Mussel-inspired PTW@PDA composites for developing high-energy gun propellants with reduced erosion and enhanced mechanical strength
Xijin Wng , Zhito Liu ,*, Pengfei Sun , Feiyun Chen , Bin Xu , Xin Lio ,**
a School of Chemistry and Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, Jiangsu, China
b China North Industries Group Corporation Limited, Beijing 100045, China
Keywords:High energy gun propellant Potassium titanate whiskers Polydopamine modification Erosion inhibitors Mechanical reinforcing fillers
ABSTRACT The severe erosion and inadequate mechanical strength are prominent challenges for high-energy gun propellants.To address it,novel PTW@PDA composites was prepared by polydopamine(PDA)-modifying onto potassium titanate whisker(PTW, K2Ti6O13), and after was incorporated into gun propellant as erosion-reducing and mechanical-reinforcing fillers.The interfacial characterizations results indicated that as-prepared PTW@PDA composites exhibits an enhanced surface compatible with propellant matrix,thereby facilitating their dispersion into propellants more effectively than raw PTW materials.Compared to original propellants, PTW@PDA-modified propellants exhibited significant less erosion, with a Ti-Kbased protective coating being detected on the eroded steel.And 0.5 wt% and 1.0 wt% addition of PTW@PDA significantly improved impact, compressive and tensile strength of propellants.Despite the inevitably reduction in relative force,PTW@PDA slightly increase propellant burning rate while exerting little adverse impact on propellant dynamic activity.This strategy can provide a promising alternative to develop high-energy gun propellant with less erosion and more mechanical strength.
1.Introduction
High-energy gun propellants containing balanced amounts of fuel and oxidants are demanded for modern high-chamber pressure gun weapons with faster muzzle velocity and longer range[1-4].Compared with conventional single-based and double-based propellants, high-energy propellants incorporate high levels of powerful energetic materials, such as nitroglycerin (NG) and cyclotrimethylenetrinitramine(RDX),which can result in increased force constant for gun propellants [1,3,4].However, the significant challenges facing high-energy gun propellants are erosion and mechanical weakness, which arises from their extremely high flame temperature and rapid pressure rise [1,3-6].The serious erosion of gun propellant lead to the enlargement of the origin of rifling or the down bore area, which can deteriorate the ammunition performance, resulting in reduced range and accuracy, fuze malfunctions, excessive muzzle flash, excessive torsional impulse and blast overpressure,resulting in a shortened lifespan of the gun barrel [2,7-9].With the increasing demand for guns with faster muzzle velocity, longer range, and greater accuracy, barrel erosion has rapidly deteriorated and become a major limitation to the development of modern gun weapons [2,7-9].On the other hand,because of the increased chamber pressure resulting from enhanced force constant,powerful propellant grains are subjected to rapid pressure rise than general propellants [5,10-12].This situation can cause to deformation, cracking and disruption of the grains, which results in pressure course p(t) deviating from the geometric burning model [13].In extreme case, the disruption of grains can strongly accelerate the pressure rise, which can cause damage to the launching system [13].Therefore, it is very important to control the erosion and mechanical properties of highenergy gun propellant.
To reduce erosion of propellants, adding wear-reducing additives in propellant charges is efficiency and economical technique,which has been widely applied in a variety of gun weapons over the last few decades [14-19].The involved materials with reducing erosion properties have included organic materials such as wax and silicone, and inorganic materials such as talc, titanium dioxide(-TiO2), potassium sulphate (K2SO4), calcium carbonate(CaCO3)[14,15,18,19].Some previous studies have proven that these inorganic materials had excellent anti-erosion ability on general propellants, including single-based, double-based, triple-based propellants [18-21].However, high-energy RDX propellants will generate higher flame temperature and erosion, which resulted in conventional inorganic materials hardly meeting to the requirement for effectively reducing erosion caused by high-energy propellants.It seems that the flame temperature of propellants has an impact on the effectiveness of inhibitors in preventing erosion.To mitigate the erosion of high-energy RDX propellants, some recent researches have focused on synthesizing multi-component composite erosion inhibitors [15-17,22-24].And there will be an ongoing need to develop more efficiency inhibitors, which can be prepared in an economical and scalable approach.
As compared to general single-based and double-based propellants, the increasing content of RDX and NG in composition of high-energy propellants indicates a reduction in binder content,that may potentially have a detrimental impact on mechanical strength of propellant.To solve this problem, strategies of adding fillers [12,25,26] and fillers surface modification [11,12,27] have been attempted to increase mechanical strength of gun propellant,which are usually investigated by impact testing, tensile and compression testing in the range of quasi-static loading conditions[11-13],as well as at the dynamic loading range in a higher strain rates conditions [13].
In comparison to various inorganic fillers, such as glass fibers,carbon fibers, inorganic ceramic whiskers are short fiber-shaped single crystals with very large length-to-diameter ratios and high perfection almost free of defects[28].Among the available ceramic whiskers, potassium titanate whiskers (PTW, K2Ti6O13) has attracted widely interests and been found useful as reinforcing materials for polymer composites[28,29],wear resistant composites[30,31],heat-insulating paints [32,33] and photocatalysts [34].PTW displays distinct properties of lubricity,wear resistance,chemical and thermal stabilities,small size,high ratio of length-to-diameter,high strength and modulus, low hardness(the Mohs hardness is 4),and economical price[28,30,35].Meanwhile,PTW exhibits an inorganic chemical composition containing Ti and K elements that are similar to those found in conventional erosion inhibitors such as TiO2and K2SO4.All unique properties of PTW are contributing to its potential application in the field of propellants, resulted in reinforced mechanical strength and decreased erosion for propellant, as well as providing lubricity and wear resistance for gun barrels.In this work, we attempted to incorporate PTW in propellants matrix using a conventional solvent-based propellants manufacturing approach [2,12,17].However, further investigation was limited by aggregation and poor dispersion of PTW in propellant matrix, due to the poor interfacial interaction between PTW and polymer[35,36].Based on the considerations,a necessary approach that can be used to modify surface properties of PTW and improve interfacial compatibility between PTW and propellant binder, should be studied.
Fortunately,inspired by excellent adhering functional of mussel adhesive proteins to different substrates, polydopamine(PDA) is employed as a powerful bonding agent that can readily attach on various inorganic and organic substrates by self-polymerization reaction without any complicated reaction conditions [36-41].In the last decade, it has been widely reported that PDA can improve interfacial interaction and promote dispersion of micron or nanosize fillers in polymer matrix, resulting in an outstanding improvement of the mechanical properties of polymer matrix[42,43].Besides, due to the numerous functional groups of PDA layer,including primary,secondary amino groups,hydroxyl groups,carboxylic acid and catechol groups,PDA can present an amounts of active sites on the substrates coated to form hydrogen or covalent bonds which characteristics provide a promising strategy for synthesizing novel scalable core@double shell structure composites[38,41,42].Considering all above characteristics of PDA, it can be a suitable candidate for modifying surface properties of PTW and help us further manufacturing well-dispersive PTW@PDA gun propellants with reduced erosion and enhanced mechanical strength.
In this work,to overcome the erosion and mechanical weakness associated with high-energy propellants, we adopted a novel bioinspired coating strategy to synthesize an organic-inorganic PTW@PDA composites by the PDA self-polymerization onto PTW.After that,raw PTW and modified PTW@PDA with various content were dispersed into propellant to prepare a series of modified highenergy propellant samples, which were manufactured using conventional solvent-based propellant processing [2,12,17].Based on comprehensive interfacial characterizations including SEM-EDX,FT-IR, Raman, XPS and contact angles, PTW@PDA composites exhibited an enhanced surface compatible with propellant matrix,thereby facilitating their dispersion into propellants more effectively than raw PTW materials.Besides, the PTW@PDA-modified propellants exhibited obviously reduced erosion, with a Ti-Kbased protective layer being detected on the eroded steel.And the addition of PTW@PDA at 0.5 wt% and 1.0 wt% significantly enhanced the impact, compressive, and tensile strength of propellants.Despite the inevitably reduction in relative force,PTW@PDA slightly increase propellant burning rate while exerting little adverse impact on propellant dynamic activity.To the best of our knowledge,this is the first report on the application of PTW as erosion inhibitor and mechanical reinforcing materials in the field of gun propellants.The present work would provide an alternative scalable approach for development of powerful gun propellants with less erosion and more mechanical strength that can meet requirement of modern high chamber pressure guns.
2.Experiment
2.1.Materials
Potassium titanate whiskers(PTW, K2Ti6O13, 95.5%) with a diameter of 0.2-1 μ m and a length of 10-50 μ m were provided by Nanjing University of Science and Technology Chemical Reagent Group.The raw PTW used in this paper is commercial product with multiple sources.Dopamine(DA, 98%) was purchased from Xiya Reagent Co., Ltd (China).Hydrochloric acid(HCl,1 N) solution was provided from Codow Co.,Ltd(China).Tris-base(98%)was obtained from Scientific phygene Reagent Co.,Ltd(China)used to prepare tris buffer solution.Anhydrous ethanol(99.7%), acetone(99.7%) and TiO2(100 nm, anatase) were purchased form Sinopharm Chemical Reagent Co.Ltd(China).Propellant tablets are composed of 44.9 wt%nitrocellulose(NC, N = 13.0 wt%), 25 wt% nitroglycerine(NG),14.3 wt% diethylene glycol dinitrate(DEGDN), 14.5 wt% cyclotrimethylene trinitramine(RDX, 10-50μm) and 1.3 wt% centralite,provided by Liaoning Qingyang Chemical Industry Group Co.,LTD.(Liaoning,China).The force constant and flame temperature of the propellant tablets are 1200.6 J/g and 3663 K in theoretical by REAL software, respectively.Nitrocellose(N = 11.88-12.40 wt%)was used to prepare the ignition powder,provided by Luzhou North Chemical Industry Co.LTD.(Luzhou, China).
2.2.Preparation of PTW@PDA composites
Scheme 1(a) illustrates the preparation process of PDAmodifying PTW@PDA composites.Firstly, a 500 mL Tris-HCl buffer solution (10 mM, PH = 8.5) was prepared using Tris-base,HCl solution and deionized water.Secondly, 25 g PTW was subjected to ultrasonic treatment in a 500 mL Tris-HCl buffer solution for 15 min,resulting in the formation of a suspension.After that,the suspension solution was stirred at 400 rpm and 1.0 g DA was added into the suspension solution.The reaction was kept for 24 h at 25°C.In reaction process, the color of the suspension solution changed from white to brown.Finally, the brown PTW@PDA composites were filtered, washed with ethanol and deionized water until the PH was 7.0, and dry at 50°C for 24 h and the PTW@PDA composites can be obtained.
