–D). In addition,changes in the activities of the pyrophosphorylase enzyme and acid phosphatase,which are involved in starch synthesis and sucrose decomposition,respectively,were consistent with the trend of sugar concentrations in roots under the different N forms (Fig.5-E and F). These results further suggested that the increase in lateral root length under mixed N may not be directly regulated by the transportation of C from shoots to roots.
3.4.Auxin synthesis and transportation regulate lateral root elongation of maize under a mixed N supply
To further prove whether the lateral root elongation under mixed N was related to auxin synthesis and transportation,we used 1st whorl crown root as the material in the experiment. The tryptophan concentrations in the root tip (<5 mm;Fig.6-A) and the growing lateral root tips (<10 mm;Fig.6-B) under the mixed N supply were significantly increased compared with those under sole NO3–and NH4+treatments. Furthermore,although the tryptophan concentration of the lateral root tips under mixed N was significantly increased compared with the sole NO3–supply,there was no significant difference between those under mixed N and the sole NH4+supply (Fig.6-B). Furthermore,we measured the auxin concentrations in the root tip (part I,root tip length<5 mm),the axial root of the lateral root initiation zone(part II,where no lateral root could be seen),the axial roots corresponding to the growing lateral roots (part III,lateral root length <10 mm) and the growing lateral roots (part IV;lateral root length <10 mm) (Fig.7-A).Compared with the