2.3.Preparation of PTW-and PTW@PDA-Modified high energy gun propellants
Scheme 1(b)illustrates the manufacturing process of PTW@PDA modified gun propellants samples.High energy modified propellants containing PTW and PTW@PDA were manufactured by a solvent-based propellant processing and extrusion technique.The propellant compositions are shown in Table S1 in Supplementary.The quantity of utilized propellant tablets material for each propellant sample was 200 g.Firstly, a certain amount of PTW or PTW@PDA were ultrasound treated for 5 min in mixture-solvent made up of acetone and ethanol (1:1 by volume), which equal to 18 wt% utilized propellant tablets for each propellant sample.Secondly,dried propellants tablets and mixture solvent were mixed and kneaded in a kneading machine for 3 h at 35°C.After gelatinizing process, a homogeneous propellant dough is obtained and added into a tubular forming mold to extrude a propellant strand by a hydraulic machine.After that, the received propellant strand was cut into 4.0 ± 0.1 cm length tubular propellant grains with an inner and outer diameter of 2.8 ± 0.1 mm and 6.5 ± 0.1 mm, and dried in air at 25°C for 2 days and in an oven at 50°C for 7 days.The original control propellant sample was labeled as GP-1#.The PTWmodified propellant samples containing additional 0.5 wt%,1.0 wt%and 3.0 wt% PTW were labeled as GP-2#, GP-3#, GP-4#.The PTW@PDA-modified propellant samples containing additional 0.5 wt%,1.0 wt%and 3.0 wt%PTW@PDA were labeled as GP-5#,GP-6#, GP-7#.The referred control propellant samples containing additional 0.5 wt%and 3.0 wt%TiO2were labeled as GP-8#and GP-9#.
2.4.Measurements
2.4.1.Interfacial characterization
In this work, the surface morphology, functional groups and element compositions of untreated PTW and as-prepared PTW@PDA samples were first investigated by scanning electron microscopy(SEM, QUANTA250FEG, FEIGECH) with X-ray energy dispersive analysis(EDX), flourier transform infrared spectrometer(FT-IR, Bruker, VERTEX70), Raman spectrometer(Renishaw inVia-Qontor Raman Microscope) and X-ray photoelectron spectrometer(XPS, Kratos, AXIS-Ultra DLD).Subsequently, optical contact angle measuring instrument(Dataphysics,OCA20) was employed to investigate the surface adhesion properties of PTW and PTW@PDA samples.In this testing,pure water and ethanol-acetone mixture-solvent contact angles were employed to characterize the surface adhesive properties of PTW and PTW@PDA.The water contact angle is a typical parameter to evaluate the surface properties, while ethanol-acetone mixturesolvent of volume ratio of 1:1 is a typical plasticizing solvent using in solvent-based gun propellants processing.Finally,the dispersion of PTW and PTW@PDA in modified propellants was assessed through SEM and EDX mapping analysis by identifying the positions of titanium(Ti) and potassium (K) elements.
2.4.2.Gun propellant erosion testing
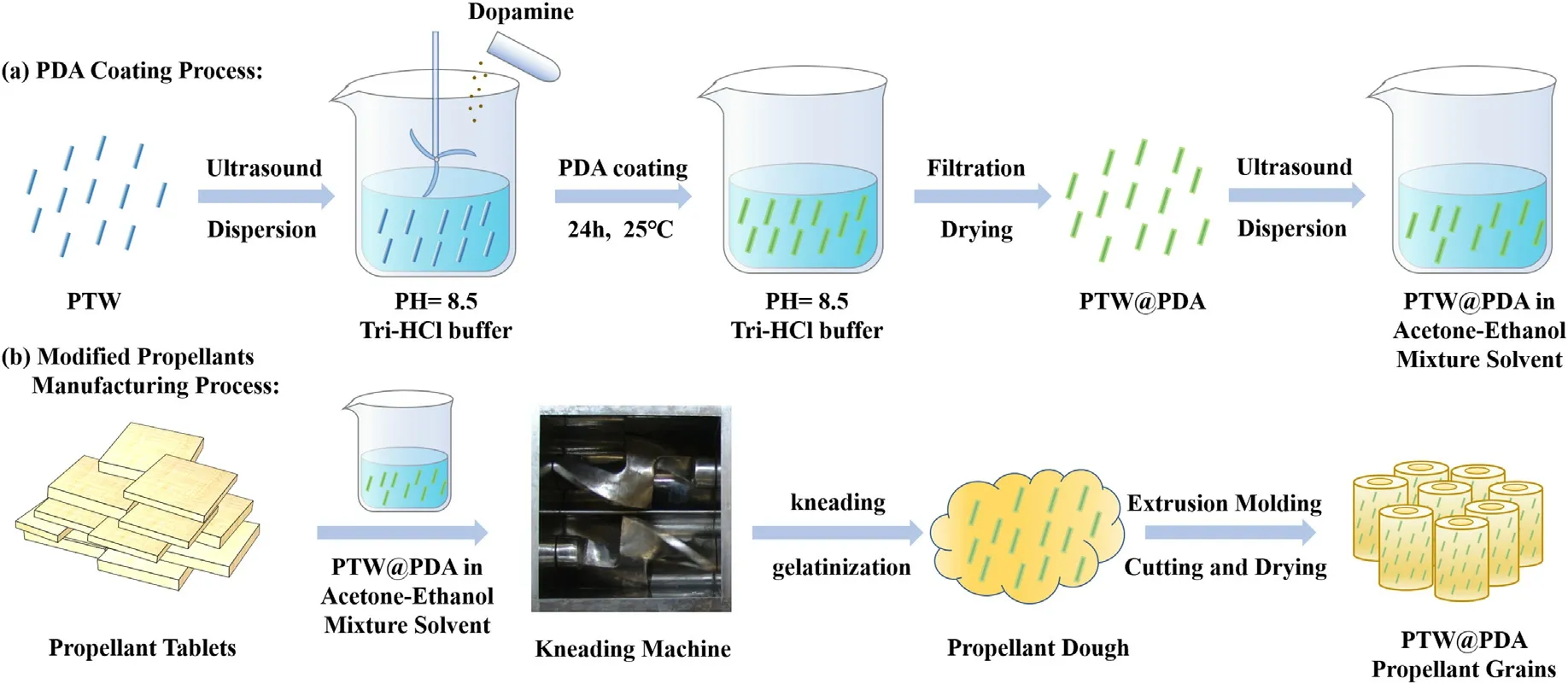
Scheme 1.Schematic illustration of preparation process of (a) PTW@PDA composites and (b) modified propellants containing PTW@PDA composites.
The erosion performances of propellants were investigated by a vented bomb erosion tester at 20°C.The tester is a standard 100 cm3closed boom tester that modified by placing a copper sheet and an erosion tube in it [16].Scheme S1(a) in Supplementary depicts the vented bomb erosion tester, designed to simulate the erosive environment of gun barrels by characterizing the erosion of burning propellant gases on steel erosion tubes.In erosion testing,the tubular propellants grains with 4 cm length were placed in combustion chamber of vented erosion tester,and initiated using a nickel-chrome ignition wire and 1.0000 ± 0.0005 g ignition powder(nitrocellulose, 11.88-12.40 wt% N) for each testing.The produced propellants gas products would break the copper sheet,then leak out from the erosion tube.The thickness of each copper sheet was 1.50±0.02 mm,which would break empirically at a pressure of 240 MPa.The inner surface of the erosion tube would suffer severe erosion damage,resulting in a decrease in weight.The weight of the erosion tube was recorded before and after three successive firings,and the weight loss was used to indicate the erosivity of the tested propellants.Before weighing,the erosion tube would be ultrasonic treated for 5 min in mixture of ethanol and acetone (1:1).In the work, GB45# quality carbon structural steel [44] was used to manufacture erosion tube samples.Additionally, to mitigate the impact of energy and pressure variables on erosion results, it is empirically possible to adjust the quantity of propellant charges tested so as to achieve a comparable maximum burning pressure within a range of 300±10 MPa,which is determined by a pressure sensor.
To better understand the erosion reducing mechanism of PTW and PTW@PDA inhibitors, the morphology and element composition of the inner surface of erosion tube were analyzed by SEM-EDX and XPS.After three propellant firing, each erosion tube sample would be cut longitudinally and ultrasonic treated for 5min in ethanol-acetone mixture to facilitate subsequent characterizations for its inner surface.
2.4.3.Gun propellant mechanical testing
The impact,tensile and compression strength testing were used to characterize mechanical properties of modified propellants.The impact strength testing[45]was performed on a pendulum impact simply supported beam tester(JJ-20, Changchun Intelligent Instrument Equipment CO., Ltd.) with a 4 J impact energy at -40°C operating temperature.The impact strength at 20°C and 50°C are not presented because the tested propellant did not break thoroughly resulting from the high toughness at room or higher temperature, leading to invalid data.The tensile and compression strength testing were conducted on an American Instron (Model-3367) at across-head speed of 10 mm/min at -40°C, 20°C and 50°C operating temperature.For impact testing, the shapes of propellant grains were tubular with length of 6 cm and with an inner and outer diameter of 2.8 mm and 6.5 mm.For compressive testing, the tubular propellant grains were with length of 6.5 mm and with an inner and outer diameter of 2.8 mm and 6.5 mm.Moreover, for tensile testing, the propellant appearance was dumbbell-shaped with rectangular stretch cross-sectional with a length of 3.75 mm and a width of 0.9 mm.For each propellant composition, at least three specimens were tested.
2.4.4.Gun propellant closed bomb testing
The combustion behaviors of propellants samples were investigated by a closed bomb tester[46]at 20°C.The diagram of tester is showed in Scheme S1(b)in Supplementary and the test loading density was 0.20 g/cm3.For each testing,tubular propellants grains with a length of 4 cm were initiated using 1.0000 ± 0.0005 g ignition powder(nitrocellulose,N=11.88-12.40 wt%)and a nickelchrome ignition wire.The combustion of ignition powder can generate a theoretical ignition pressure of 10.98 MPa,ensuring the subsequent burning of propellant.The theoretical ignition pressure can be determined by Eq.(1) [46].
where Pigis theoretical ignition pressure (MPa) resulted from ignition powder;migand figare the mass(g)and force constant(kJ/kg) of nitrocellulose ignition powder with values of 1 g and 994.46 J/g(theoretical);V0is the actual volume of the closed bomb vessel with a value of 103.5 cm3;Δ and ρ are the loading density(g/cm3)and density(g/cm3)of testing propellant grains with values of 0.20 g/cm3and 1.6 g/cm3.The pressure-time history during the propellant burning process was recorded by a pressure sensor in a closed bomb tester, enabling the calculation and plotting of the pressure-time (p-t) curve, burning rate-time (u-p) curve, and dynamic vivacity-relative pressure (L-B) curve for each propellant sample [46].
2.4.5.Gun propellant thermal compatibility testing
The thermal compatibility of a new additives to gun propellant are important for safety reasons.The DSC thermal compatibility testing was employed according to method [47], and the related dates are presented in Fig.S1 and Table S2 in Supplementary.The results suggested that pure PTW and PTW@PDA are safe in the contact of NC-NG-DEGDN-RDX gun propellant compositions.
3.Results and discussion
3.1.Enhanced interfacial interaction and surface modification mechanisms
It is widely accepted that PTW as reinforcing filler can modify the mechanical properties of various polymers,such as epoxy[28],poly(ether ether ketone)[29]and polytetrafluoroethylene[30]due to its nearly perfect crystal structure and high strength.
In this work, we attempted to incorporate PTW in propellant polymer matrix.However,PTW often exhibits easy aggregation and poor dispersion in polymer matrix[35].The surface modification is widely acknowledged for its ability to enhance the interfacial interaction between the inorganic components and the polymer matrix [35].Nevertheless, due to the tunneling structure and chemical stability, direct coupling on PTW surface did not yield appreciable effects [48,49].Fortunately, remarkable adhesive ability of PDA-modification has proven highly effective in coating and providing active sites on the various inorganic and organic substrate[37].As illustrated in Scheme 2(a),following the application of PDA coating on PTW, the novel PTW@PDA composites was incorporated into propellants matrix.Although PDA can be obtained through polymerization under facile and mild conditions,the exact related mechanisms remain elusive hitherto,thus far due to the involvement of intricate redox reactions and the generation of a series of intermediates.Several popular models suggested the possible mechanisms of DA polymerization into PDA by covalent and/or non-covalent interactions [37,50], as illustrated in Scheme 2(b).The first hypothesis proposes that under aerobic and alkaline conditions, DA undergoes covalent polymerization to form dopaminequinone, which subsequently transforms into 5, 6-dihydroxyindole (DHI), followed by deprotonating and intermolecular Michael addition to produce cross-linked PDA [37,51].The second model integrates covalent polymerization and non-covalent self-assembly, with the latter encompassing ionic, charge transfer,T-shape,hydrogen-bonding and π-π interactions[51,52].The PTW crystals were PDA-functionalized with active sites including hydroxyl groups, amino groups and catechol groups, which acted as anchor nodes to further facilitate the formation of intermolecular hydrogen-bonding interactions, as illustrated in Scheme 2(c).In order to verify that the actives sites are presented by PDA,the FT-IR,Raman and XPS spectra were conducted.With further coating of PDA,the propellant matrix could be tightly adhered to PTW crystals as confirmed by following SEM-EDX analysis.
3.2.Surface morphologies and chemical compositions of PTW and as-prepared PTW@PDA composites
Fig.1 shows the SEM images of surface morphologies of PTW Scheme 2.Schematic illustration of(a) preparation process of modified propellants containing PTW@PDA composites; (b) Polymerization mechanism of dopamine; (c) Enhanced interfacial interactions in PTW@PDA propellants.and as-prepared PTW@PDA composites.As shown in Figs.1(a)and 1(c), it can be seen that PTW and as-prepared PTW@PDA composites exhibit a needle-like appearance.As shown in Figs.1(b)and 1(d),upon comparison with the image of PTW and PTW@PDA at a higher magnification, the PTW exhibited a smooth surface appearance, whereas the PTW displayed a rougher surface appearance covered with nano-scale particles, indicating the successful surface PDA-grafting on whiskers.
The FT-IR spectra of PTW,PTW@PDA,DA and PDA are displayed in Fig.2.The appearing absorption peaks at the range of 500-1600 cm-1were attributed to the characteristic peaks of PTW that agree well with the previously reported date [35,36,53,54].Absorption bands around 686 cm-1and 934 cm-1for PTW and PTW@PDA samples were corresponded to O-Ti-O bending vibrations(δ) and Ti-O stretching vibrations (ν) of TiO6octahedral groups.In comparison to PTW, the marked peaks within the rectangular box in the PTW@PDA spectrum exhibited diminished intensity and eventually vanished.It suggests that a certain degree of chemical interaction has occurred at the interface between PTW and PDA.On the other hand,the IR peaks of DA and PDA are similar to that in previous report [55].A broad absorption band was both observed in the DA and PDA regions of 2800-3500 cm-1,which are attributes to stretching modes(ν)of CH2,CH,OH and NH2groups.A few intensive peaks in the region of DA at 525-1599 cm-1are attributed to various models of CC,CO,CH,CH2,NH and NH2groups[55].The peak at 1599 cm-1is ascribed to stretching(ν)of NH and scissoring (ρ) of CC; The peak at 1497 cm-1is ascribed to plane bending(β)of CH and stretching(ν)of CC;The peaks at 1286 cm-1and 813 cm-1are attributed to stretching(ν)of CO and rocking(μ)of CH2and wagging(ω)of NH2;The peaks at 525 cm-1is attributed to twisting (τ) of CC and plane bending (β) of CO.The adsorption peaks in the region of PDA at 525-1594 cm-1are conformed with previous report [56].That is different with that of DA, which difference should result from the self-polymerization.In comparison to region of PTW,a new broad adsorption band observed in that of PTW@PDA at 525-686 cm-1suggest that PDA was deposited onto the surface of PTW.Fig.3 presents the Raman analysis.The Raman spectra of PTW exhibited four intense peaks at 447 cm-1,508 cm-1,642 cm-1and 848 cm-1,which are attributed to the characteristic region of PTW.These observed peaks are quite similar to that in Raman spectra in report [54,57], which suggested that are attributed to the Ti-O bond whose oxygen is not shared among the TiO6units [54,57].According to Raman region of DA in previous report[55],the observed peak at 743 cm-1in this work is mainly ascribed to the stretching(ν)of CC and the twisting(τ)of CH2;The peaks at 787 cm-1is mainly attributed to the twisting (τ) of CO and the wagging (ω) of NH2; The peaks at 1279 cm-1is derived from the stretching (ν) of CO and CC, the rocking (μ) of CH2and the plane bending(β)of CH.After PDA coating,the intensity of four peaks at 447 cm-1,508 cm-1,642 cm-1and 848 cm-1were decreased that are ascribing the presence of PDA coating on the whiskers, which weakens the characteristic peaks of PTW.
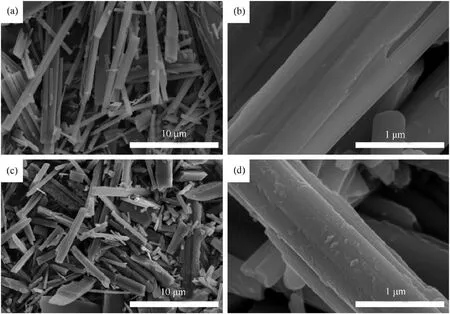
Fig.1.The SEM images of (a), (b) PTW and (c), (d) PTW@PDA composites samples.
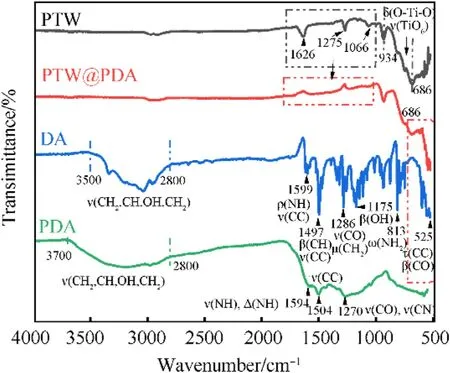
Fig.2.The FT-IR spectra of pure PTW,DA,PDA and as-prepared PTW@PDA composites.
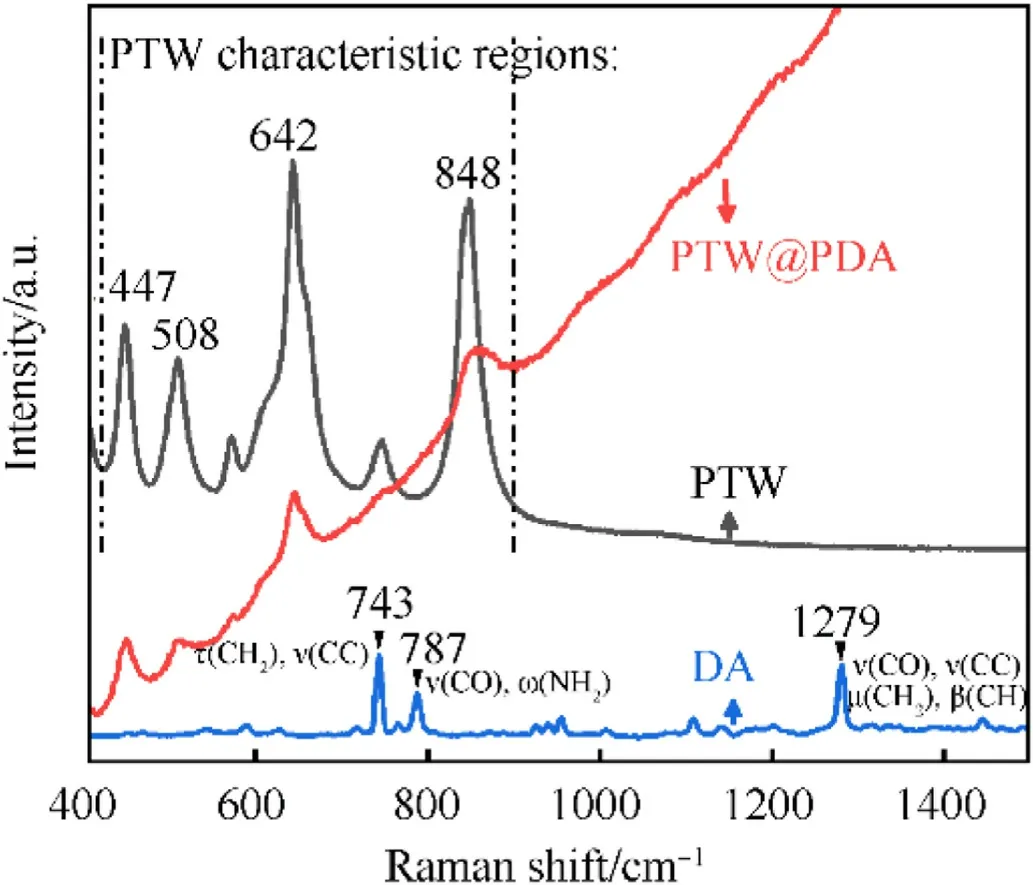
Fig.3.The Raman spectra of pure PTW, DA and as-prepared PTW@PDA composites.