sole NO3–or NH4+treatments,mixed N significantly increased the auxin concentrations in parts I and IV (Fig.7-B and E). At the same time,although the auxin concentrations in parts II and III were increased significantly under mixed N compared to the sole NO3–treatment,there was no significant difference between those under the mixed N and sole NH4+supplies (Fig.7-C and D). These results indicated that the promotion of auxin synthesis and transportation in root tips under mixed N may be the main driver for the regulation of lateral root elongation.
A series of auxin transporters have been reported to regulate the changes in root morphology of maize (Yuet al.2016,2019;Liet al.2018). In this study,there was no significant difference in the expression ofZmPIN1Ain parts I,II and IV between the mixed nitrogen and sole NO3–supplies (Fig.8-A). Compared with sole NO3–,the expression ofZmPIN1Bin part II was increased significantly under the mixed N supply. Meanwhile,in parts III and IV,the expression ofZmPIN1Bwas not upregulated under mixed N (Fig.8-B). In addition,theexpression ofZmPIN9increased in parts II and IV under mixed N.
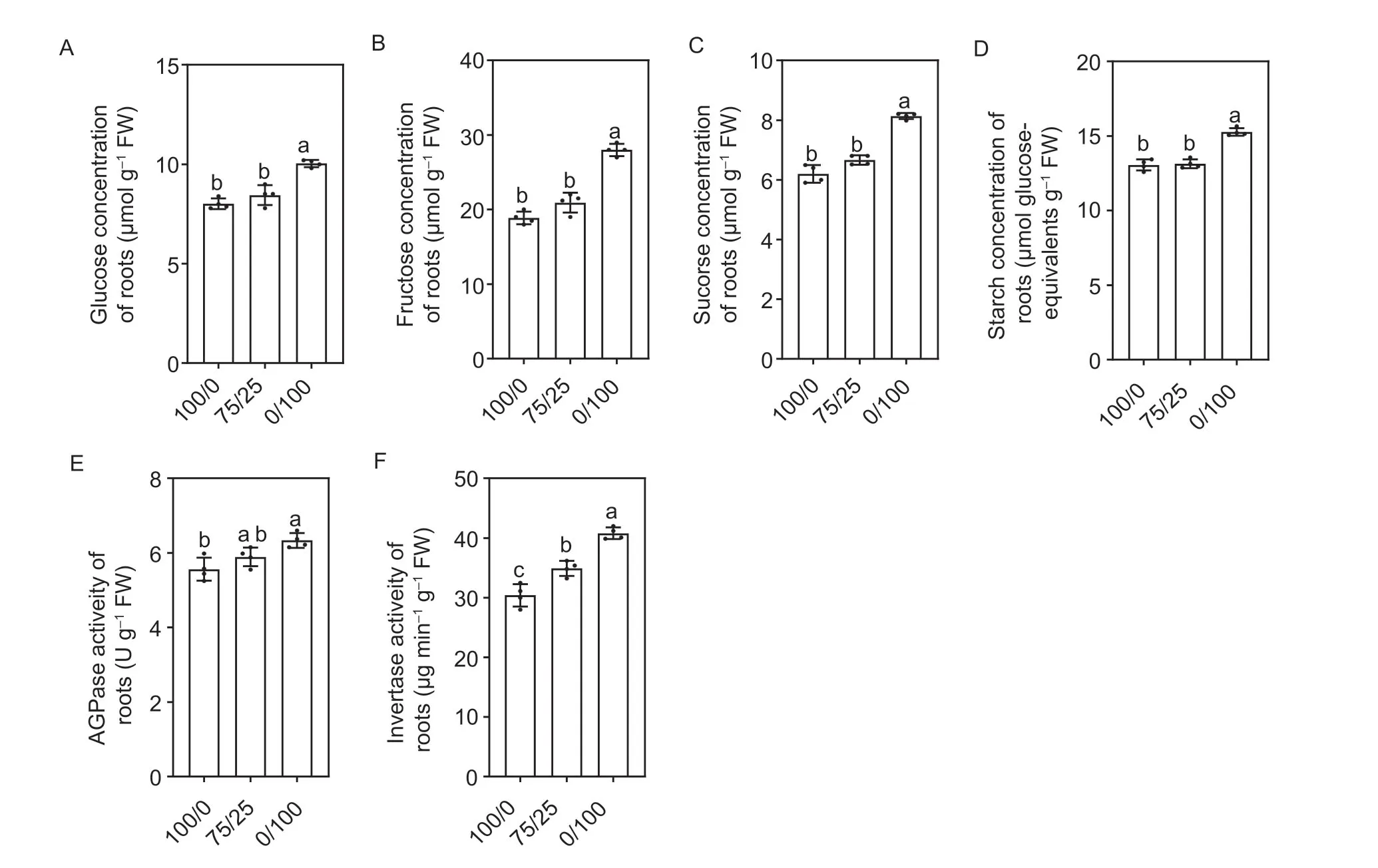
Fig. 5 Changes in sugar contents and relate enzyme activities in roots under different N forms at 12 d. A,glucose.B,fructose. C,sucrose. D,starch. E,AGPase activity. F,invertase activity. Treatment notations of 100/0,75/25 and 0/100 represent sole NO3– supply,75/25 NO3–/NH4+ and sole ammonium supply,respectively. Values are mean±SE(A–F: n=4). Significant differences at P<0.05 are shown with different letters.