By means of XPS analysis, the surface element states of PTW,PTW@PDA and PDA were further investigated.Fig.4(a) illustrates that the survey XPS spectra of PTW sample exhibit O1s, Ti2p and K2p peaks;while those of PDA show O1s,N1s,and C1s peaks.After PDA modifying, the spectrum of PTW@PDA composites displayed additional N1s and C1s peaks.Figs.4(b)-4(d)displayed of C1s,O1s,and N1sregions of high-resolution XPS spectra of PTW,PTW@PDA and PDA.Through curve-fitting using XPS PEAK software, the binding energies and assigned chemical groups of each peak were fitted with a convolution of Gaussian and Lorentzian line at a ratio of 80:20 and listed in Table S3.As shown in Fig.4(b),the O1s region of PTW was fitted to two peaks occurred at 529.6 eV and 530.5 eV,which were ascribed to the lattice oxygen of PTW and adsorption oxygen of the hydroxyl group of PTW,respectively.The O1s region of PDA was fitted by C-OH peak at 533.0 eV and C=Opeak at 531.9 eV.After the PDA coating, the O1s region of PTW@PDA exhibited an increase in both binding energy (~530.7 eV) and adsorption oxygen counts.This situation could be attributed to the interaction between PTW and PDA, indicating the introduction of adsorption oxygen by hydroxyl groups of PTW@PDA.As shown in Fig.4(c), the C1s region of PDA can be fitted to three peaks at 284.8 eV,285.7 eV and 288.7 eV,which are attributed to C-C/C-H,C-OH/C-N and C=O groups, respectively.Meanwhile, for C1s region of PTW, the peak at 284.8 eV can be ascribed to C-C/C-H groups.For C1s region of PTW@PDA, two new peaks appeared at 285.5 eV and 287.7 eV,resulting from PDA introduction.Moreover,as shown in Fig.4(d), the N1s region of PDA were fitted to three peaks at 399.0 eV,399.8 eV and 401.5 eV,corresponding to C-N=C,C-NH-C and C-NH2groups,respectively.These three peaks were also visible in the N1s region of PDA@PTW,but were not observed in that of PTW, indicating the presence of C-N=C, C-NH-C and C-NH2 groups on the surface of PTW@PDA.Based on the analysis of FT-IR, Raman and XPS spectra, PDA has been successfully introduced in PTW@PDA composites, which exhibited a modified surface morphology, functional groups and element states.
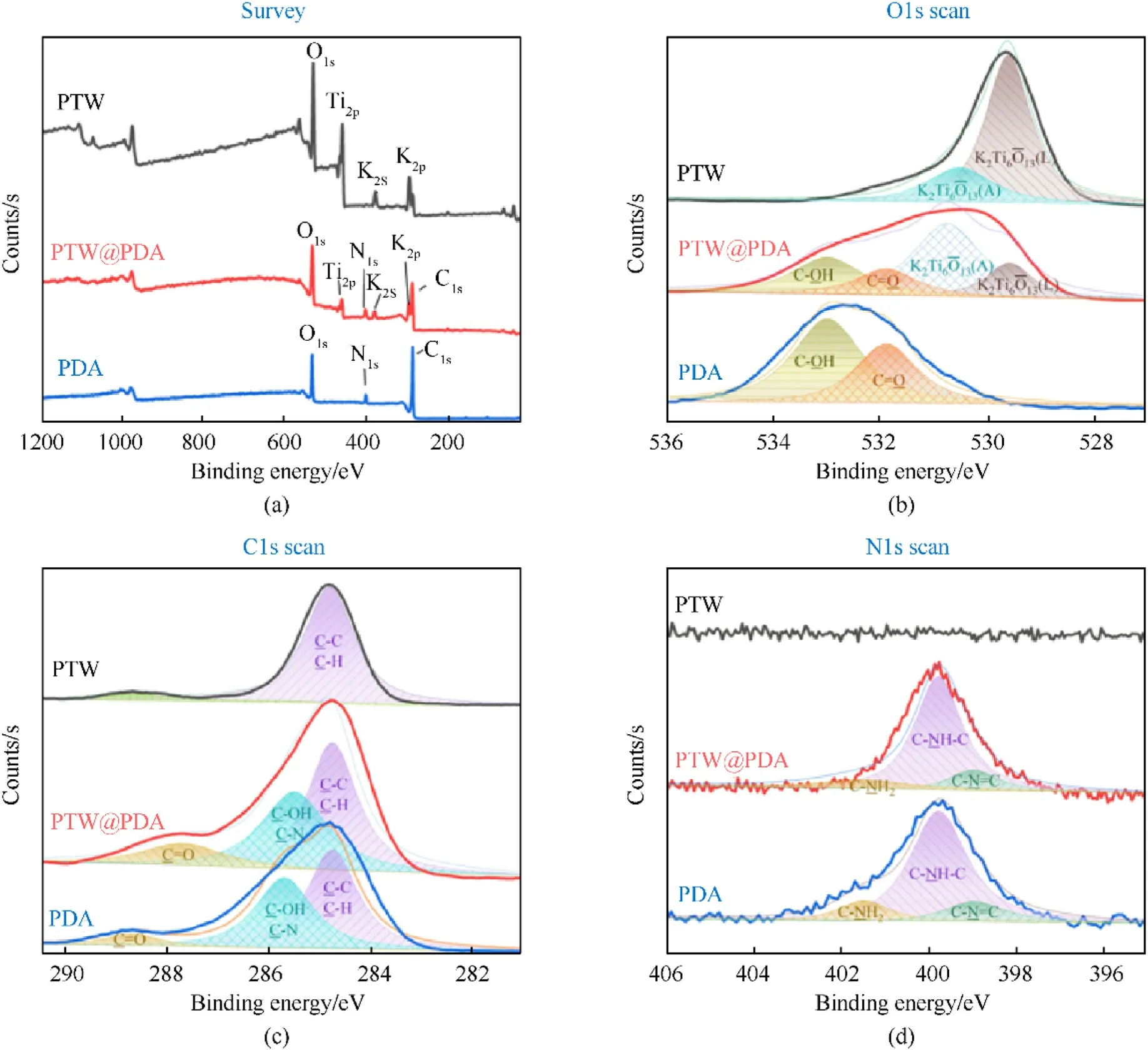
Fig.4.(a) The survey XPS spectra and high-resolution XPS spectra of (b) O1s, (c) C1s and (d) N1s regions for PTW, PTW@PDA and PDA.
3.3.Surface adhesion properties of PTW and as-prepared PTW@PDA composites
The contact angle is an important parameter to evaluate the surface adhesion abilities of materials.In this work, ethanolacetone mixture-solvent contact angles were employed to characterize the surface properties of PTW, PTW@PDA and nitrocellulose(NC), which is the main component of propellant binder.Ethanol-acetone mixture of volume ratio of 1:1 is a typical plasticizing solvent for NC in a solvent-based processing approach to manufacture gun propellants.The results are shown in Fig.5,ethanol-acetone mixture-solvent and water contact angles of PTW were 24.2°and 78.2°, respectively.After PDA modification, those two liquid contact angles of PTW@PDA were decreased to 16.7°and 45.8°, respectively.It was clear that the PTW@PDA surface exhibited greater adhesive ability and wetting behavior towards ethanolacetone mixture and water droplets compared to PTW.The phenomenon can be attributed to a considerable number of functional groups in PDA coating,including-NH2,-NH-and-OH groups,as depicted in above.On the other hand,the ethanol-acetone mixture and water contact angles of nitrocellulose were 7.6°and 119.4°.It appeared to that PDA-modifying leads to PTW@PDA ethanolacetone mixture contact angles that approached those of nitrocellulose, suggesting similar surface properties and improved surface compatibility between PTW@PDA and NC in the wetting behavior of an ethanol-acetone solvent.Therefore,PTW@PDA can be readily dispersed randomly into propellant using a convention ethanolacetone solvent-based processing approach.

Fig.5.The contact angles of PTW and PTW@PDA with water and mixture of ethanol and acetone of volume ratio of 1:1.
3.4.Dispersion of PTW and as-prepared PTW@PDA in propellant matrix
SEM with energy dispersive x-ray(EDX)mapping analysis of asprepared PTW- and PTW@PDA-modified propellants were performed to characterize the dispersion of PTW and PTW in propellant matrix.Due to the thermal decomposition of propellant matrix at high magnifications of SEM,it was not feasible to directly observe PTW@PDA composites in micron and nano sizes.With help of EDX mapping analysis, as shown in Fig.6, the position of Ti and K elements in cross section of propellant grains can be detectable.Ti and K were utilized as identifying elements for PTW and PTW@PDA,facilitating the localization of composites within propellants.It is evident that there exists significant agglomeration of PTW within the propellant matrix, which corresponds to the observed PTW agglomeration in polymers as reported in previous Refs.[28,30].In contrast, the PTW@PDA, after undergoing PDA modification,exhibited excellent dispersion in the propellant, which can be attributed to the enhanced interfacial interaction between PTW@PDA and propellant matrix.The EDX mapping analysis results were in accordance with the observed alterations in surface adhesion properties as determined by contact angle testing above.
3.5.Erosion performance of as-prepared propellants
To investigate the erosion properties of propellants containing with and without PTW and PTW@PDA additives, vented bomb erosion tester was employed.During each erosion testing, propellant gases will lead to weight loss for each erosion tube specimen.The weight variation of each erosion tube after three propellants firings can predict the erosion values of per gram propellant(w)for each propellant composition and the Erosion-reducing efficiency(E) of additives, that can be calculated by the following equations:
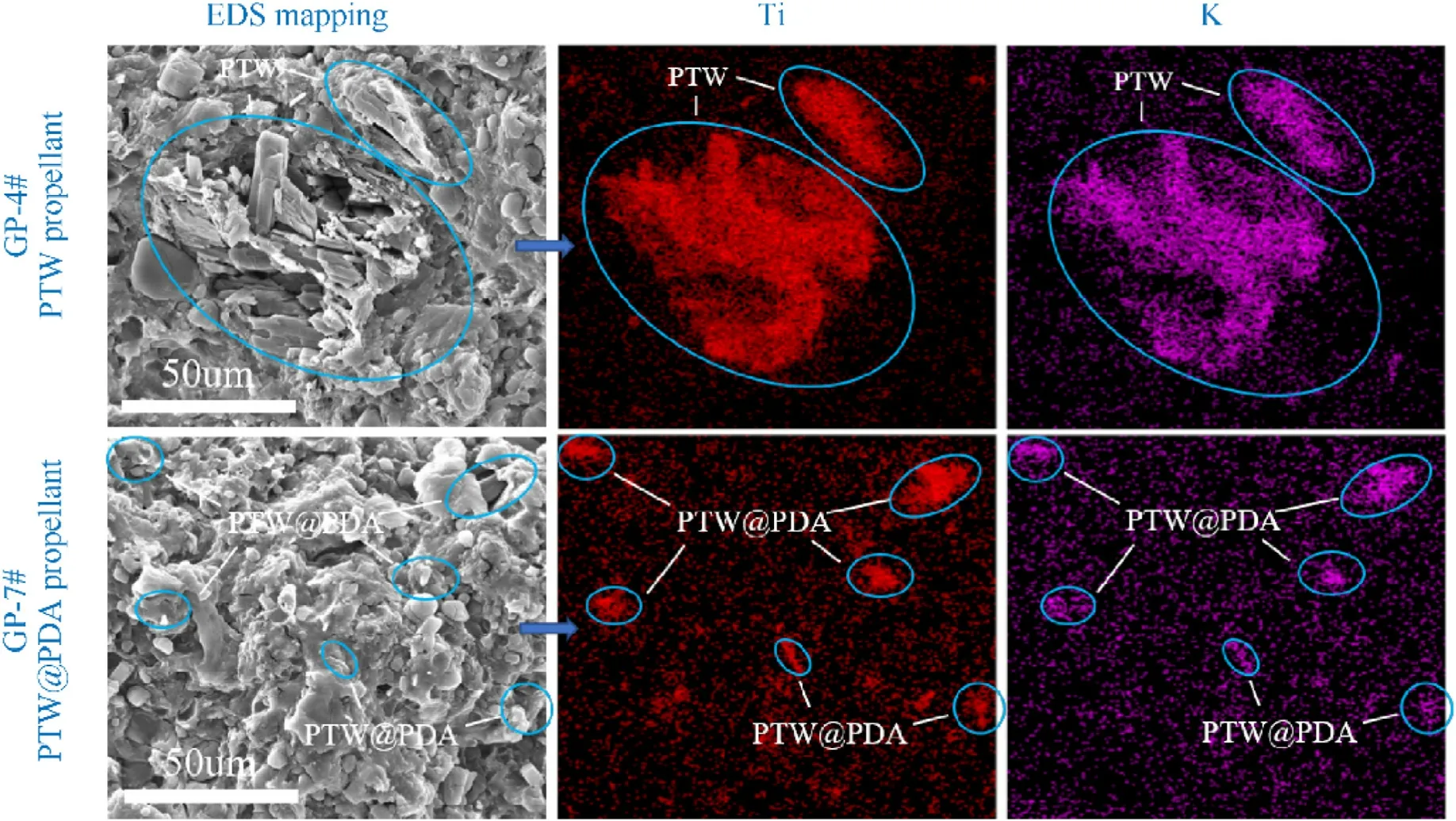
Fig.6.The SEM images with EDX mapping analysis of the cross section of modified propellant grains containing PTW and PTW@PDA
where m0and m1are the weight of the steel tube before and after three firings, respectively; mpis the weight of the propellant charges in each firing; wband wsare the w values of original propellant and the modified propellant with additives, respectively.Fig.7 shows the Erosion-reducing efficiency(E)of PTW,PTW@PDA and TiO2 additives.And the detailed results are listed in Table 1.As shown in Table 1, the loading mass of PTW-(GP2#3#4#),PTW@PDA-(GP5#6#7#) and TiO2-(GP8#9#) modified propellants were increased based on that of original propellant(GP1#).The different loading mass is to offset the loss in force constant resulting from inert additives in propellant compositions and to obtain the w values for each propellant composition under similar pressure conditions (309.7-322.9 MPa).The theoretically calculated energy properties of PTW propellant compositions are presented in Table S6.
Compared to original propellants,as shown in Fig.7,propellants containing 0.5 wt%, 1.0 wt%, and 3.0% PTW exhibited Erosionreducing efficiencies (E) value of -0.13%, 0.86%, and 8.56%,respectively.After PDA coating, propellants containing 0.5 wt%,1.0 wt%and 3.0 wt%PTW@PDA exhibited 4.30%,4.02%and 12.1%of E values.The results suggested that the as-prepared PTW@PDA exhibits higher E values than raw PTW,and can significantly reduce the erosion of high energy RDX propellant under consistent burning pressure conditions.Generally, TiO2served as a conventional military inhibitor can directly be added in propellant compositions, as well as used as inorganic component in organicinorganic compound inhibitor [58].Whereas, for the high energy RDX propellant in this work,TiO2exhibited E values of-1.97%and 1.92%in GP-8#and GP-9#samples testing.Therefore,the raw PTW and as-prepared PTW@PDA could be promising novel inhibitors,both showed better erosion-reducing effect than conventional TiO2for the high-energy RDX propellant in this work.
3.6.Surface morphologies and chemical compositions of erosion tube samples
Fig.8 shows the surface appearance of the eroded steel samples after three firings for each propellant sample.Visually, significant disparities were observed in the appearance of steel samples fired with propellants with different inhibitors and without additive.As shown in Fig.8(a), steel sample (a) fired without any inhibitors,resulted in severe erosion with a distinctive darker rust color.Steel sample(b)and(c)fired with PTW and PTW@PDA additives did not exhibit severe darkening color indicative as the observation in steel samples (a).Sample (d) fired with TiO2additives had a gray color,possibly due to the coverage of TiO2particles, which has ability to dye white.The optical observation results initially suggested that PTW and PTW@PDA additives may be preventing steel surface damaged from the contact of burning propellant gases.
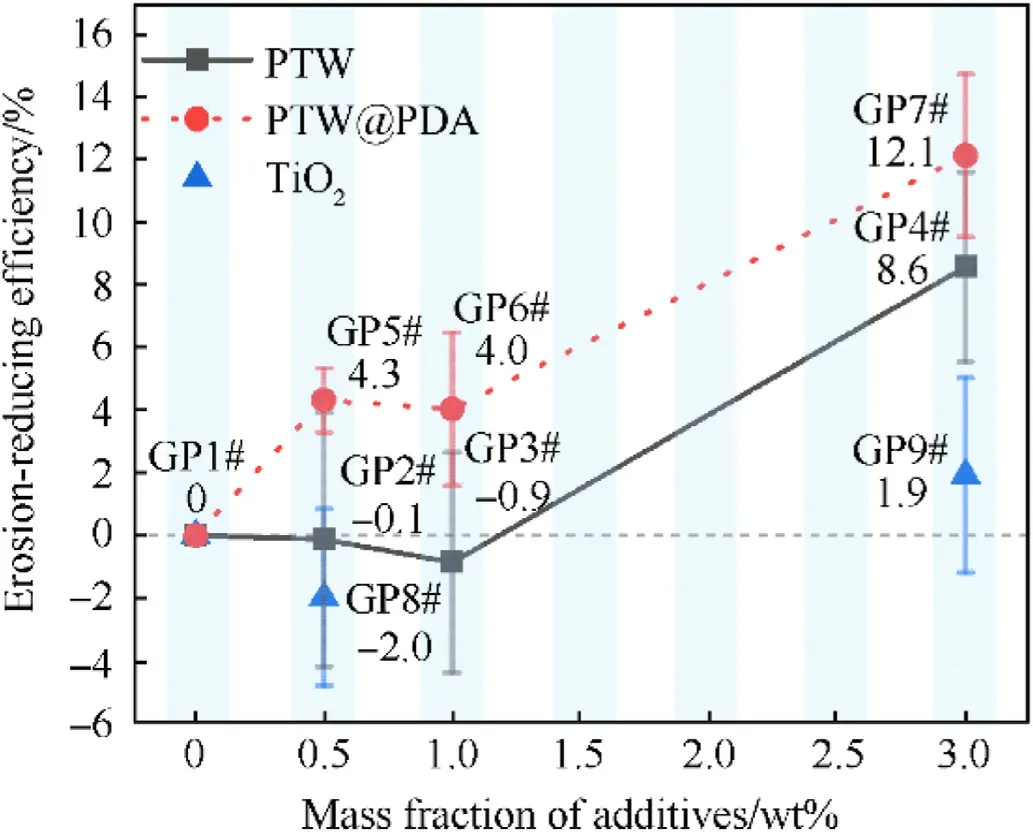
Fig.7.The Erosion-reducing efficiency of PTW, PTW@PDA and TiO2 inhibitor for propellants.
To further investigate any effects of PTW and PTW@PDA materials on reduced erosion wear and its mechanisms for gun propellant firings,the surface morphologies and chemical compositions of the steel samples were characterized by SEM-EDX analysis.As seen in Fig.9,the steel samples were both imaged at 100×,2500×and 20,000 × magnification.As seen in Figs.9(a), 9(a1) and 9(a2), the steel sample fired by original propellant without additives exhibited a rough surface under 100×magnifying observation and severe cracking under 2500 × and 20,000 × magnifying observation.As shown in Figs.9(b)-9(d),the three steels samples fired with PTW,PTW@PDA and TiO2inhibitors seemed to have the presence of particles and platelets covering the eroded steel surfaces under 100×magnification.To further observer detailed features in these areas, the magnifications were increased to 2500 ×.Fig.9(d1)shows that the propellant containing TiO2leads to particles agglomerating and covering steel surface.Moreover, Fig.9(b1)shows that the steel sample fired by PTW propellant has surface covered by needle-shaped whiskers, which are composed of K, Ti and O confirmed by EDX analysis.While Fig.9(c1)displayed that the steel sample fired by PTW@PDA propellant has what appeared to flat and smooth platelets coating on surface.The coating consisted of 19.35 wt% Ti, 7.91 wt% K, 34.57 wt% O and 29.21 wt% Fe, that detectable by EDX.Under 20,000×major observation,as shown in Fig.9(c2), the PTW@PDA coating had been found to be covered nano-sized spherical particles, that are similar with TiO2 particles(in Fig.9(d2)) but more evenly dispersed on steel without agglomeration.The SEM-EDX results suggested that the firing of PTW@PDA-propellants generates a Ti-K-based coating that apparently covers the steel surface and reduces erosion.However,it hardly provided a comprehensive explanation for the noticeable disparities observed in coating appearance between PTW and PTW@PDA.The presence of any whisker-like coating has not been observed in PTW@PDA-propellant fired steel samples.We supposed that the well-dispersed PTW@PDA within the propellant matrix leads to a more efficiently heat transfer with burning propellant matrix during propellant firing, which occurred in a very short time.The adequate heat facilitated the PTW@PDA phase transition, leading to the formation of a Ti-K-based coating attaching on steel at high-pressure and pressure.The Ti-K-based coating can provide wear resistance for steel by insulating thermal and buffering impact from propellant gases.In contrast,PTW have been found to agglomerate seriously in the propellant matrix.This situation leaded to a poor contact interaction and low heat transfer between PTW and burning propellant matrix, causing to PTW maintaining its original needle-shaped appearance.
Fig.10 and Table S4 give the results of the chemical compositions of steel samples detected by EDX elemental analysis.All fired steel samples contained Fe,Ni and O on the surface of eroded steel samples.Fe and Ni should originate from the steels; and O was originated from the propellant or additives.The surface of the steels fired by PTW-and PTW@PDA-propellants were found to contain Ti and K, indicating that these elements originated from PTW and were subsequently deposited on the steel during firing.Clearly,the steel surface fired propellant without additive exhibited most content of Fe and Ni,which was more eroded.It was apparent that no wear-reducing additive will cause the steel directly exposed to hot propellant gases, thereby increasing erosion.
The steel erosion gradually reduced as the steel surface concentration of Fe and Ni steadily decreasing and that of Ti and K increasing.The steel surface fired with PTW@PDA-propellant had less Fe and Ni,and shows relative abundance of O,Ti and K,whichcaused to the least erosion sample.It can be attributed to that the steel surface was coated more completely by particles and platelets,which originated from PTW@PDA inhibitors.Compared to conventional TiO2inhibitors, steel surface fired with PTW- and PTW@PDA-propellant presented lower Fe and Ni content as well as high erosion-reducing efficiencies, indicating that Ti-K-based layers can effectively prevent interaction between steel and hot propellant gases.