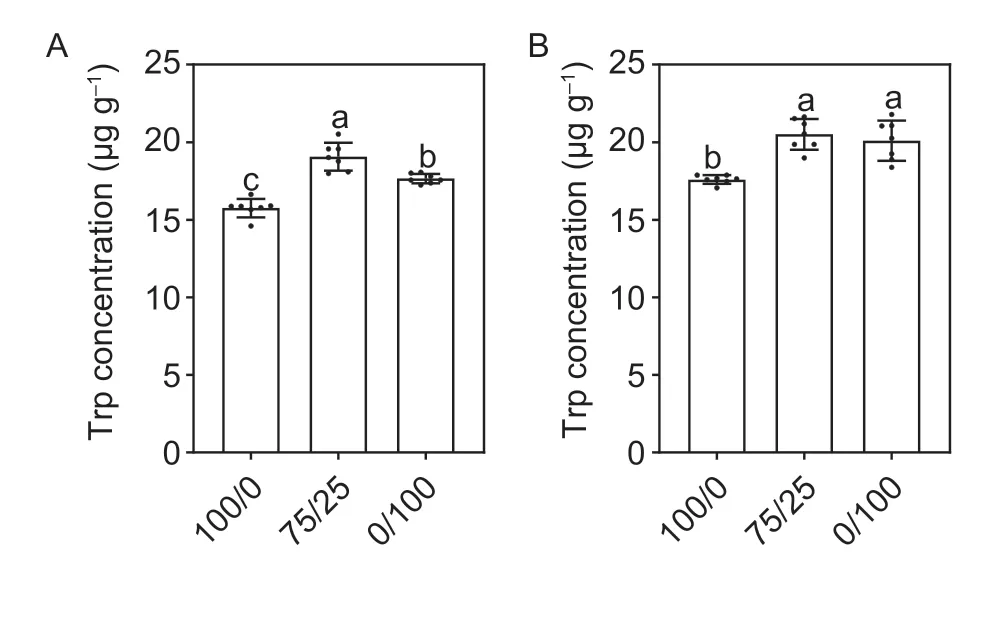
Fig. 6 Trp concentration of the root tips of axial roots (A) and lateral roots (B) in 1st whorl crown root. Treatment notations of 100/0,75/25 and 0/100 represent sole NO3– supply,75/25 NO3–/NH4+ and sole ammonium supply,respectively. Values are mean±SE (n=7). Significant differences at P<0.05 are shown with different letters.
4.Discussion
Reasonable root architecture is critical for water acquisition and nutrient uptake (Renet al.2022). Lateral roots are the key part of root architecture which begins from the pericycle cells. Studies have shown that the development of lateral roots is important for improving the total root surface area and N efficiency (Yuet al.2019). Early studies suggested that localized NO3–signaling stimulates lateral root development,especially lateral root length,in a variety of plants,such as maize,barley,rice,and Arabidopsis (Liuet al.2010;Xuet al.2012;Fordeet al.2014;Yuet al.2014;Steffens and Rasmussen 2016).Under the condition of a homogeneous NO3–supply,both nitrogen deficiency (<1 mmol L–1) and excess NO3–supply(>10 mmol L–1) can inhibit the elongation of lateral roots in maize (Zhanget al.1999,2007). Meanwhile,using NH4+as the only N source can often inhibit the elongation of the primary and lateral roots,but a local NH4+supply can stimulate the branches of lateral roots (Liet al.2010;Limaet al.2010;Arayaet al.2016). In the present study,under the condition of a homogeneous N supply,the average LR length of each axial root under a sole NO3–supply were all increased compared with sole NH4+(Fig.2),and these results were also consistent with another study in maize(Schortemeyeret al.1993). However,the average lateral root length under mixed N was further increased,which indicated that appropriate NH4+may interact with NO3–to stimulate lateral root elongation (Fig.2). Meanwhile,compared with the sole NO3–supply,the average LR densities of each axial root under the NH4+supply were promoted,and similar results have also been reported in rice (Wanget al.2021) andArabidopsis thaliana(Limaet al.2010),although there was no significant difference in average LR density under mixed N or sole NH4+treatments in this study,except for in 2nd crown roots.These results indicated that the increase in the average LR density in each axial root under mixed N compared to a sole NO3–supply may be due to the effect of NH4+.

Fig. 7 Effects of mixed N supply in auxin concentrations of root tips in the axial and lateral roots. A,schematic diagram of a root tip for sampling. I is the root tip (≤5 mm);II is the axis root part which has no lateral root emergence;III is the axis root part with lateral root lengths less than 1 cm;IV is the lateral root part with lateral root lengths less than 1 cm. B–E,the indole-3-acetic acid(IAA) concentrations of I,II,III and IV parts of roots grown in the different N forms. Treatment notations of 100/0,75/25 and 0/100 represent sole NO3– supply,75/25 NO3–/NH4+ and sole NH4+ supply,respectively. Values are mean±SE (B–E: n=4). Significant differences at P<0.05 are shown with different letters.