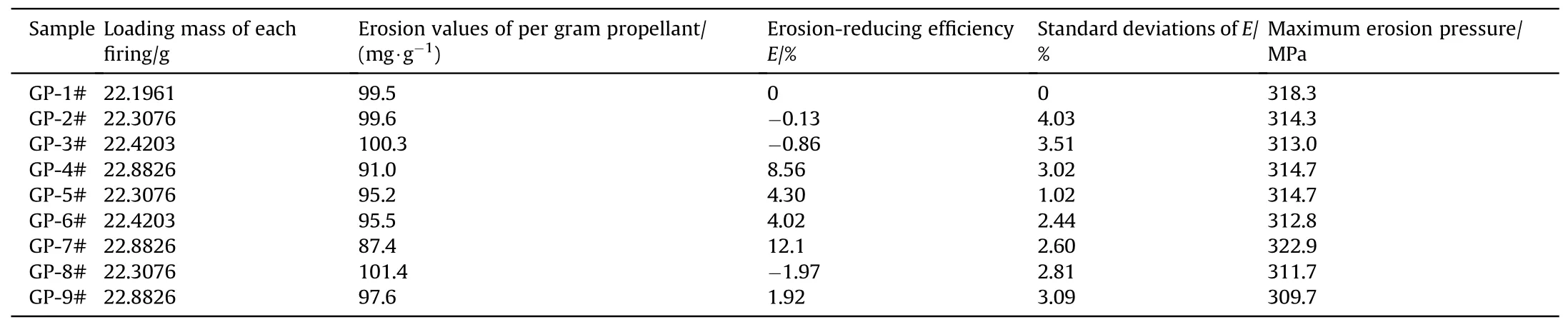
Table 1 Erosion performance of propellants with various contents of inhibitors.
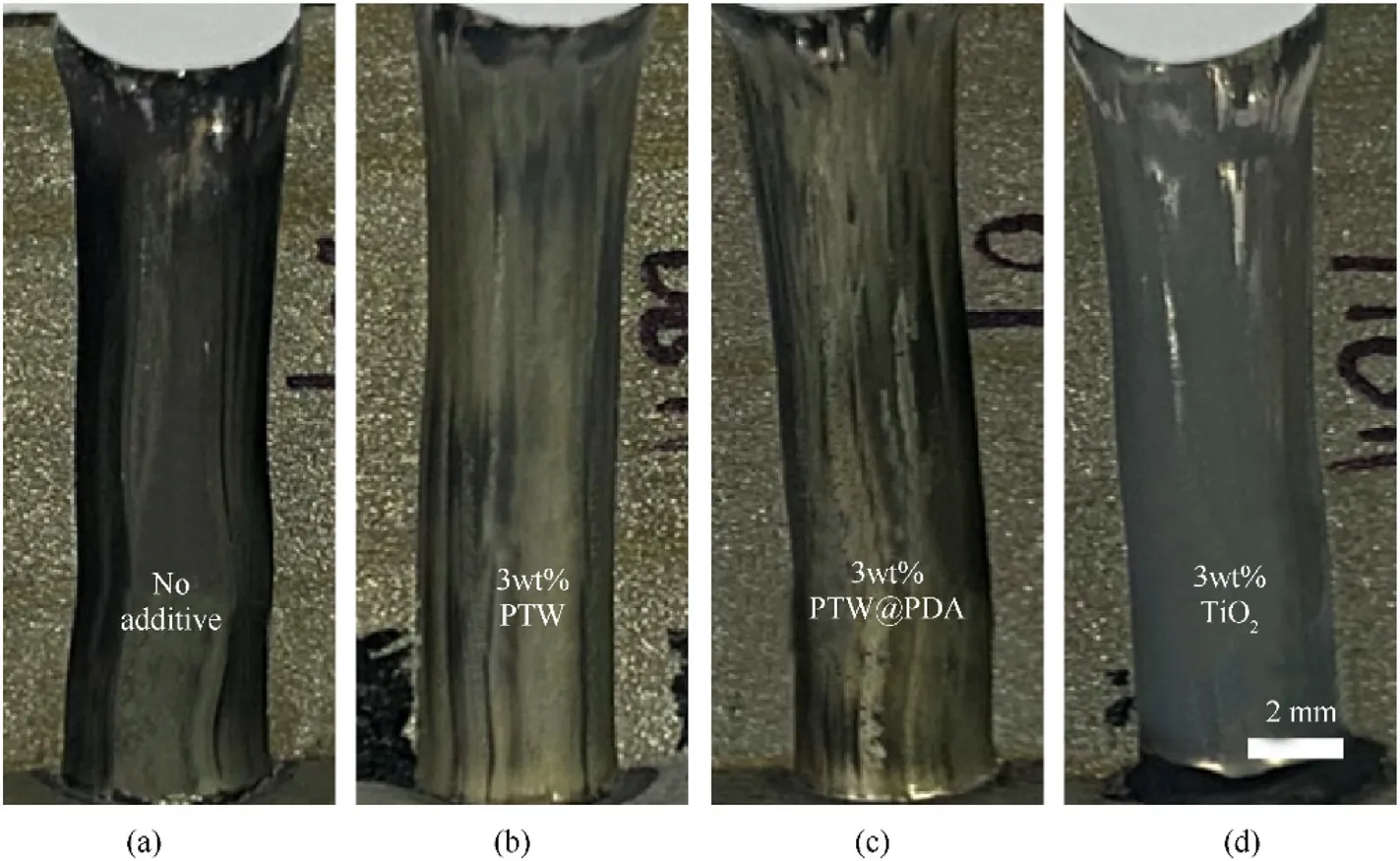
Fig.8.Photographs of eroded erosion steel samples after three propellant firings in vented bomb erosion testing.
To better understand the chemical erosive effects by modified propellants, the steel samples were characterized by XPS.As seen from Fig.11(a),after adding PTW and PTW@PDA in propellants,the Ti2P peaks can be observed in survey XPS spectra of steel samples.However, Fig.11(b) suggested that the presence of PTW and PTW@PDA seem to hardly affect the iron oxidation state.It is still not clear that whether any exact chemical reaction occurred between steel and Ti-K-based layer in a high-temperature and highpressure condition.
To sum up, both PTW and PTW@PDA exhibited significant erosion-reducing effects compared to the conventional TiO2inhibitor.Furthermore, the well-dispersed PTW@PDA demonstrated even further enhanced erosion resistance in comparison to raw PTW.We attributed the significant erosion resistance to the various protective coating observed on the steel piece.Especially after using PTW@PDA, the coating is flat and smooth in 20,000×magnification.We believed that the further heat transfer calculation can potentially explain the coating erosion resistance against the propellant gases.And the coating formation process possibly involve both the unique lattice structure of K2Ti6O13and the structure rearrangement at high temperature and high pressure, which should be further studied in theory in the future.Furthermore, theoretical flame temperature of the high-energy RDX-based propellant in this work is 3663 K.Therefore, it is not sure that whether similar Ti-K-based coating can be founded on the steel surface following the using of cool flame temperature propellant containing PTW@PDA.Based on erosion results and analysis of the morphologies and compositions of the coating,it is concluded that PTW@PDA can serve as an effective inorganic erosion inhibitor for high-energy gun propellant.
3.7.Mechanical properties of as-prepared propellants
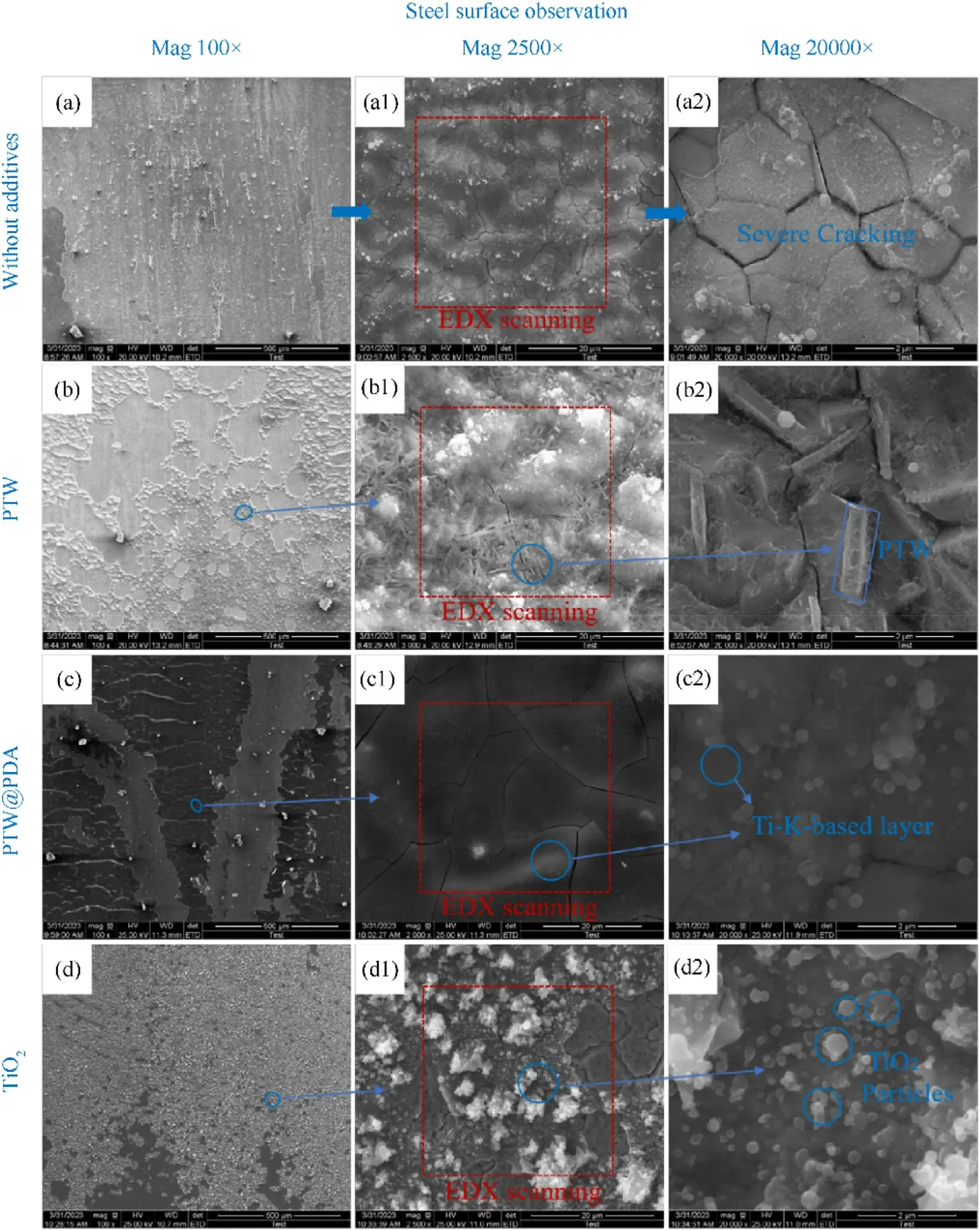
Fig.9.SEM images of erosion steel tube samples (inner surface) fired by propellants without additives and with PTW,PTW@PDA and TiO2 inhibitors in 3 wt%addition content at 100 × , 2500 × and 20,000 × magnification.