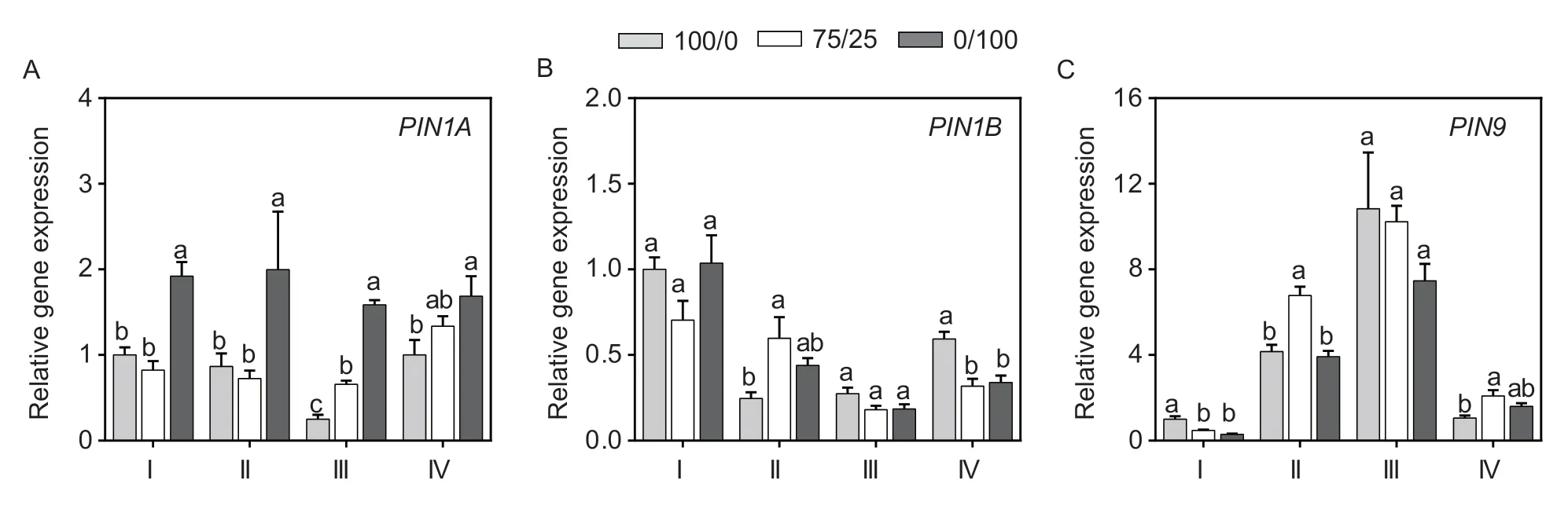
Fig. 8 Gene expression of ZmPIN1A,ZmPIN1B and ZmPIN9 in the roots supplied with different N forms. I is the root tip (≤5 mm);II is the axis root part which has no lateral root emergence;III is the axis root part with lateral root lengths less than 1 cm;IV is the lateral root part with lateral root lengths less than 1 cm. Treatment notations of 100/0,75/25 and 0/100 represent sole NO3– supply,75/25 NO3–/NH4+ and sole ammonium supply,respectively. Values are mean±SE (n=3). Significant differences at P<0.05 are shown with different letters. The statistical analyses of the I,II,III,and IV parts were independent.
AtNRT1.1andAtAMT1.3have been shown to play key roles in the regulation of lateral root development by“local” N signaling inArabidopsis(Hoet al.2009;Limaet al.2010). Furthermore,several studies have illustrated that some key NO3–or NH4+transporters may also attend the “system” for N signaling on root development in plants (Jia and von Wirén 2020;Jiaet al.2022). In the present study,compared to a sole NO3–supply,maize had a higher N uptake rate under sole NH4+,while mixed N further increased N uptake (Fig.3-A). Among them,the increased uptake of both NO3–and NH4+may be the main reason for the increased uptake of N under mixed N,especially the latter. Our previous study showed that low affinity transporters in NO3–transportation (such asZmNRT1.1A-ZmNRT1.1C,ZmNRT1.2andZmNRT1.3)and NH4+transporters (such asZmAMT1.1A) are important for NO3–and NH4+uptake under a mixed N supply (Wanget al.2023). However,whether these genes are involved in the lateral root elongation mediated by a mixed N supply needs further investigation.
In this study,the NR activity of roots under a mixed N supply showed the highest value among the three N forms,and this result was consistent with earlier studies in maize (Oakset al.1982). Studies have shown that a mixed N supply could reduce the protein content of nitrate reductase (NRP) but increase the nitrate reductase activity (NRA) compared to those under a sole nitrate supply (Oakset al.1982). Therefore,we speculated that the change in the NR enzyme activity in roots may be involved in regulating the changes in root morphology.Meanwhile,the average LR lengths of primary roots,seminal roots and 1st whorl crown roots were all inhibited and had no significant differences in the three N forms after the addition of the NR inhibitor (Fig.3-G and H),and these results further showed that NR-mediated NO3–assimilation may drive the process of lateral root development. In addition,exogenously added GS inhibitors and Gln also affected lateral root elongation under the different N supplies (Fig.3-G and H). We found that inhibition of the GS enzyme activity could lead to high NH4+ion accumulation in roots,and the average LR length could also be further inhibited with an increase in the NH4+concentration (Fig.4-B). Therefore,there may be a dynamic equilibrium of endogenous NH4+under the mixed N supply,which not only ensures that the root has a higher N assimilation rate,but also ensures that the root does not produce too much NH4+ion which would inhibit root elongation under mixed N.
In recent years,NR-mediated NO production had been shown to play a critical role in regulating the initiation and elongation of lateral roots inArabidopsisand tomato(Kolbertet al.2010;Caoet al.2017). Generally,NO synthesis in plants mainly occurs through two pathways,the NR and NOS pathways (Kolbert and Erdei 2008). In the present study,after exogenous addition of the NOS inhibitor,the lengths of the average lateral roots of each axial root under mixed N were still higher than under the sole NO3–supply (Fig.3-G and H). Therefore,we speculate that NOS-mediated NO synthesis may not be the main pathway for the regulation of lateral root synthesis under a mixed N supply. However,whether there is a compensating effect in the production of NO in the NR pathway or NO production directly from the NR pathway to regulate the elongation of lateral roots needs further exploration.
N absorption and assimilation in plants has the power to increase “sink-strength” (Tegeder and Masclaux-Daubresse 2018),and the process is closely related to N–C metabolism. Previous studies have shown that the enhancement of root growth under a mixed N supply is related to the high ratio of C transported from shoot to root,so the high C utilization by the plants was necessary(Wanget al.2019a,b). Further experiments were conducted in order to prove whether N absorption and assimilation in the plant could affect the changes in root morphology by affecting C transportation and unitization under a mixed N supply. The results of the root/shoot ratio,root C concentration and root sugar concentrations in the present study suggested that the changes in the N assimilation process did not affect the changes in the C trends under the different N forms (Figs.4-C and D and 5). This result further suggests that lateral root elongation under a mixed N supply may be not regulated by total C transportation and utilization.