To meet the demands of high-energy propellants using in highchamber-pressure guns, propellants should have excellent mechanical properties to achieve controllable burning behaviors within an operational temperature range spanning from-40°C to 50°C [5,6,11,12].Fig.12 illustrates the impact strength results of modified propellant at-40°C,along with compressive and tensile strengths at -40°C, 20°C and 50°C, as PTW and PTW@PDA contents increase from 0 wt% to 3 wt%.The detailed results are presented in Table S5.The impact,compressive,and tensile strength of PTW and PTW@PDA-propellants exhibited an initial increase followed by a subsequent decrease upon the addition of both additives, that trends well agree with the mechanical improvement of carbon nanofibers(CNFs) and graphene nanoplates(GNPs) on NCTEGDN-RDX gun propellant in previous report[12,25].As shown in Figs.12(a),12(c)and 12(e),when the PTW content was 0.5 wt%,the PTW-propellant exhibited significantly enhanced impact,compressive, and tensile strength compared to the reference propellant without additives.When the PTW content reached to 3.0 wt%,the mechanical properties are poor than original propellant.The variation trends can ascribe to following reasons: Firstly, when addition content was in 0.5 wt%, raw PTW can still randomly dispersed in the propellant matrix, resulting in enhanced mechanical strength for propellants, which are similar with the reinforcement of PTW in other polymer composites [28,29].However,the presence of 3 wt% PTW agglomerations disrupts the homogeneity of the propellant binder, thereby compromising its integrity.The resulting defects in the propellant matrix are susceptible to localized stress concentration at the interface between PTW and the binder, ultimately leading to shear band formation and craze development under external stress.
On the other hand, as shown in Figs.12(b), 12(d) and 12(f),PTW@PDA-modified propellants exhibited significantly enhanced impact and tensile strength compared to PTW-propellants with the same additives content.Meanwhile, it was clear that the impact,compressive, and tensile strength of modified propellants with 0.5 wt%and 1.0 wt%PTW@PDA are both higher than that of original propellants without additives.A maximum impact and compressive strengths of propellants can be obtained after adding 0.5 wt%PTW@PDA.And a maximum tensile strengths of propellants samples were observed after adding 1.0 wt% PTW@PDA.The above results indicated that PTW@PDA can be used as superior mechanical reinforcer to improve the mechanical properties of high energy propellants, including impact, compressive and tensile strength.The improvement can be attributed to the following reasons:Firstly, the complementary advantages can be reflected in PTW@PDA composites, which combine both the distinct mechanical properties of high length-to-diameter ratio,low hardness,high strength and modulus of PTW and strong chemical adhesion of PDA coating layer.The unique needle-shaped structure of PTW@PDA restricts the expansion in transverse and longitudinal direction and provide resistance to stress and impact force under axial or radial loading for propellant.Besides, the presence of functional groups such as -NH2, -NH-, and -OH in PDA layer facilitated the formation of hydrogen bonds with -OH and -NO2functional groups in propellant binders, thereby promoting robust interfacial adhesion between PTW@PDA and the propellant matrix.Furthermore,the compression testing in this work was conducted in a quasistatic loading conditions with average strain rate of order of 0.01/s.And the higher strain rates (of order of 1000-6000/s) strength tests should be furtherly employed to evaluate the cracks and disruption of grains that can simulate the dynamic fast pressure rise during the shot[13].
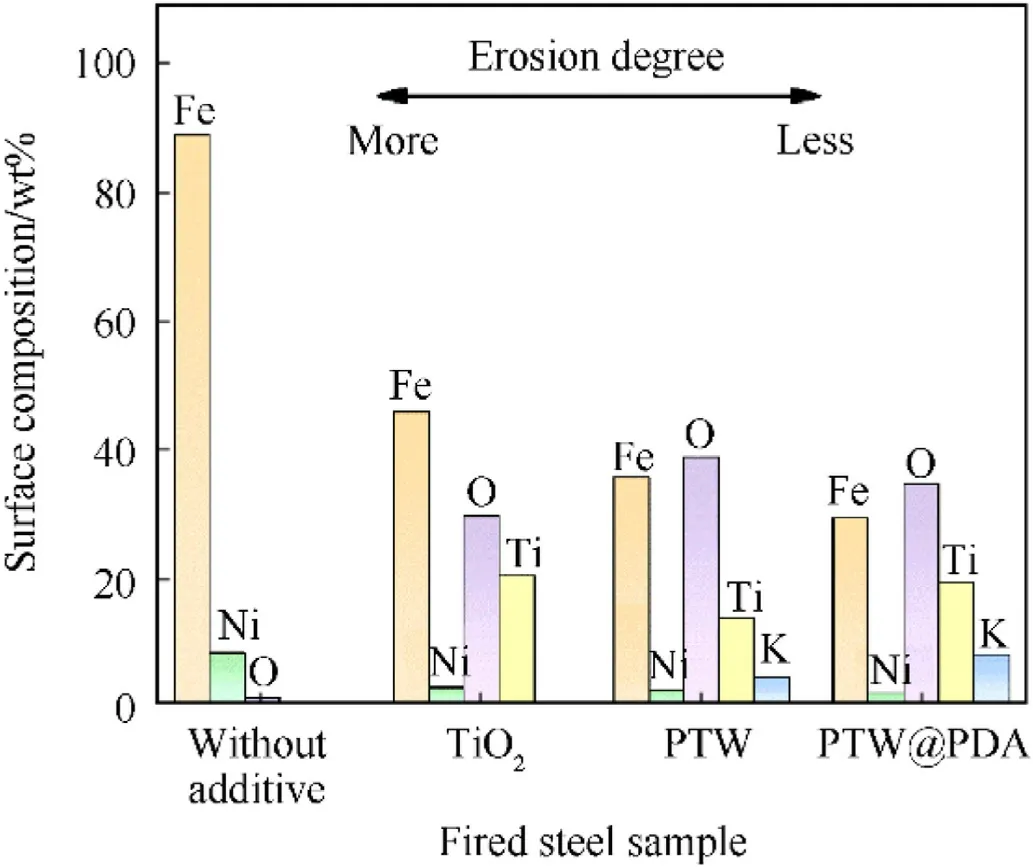
Fig.10.EDX analysis of surface chemical compositions for steel samples after firing propellants without additive and with TiO2, PTW and PTW@PDA in 3 wt% addition.
3.8.Combustion behaviors of as-prepared propellants
The 100 cm3closed bomb testing was employed to assess the impact of PTW and PTW@PDA on propellant combustion characteristics.Fig.13 shows the burning pressure-time (p-t), burning rate-pressure (u-p) and dynamic vivacity-relative pressure (L-B)curves of the propellants samples in the closed bomb vessel with the loading density of 0.20 g/cm3.Dynamic vivacity(L)and relative pressure (B) were used to reflect the changes in actual burning surface areas and burning progressivity of propellant, calculated according to Eqs.(4) and (5) [17,25,46]:
where pmand p(t) are the maximum pressure and the pressure at some time in the closed vessel experiment, respectively.The relative force (RF) is a comparison of the maximum burning pressure(Pm) between modified propellants containing additives and original reference propellants in a closed bomb vessel[2,46].
As shown in Figs.13(a) and 13(b), the value of maximum pressure(pm) of modified propellants are lower than that of original propellant.Table 2 shows that the RF values of modified propellant in 0.2 g/cm3loading density are less than 100%value.And Table S6 in Supplementary provided the calculated force constant,covolume and flame temperature by REAL software, which can calculate the thermophysical properties of propellants at high pressure and temperature.The observed energy loss can be explained as due to various percentage of inert PTW and PTW@PDA incorporated into the propellant composition.To satisfy the force requirements of powerful propellants, the content of inert additives should be limited to a low level.
From the u-p curves as shown in Fig.13(c) and 13(d), the modified propellants containing 0.5-3.0 wt% PTW, 0.5-1.0 wt%PTW@PDA additives exhibit increased burning rate with increasing pressure from 120 MPa to 240 MPa compared to original propellant without any additives.The results indicated that a probable catalytic effect of PTW and PTW@PDA can influence on combustion process of propellants.However, the exact mechanism involved in these processes remain unclear.
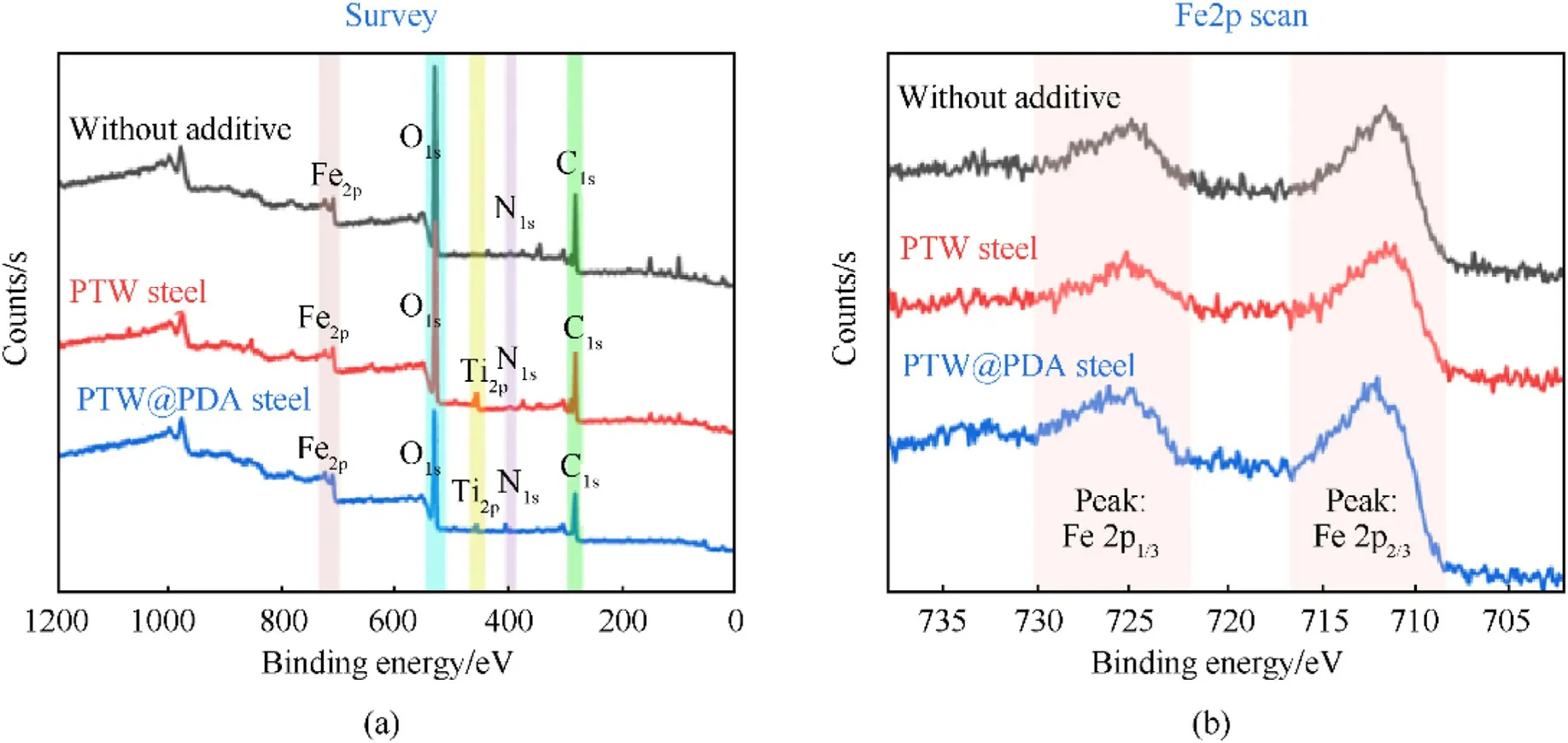
Fig.11.The (a) survey XPS spectra and(b) high-resolution XPS spectra of Fe2p region of erosion steel tubes (inner surface)fired with propellant without additives and with PTW and PTW@PDA erosion inhibitors.