Plant hormones play an important role in regulating the development of lateral roots in maize,such as abscisic acid (ABA) (Maet al.2018),Cytokinin (Rivaset al.2022) and Auxin (Alarcónet al.2019). In early research,the auxin concentration of maize roots was specifically promoted under mixed N compared to a sole NO3–or NH4+supply (Wanget al.2019a). This is because N metabolism under the mixed N supply caused part of the carbon to flow to the shikimic acid pathway and eventually form tryptophan and auxin (Wanget al.2019a). In present study,the tryptophan and auxin concentrations in root tips of 1st whorl crown root were specifically enhanced under mixed N compared to the sole NO3–supply (Figs.6 and 7-A). In addition,the Trp concentration under mixed N had no difference from that under the sole NO3–supply in the tips of lateral roots,but the IAA concentration was significantly higher than that under the sole NO3–supply (Figs.6 and 7-D). Therefore,we speculate that lateral root elongation under mixed N may be related to IAA synthesis in the root tip of the axis root. The auxin in lateral roots may also from shoots,which may also be a possible reason. In addition,we found no significant difference in auxin concentrations between the mixed N and sole NH4+supplies at part II or part III of the root,but it was significantly higher than that under the sole NO3–supply (Fig.7). These results may explain why lateral root density under mixed N and sole NH4+supplies were higher than the sole NO3–supply.
Auxin polar transporters are essential for the initiation and development of lateral roots,such asPIN1andPIN2inArabidopsis(Tanakaet al.2006).ZmPIN1AandZmPIN1Bhave different roles in maize root development;withZmPIN1Aincreasing the number of lateral roots and inhibiting their elongation,thus forming a developed root system,whileZmPIN1Bshows increased root biomass by promoting the growth of both lateral and seminal roots(Liet al.2018). Another study showed that the monocotspecificZmPIN9gene in phloem pole cells of shoot-borne roots at silking modulates auxin efflux to the pericycle cells and subsequent cell cycle activation by alleviating the inhibition of Kip-related protein coding genes in maize (Yuet al.2016). In the present study,compared to the sole NO3–or NH4+supplies,mixed N significantly increased the expression ofZmPIN1BandZmPIN9in part II. Furthermore,a mixed N supply significantly increasedZmPIN9expression in lateral root tips (part IV).According to the roles ofZmPIN1BandZmPIN9in regulating root morphology (Yuet al.2016;Liet al.2018),we speculated thatZmPIN1BandZmPIN9may participate in the initiation of lateral roots,but whetherZmPIN9is involved in the elongation of lateral roots needs further exploration.
5.Conclusion
The average LR length of each axial root under a mixed N supply was moderately increased,and this process may relate to an increase in the total N absorption ability,thus further promoting N assimilation in the roots. Among them,NR-and GS-mediated N assimilation under a mixed N supply was critical. In addition,the endogenous NH4+concentration may be one of the main factors regulating lateral root elongation,while excessive ammonium ion will significantly inhibit lateral root elongation. Furthermore,the enhancement of N metabolism under a mixed N supply may not be the main factor mediating lateral root elongation through C transportation from shoots to roots. The enhanced N metabolism under a mixed N supply may affect auxin synthesis and transportation to axial root tips,and among the factors,ZmPIN9andZmPIN1Bmay be involved in this process. The present study elucidated the changes in root morphology and possible physiological mechanism under a mixed N supply,which may improve the theoretical basis for cultivating maize varieties with high N efficiency.
Acknowledgements
The study was supported by the National Natural Science Foundation of China (31421092) and the Central Publicinterest Scientific Institution Basal Research Fund,China(1610232023023).
Declaration of competing interests
The authors declare that they have no conflict of interest.
Appendixassociated with this paper is available on https://doi.org/10.1016/j.jia.2023.07.012
 Journal of Integrative Agriculture2024年3期
Journal of Integrative Agriculture2024年3期
- Journal of Integrative Agriculture的其它文章
- Molecular mechanisms of stress resistance in sorghum: lmplications for crop improvement strategies
- Artificial selection of the Green Revolution gene Semidwarf 1 is implicated in upland rice breeding
- Dynamics and genetic regulation of macronutrient concentrations during grain development in maize
- The NAC transcription factor LuNAC61 negatively regulates fiber development in flax (Linum usitatissimum L.)
- The underlying mechanism of variety–water–nitrogen–stubble damage interactions on yield formation in ratoon rice with low stubble height under mechanized harvesting
- Rice canopy temperature is affected by nitrogen fertilizer