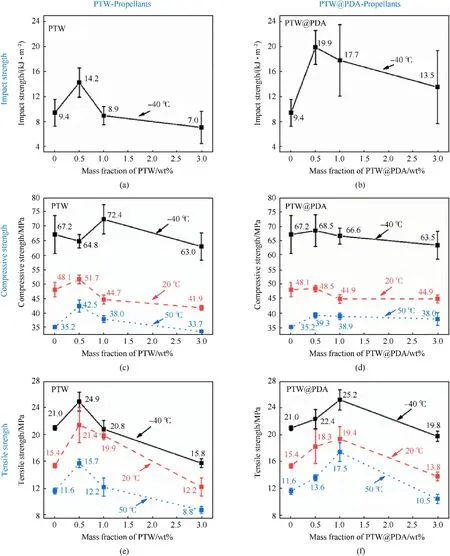
Fig.12.Variation of impact, compressive and tensile strengths versus mass fraction of PTW and PTW@PDA.
According to L-B curves as shown in Figs.13(e) and 13(f), the L values of modified propellants containing 0.5 wt%PTW,0.5-1.0 wt% PTW@PDA are slightly higher than those of original propellant within the B-value range of 0.2-0.8.The disparities feature that are likely attributable to non-uniform thickness of the tubular grains in different propellant samples.The L-values of PTW-and PTW@PDAmodified propellant samples exhibit a stably neutral burning behavior,with values ranging from approximately 1.0 to 1.2 within a B-value range of 0.2-0.8, consistent with the characteristics of typical tubular propellant grains [25].Moreover, the peaks appeared on the L-B curves at the beginning of combustion within a B-value range of 0.2-0.8,resulted from the burning of NC ignition powder, which exhibited a high dynamic vivacity (L) than tubular propellant grains.Meanwhile, the phenomenon can be also observed in burning of gun propellants with a tubular- [25] and multi-holes-shapes [59] without coating.The reason is probably that tubular and multi-hole shapes propellant usually have lower dynamic vivacity (L) at the beginning of combustion, compared to NC ignition powder.
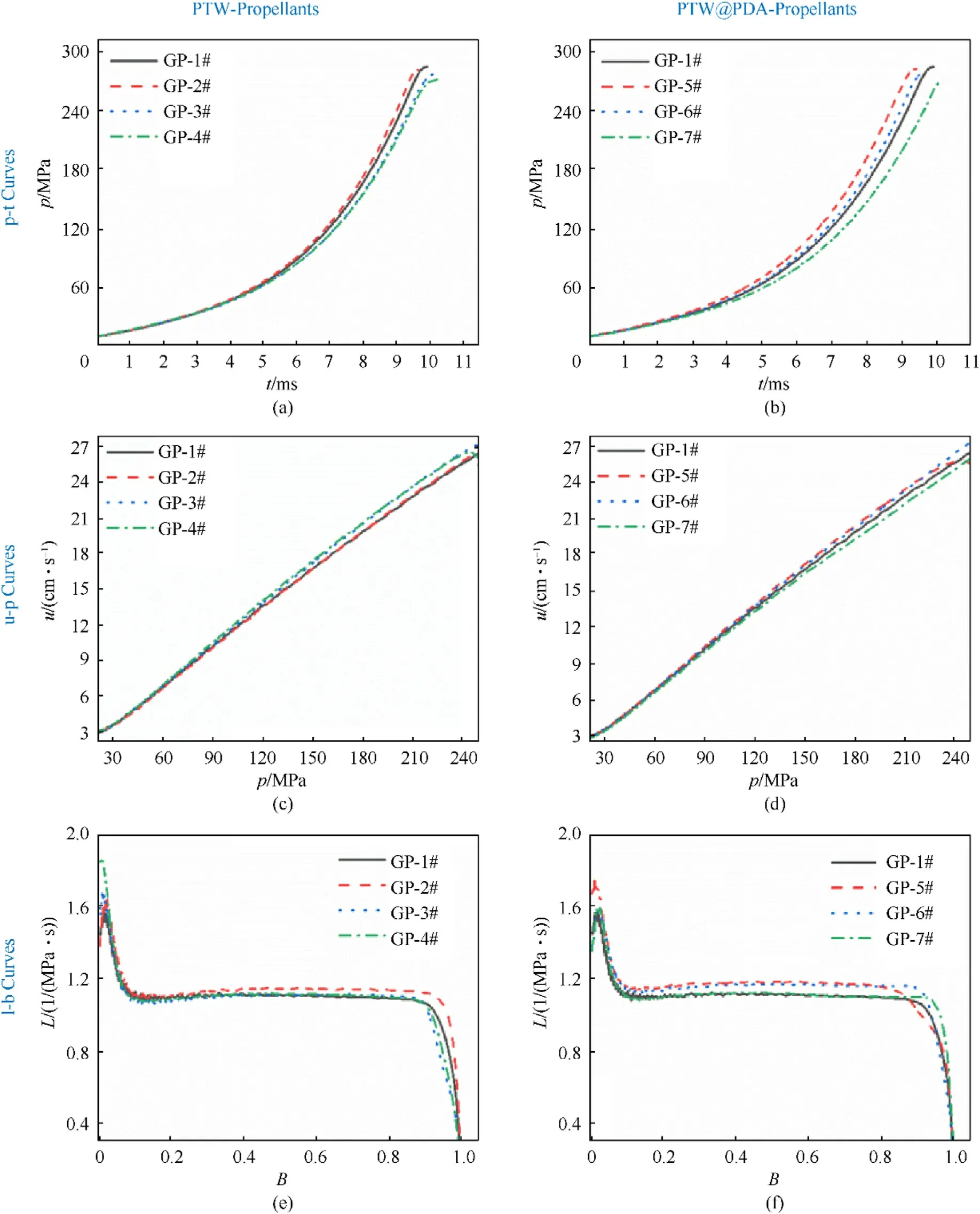
Fig.13.The (a), (b) p-t, (c), (d) u-p and (e), (f) L-B curves of propellants modified with various contents of PTW and PTW@PDA additives.
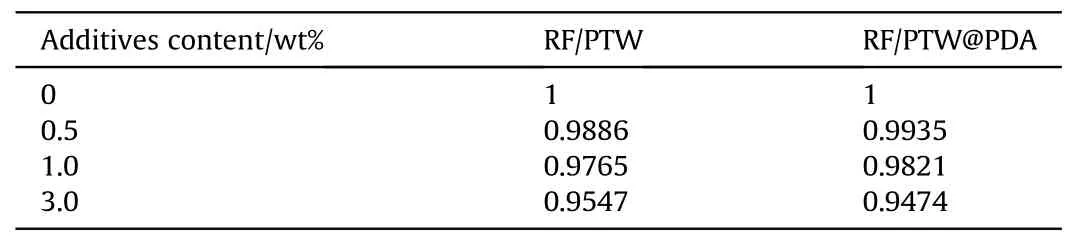
Table 2 Relative force(RF)of modified propellants with PTW and PTW@PDA additives.
4.Conclusions
In this work, novel bio-inspired PTW@PDA composites were prepared through the self-polymerization of PDA on the surface of PTW materials,resulting in enhanced interfacial compatibility with propellant binders and facilitating their random dispersion within the propellant matrix.After that, a series of gun propellants were manufactured by incorporating raw PTW and bio-inspired PTW@PDA composites, with the objective of mitigating erosion and enhancing mechanical strength in high-energy gun propellants.
It appeared that the complementary advantages can be reflected in PTW@PDA composites, which combines both the distinct properties of wear resistance and mechanical reinforcing of PTW material, and strong chemical adhesion of PDA layer.As an erosion testing result, PTW- and PTW@PDA-modified propellants shows obvious reduction in erosion than original referred propellant samples containing without inhibitors and with conventional TiO2inhibitor.In erosion testing, PTW@PDA-modified propellants containing with 0.5 wt%, 1.0 wt% and 3.0 wt% additives showed the least erosion for steel,resulting in erosion reductions of 4.3%,4.02%and 12.1% than original high energy propellant without inhibitors.Detailed SEM-EDX and XPS characterization of eroded steel samples after firing PTW@PDA propellants presented a protective coating containing Ti and K elements upon steel surface.The presence of the protective Ti-K-based coating is believed to be the primary factor accountable for the observed reduction in erosion of PTW@PDA-modified propellant.From the mechanical testing result, the PTW@PDA-modified propellants exhibited significantly higher impact, compressive and tensile strength with addition of 0.5 wt%and 1.0 wt%PTW@PDA composites in-40°C,20°C,50°C operating temperature compared to original propellants.Additionally,the results of combustion testing indicated that despite the inevitably reduction in relative force caused by PTW and PTW@PDA as inert additives, the two materials slightly increase propellant burning rate while exerting little adverse impact on propellant dynamic activity.Besides, the manufacturing approach for PTWand PTW@PDA-modified propellants is both scalable and costeffective, employing a conventional solvent-based propellant processing.This strategy potentially provided a promising route for further advancement in the development of high-energy gun propellant with less erosive and more mechanical strength,to meet the requirements of modern high chamber pressure guns.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge the support of the instrument and equipment fund of the Key Laboratory of Special Energy,Ministry of Education, Nanjing University of Science and Technology, China.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2023.11.018.
- Defence Technology的其它文章
- Ground threat prediction-based path planning of unmanned autonomous helicopter using hybrid enhanced artificial bee colony algorithm
- Layered metastructure containing freely-designed local resonators for wave attenuation
- Predicting impact strength of perforated targets using artificial neural networks trained on FEM-generated datasets
- Construct a 3D microsphere of HMX/B/Al/PTFE to obtain the high energy and combustion reactivity
- Ignition processes and characteristics of charring conductive polymers with a cavity geometry in precombustion chamber for applications in micro/nano satellite hybrid rocket motors
- Recent research in mechanical properties of geopolymer-based ultrahigh-performance concrete: A review

